Abstract
Photodynamic therapy (PDT) holds great potential to overcome limitations associated with common colorectal cancer (CRC) treatment approaches. Targeted photosensitiser (PS) delivery systems using nanoparticles (NPs) with targeting moieties are continually being designed, which are aimed at enhancing PS efficacy in CRC PDT. However, the optimisation of targeted PS delivery systems in most, in vitro PDT studies has been conducted on two dimensional (2D) monolayers cell cultures. In our present study, we developed a nano PS delivery system for in vitro cultured human colorectal three-dimensional multicellular spheroids (3D MCTS). PEGylated gold nanoparticles (PEG-AuNPs) were prepared and attached to ZnPcS4PS and further functionalised with specific CRC targeting anti-Guanylate Cyclase monoclonal antibodies(mAb). The ZnPcS4-AuNP-Anti-GCC Ab (BNC) nanoconjugates were successfully synthesised and their photodynamic effect investigated following exposure to laser irradiation and demonstrated enhanced anticancer effects in Caco-2 cells cultivated as 3D MCTS spheroids. Our findings suggest that targeted BNC nanoconjugates can improve the efficacy of PDT and highlight the potential of 3D MCTS tumour model for evaluating of targeted PDT.
Introduction
Colorectal cancer (CRC) is a highly lethal type of cancer that accounted for 9.4% of cancer-related deaths in 2020 and its prevalence is expected to rise to 3.2 million new CRC cases in 2040 [Citation1]. Conventional treatments like surgery, chemotherapy, and radiotherapy, while widely used, often fall short due to their limitations such as non-specific targeting and toxicity to healthy tissues [Citation1,Citation2]. In this regard, there is an urgent demand to explore alternative therapies for CRC, among which photodynamic therapy (PDT) has gained attention [Citation3]. Photodynamic therapy is a relatively non-invasive therapeutic approach that selectively damage malignant cells while minimising side effects [Citation4,Citation5]. It relies on the activation of a light-sensitive photosensitiser (PS) using visible light in the presence of molecular oxygen, causing reactive oxygen species production [Citation4]. The process then leads to irreversible cell damage and localised cell death [Citation4]. Zinc phthalocyanine tetrasulfonate (ZnPcS4) is a second generation photosensitiser with strong absorption in the near infra-red region (670–680 nm), making it a suitable candidate for PDT applications [Citation6,Citation7]. Notably, these photosensitising properties makes it proficient in singlet oxygen generation, which is crucial for inducing cell death [Citation7]. Studies suggest that ZnPcS4 PS has lower affinity for healthy tissues compared to cancerous colon tissues, indicating its potential value in CRC photodynamic therapy [Citation8]. However, classical photosensitizers can be compromised by poor cellular uptake, hydrophobic nature and unfavourable biodistribution, resulting in suboptimal PDT therapeutic outcomes [Citation9].
To improve the delivery of PSs, targeted delivery approaches using nanoparticles (NPs), notably gold nanoparticles (AuNPs) have been explored where various photosensitizers including ZnPcS4 PS, are encapsulated or attached onto nanoparticles [Citation5]. AuNPs stand out among various nanoparticles due to their unique properties and ability to improve PS delivery and photodynamic anticancer effects in CRC-PDT treatment [Citation3,Citation5]. AuNPs, with their tuneable surface properties and large surface area, allow for efficient functionalisation with targeting moieties like antibodies, facilitating specific accumulation at cancerous sites [Citation10–12]. Within our study, polyethylene glycol (PEG) was utilised to shield AuNPs, rendering them less prone to elimination by the reticuloendothelial system (RES) and biological barriers, as well as enhance their hydrophilicity [Citation12–14]. Additionally, monoclonal antibodies (mAbs) Anti-Guanylate Cyclase mAb (Anti-GCC) were attached to ZnPcS4 PS loaded-pegylated amine stabilised AuNPs, forming ZnPcS4- PEG -AuNPs- Anti-GCC nanoconjugates (denoted BNC nanoconjugates). These nanoconjugates were designed to specifically target CRC cells, which often overexpress Guanylyl cyclase C (GUCY2C) protein genes and GCC receptors on their surfaces [Citation15], therefore could potentially improve the efficacy of photodynamic therapy (PDT) by enhancing PS uptake while minimising damage to healthy cells [Citation3]. For example, research studies from a study suggest that employing HER2 receptor or jacalin, a lectin targeting carbohydrate T antigen, with PEG-AuNPs and ZnPc (C11Pc) on HT-29 CRC cells can enhance targeted PDT, leading to reduced cancer cell proliferation [Citation16]. Therefore, actively targeted nano PS delivery approaches may hold promise for potentially improving CRC PDT efficacy [Citation5].
Ideally, studies conducted on in vitro cell culture models have been instrumental in optimising, predicting, and assessing the potential therapeutic outcomes of nano-PS delivery therapeutic in pre-clinical research [Citation17]. Although actively targeted PDT treatments have shown promise in two dimensional (2D) cell culture models, their translational potential to clinical research is limited due to their inability to closely mimic the 3D architecture of tumour tissues [Citation17,Citation18]. In recent years, three-dimensional (3D) culture systems, such as multicellular tumour spheroids, have gained recognition as more reliable models for predicting in vitro PDT outcomes [Citation18,Citation19]. The use of multicellular tumour spheroids (MCTS) in anticancer drug testing and PDT is prevalent due to their ability to mimic in vivo microenvironments, relative to traditional 2D cell cultures [Citation19,Citation20]. MCTS exhibit heterogeneity with distinct layers – proliferation, quiescent, and necrotic – resembling physiological tumour conditions, thus providing a more accurate cell culture model [Citation21]. Moreover, through 3D structural replication, cellular interactions and growth kinetic properties, MCTS mirror key aspects of the tumour microenvironment [Citation18,Citation19]. Notably, studies emphasise the significance of MCTS in understanding molecular activities and PDT responses [Citation22]. In their studies, Pereira et al. observed that ROS-induced phototoxicity was higher in HCT-116 monolayer cultures treated with PorGlu4 compared to their spheroid cultured counterparts [Citation22]. This discrepancy may suggest that spheroid cultures exhibited resistance to phototoxicity than monolayer cultures [Citation22]. Generally, optimisation of targeted PS delivery systems in many in vitro PDT studies has focused on 2D monolayer cell cultured models [Citation18]. However, despite the availability of studies utilising 3D cellular models like spheroids, limited research on targeted NP-PSs-antibodies delivery systems in PDT for CRC within in vitro 3D MCTS Caco-2 cell culture model has been reported, which warrants the need for additional studies in this area [Citation18]. Thus, in our present study, we have set out to synthesise and characterise the active targeting BNC multicomponent system and investigate the photodynamic response of in vitro 3D multicellular spheroids, which were generated from in vitro Caco-2 human colorectal cancer cell lines post exposure to ZnPcS4 PS loaded to pegylated amine stabilised AuNPs conjugated to CRC-specific targeting antibodies- Anti-Guanylate Cyclase Ab; Anti -GCC (denoted BNC) -PDT.
Materials and methods
Materials
Gold (III) chloride trihydrate (HAuCl4.3H2O, ≥99.9% trace metals basis, Mw = 393.83 g/mol), tri-sodium citrate (for molecular biology, ≥99%), tannic acid (ACS reagent), SH-PEG2k-NH2 (HCl salt, average Mn: 2000) and phosphate-buffered saline (PBS, 10× concentrate, BioPerformance Certified, suitable for cell culture) were purchased from Sigma Aldrich. Zinc phthalocyanine tetrasulfonate (ZnPcS4 PS) was purchased from Santa Cruz® Biotechnology sc-264509A). Human colon cancer cells (CaCo-2 Cellonex Cat SS1402 CCAC-FL; CCAC-C) were purchased from the (American Type Culture Collection, Manassas, VA, USA). Monoclonal Anti-GCC Ab (Abcam: ab122404). 96-well ultra-low attachment plates (174,929) were acquired from ThermoFisher, Johannesburg, South Africa. All chemicals and reagents utilised in the study were of analytical grade. Millipore water with a resistance of 18.25 MΩ cm was utilised to prepare aqueous solutions. All glassware were autoclaved and rinsed with aqua regia solution before synthesis.
Characterisation of nanoparticles and conjugates
Fourier-transformed infrared (FT-IR) spectroscopy (FTIR)
Homogenous samples underwent Fourier-transformed Infra-red (FT-IR) spectroscopy analysis, performed using a Spectrum 100 FT-IR Spectrometer (PerkinElmer Spectrum). Briefly, samples were mixed with dry Potassium Bromide (KBr) powder to form semi-thick round discs. The infra-red spectra data was read at 4000–400 cm−1 frequency range with 25 scans using infra-red solution software.
High resolution transmission electron microscope (HR-TEM)
TEM investigations were carried out on a JEM-2100 High Resolution Transmission Electron Microscope (HR-TEM) (JEOL Ltd.) equipped with Energy Dispersive X-ray Spectroscopy (EDX) detector beams was used.to obtain images and analyse elemental composites of the sample respectively, where samples were loaded onto carbon-coated 200-mesh copper grid, air dried in the dark and analysed using the microscope.
DLS and Zeta potential
The dynamic light scattering technique was used to perform particle size and distribution measurements, using the Malvern Zetasizer Nano ZS (Malvern Instruments, Malvern UK), equipped with a 4 mW He-Ne laser of 633 nm wavelength. Samples were loaded into Zeta 3 × 3 mm dip cell cuvette, which had in-built electrodes that was used for DLS and Zeta measurements.
Cell culture
Human colon cancer cells- Caco-2 (Caco-2 Cellonex Catalog SS1402 CCAC-FL; CCAC-C) were obtained from the American Type Culture Collection. Caco-2 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) media, with 10% foetal bovine serum (FBS), 100 U penicillin and 100 μg/ml streptomycin solution, 2.5 μg/ml amphotericin B and 1 mM sodium pyruvate. When 90% confluence was reached, the cells were trypsinized with 0.25% trypsin solution to collect and counted. Caco-2 cells were then inoculated into 96 well nuclon low attachment U bottom plates (Thermo Scientific™) at density of 5,000 cells in 200 µl/well and incubated for 48 h at 37 °C, 85% humidity and 5% CO2 conditions to allow for the generation of MCTS prior to treatment.
Photodynamic treatment on MCTS
The experimental groups were divided into two subgroups, with and without laser irradiation. Photodynamic irradiation was carried out after incubation of spheroids with varying doses of (0.125, 1.5, 3 and 5 µM) ZnPcS4 PS within dose response studies or predetermined IC 50 value of 3 µM of ZnPcS4 in all nanoconjugates (ZnPcS4- AuNPs or ZnPcS4- AuNPs- GCC ab (BNC). 24 h post PS incubation, Caco-2 multicellular spheroids were exposed to the Roithner 1000 mA 673 nm high power diode laser (Arryo 4210) supplied by National Laser Centre (NLC) of South Africa, at fluence of (10 J/cm2). Non irradiated spheroids used as controls consisted of spheroids kept in the dark for the same durations of time as irradiated spheroids. Experiment was repeated n = 3. PDT experiments were conducted in the dark.
Assessment of ZnPcS4 and ZnPcS4- AuNPs-GCC ab (BNC) uptake and distribution
After seeding onto 96 well nuclon low attachment U bottom plates, uniform and compact spheroids were chosen, after observation under an inverted light microscope (Wirsan Olympus, CKX 41, Johannesburg, South Africa) with an attached digital camera (Olympus, C5060-ADUS, Johannesburg, South Africa). The selected MCTS were then incubated with DMEM media containing free ZnPcS4, ZnPcS4- AuNPs or ZnPcS4-AuNPs-GCC ab (BNC) at ZnPcS4 concentration of predetermined IC50 of 3 µM, respectively. Post incubation, Caco-2 spheroids were rinsed with PBS and Z-stack images of the spheroids were acquired using Carl Zeiss Axio Z1 microscope and images were analysed by using ZEN 3.1 (ZEN pro) software (Carl Zeiss, Göttingen, Germany).
ZnPcS4-AuNPs-GCC ab (BNC) mediated PDT effects
Morphological assessment
Morphological changes in Caco-2 MCTS were observed under the inverted light microscope (Wirsan Olympus CKX 41, Johannesburg, South Africa) connected to a digital camera (Olympus C5060-ADUS), before and post 24 h of PDT treatment. Morphological changes were evaluated by observing the treated control population and comparing them to the untreated population. CellSens version 2.3 imaging software was used to capture images and analyse morphological changes.
Lactate dehydrogenase (LDH) assay
The release of LDH into the supernatant culture medium shows the occurrence of cell membrane damage [Citation23]. To further confirm cytotoxicity and cell damage post PDT treatment, the medium supernatant was collected and measured following manufacturer’s protocol. The amount of LDH released into medium is directly proportional to the degree of cell damage, which was the measured using the 3D Cyto Tox 96® Non-Radioactive Cytotoxicity assay (Promega, G179A), according to the manufacturer’s instructions. Absorbance was measured at 490 nm using PerkinElmer, VICTOR NivoTM.
Statistical analysis
All data were statistically analysed with Sigma software (version 12.0 software). One-way ANOVA with Dunnett test was used for statistical analyses to determine the significance of the difference between the cells only control and experimental groups. Results were presented as mean ± standard error (SEM), unless stated otherwise, of at least three (n ≥ 3) independent experiments. Values in the 95% confidence interval (p < 0.05 *, p < 0.01 **, or p < 0.001 ***) were accepted as statistically significant and the standard error was defined by dispersion bars.
Results
Characterisation of nanoparticles and BNC conjugates
HRTEM analysis
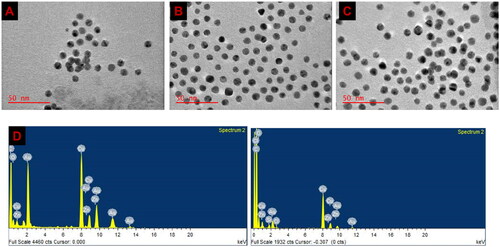
The morphological and size distribution analysis relating to the nanoconjugates were obtained using HRTEM and were presented as digital images () (A) PEG AuNPs, (B) ZnPcS4-PEG-AuNPs and (C) ZnPcS4-PEG-AuNPs-Anti GCC Ab. TEM imaging showed that the nanoconjugates were < 100 nm in size and spherical as well as revealed an almost homogenous structure of the BNC, suggesting that the interactions of the compounds and NPs did not induce aggregated. Furthermore, the elemental composition of BNC was also determined by employing TEM equipped with an EDX detector. As seen in , the analysis of EDX showed the appearance of Zn atom peaks at ∼1 keV and ∼8.7 keV and Au atom peaks at ∼2.2, 9.5 and 11.5 keV on the BNC EDX spectrum, confirming the presence of these elements after conjugation.
Dynamic light scattering (DLS) and Zeta potential (ZP)
The size distribution and the surface charge of the final nanoconjugate in aqueous solution was further studied using the dynamic light scattering method. As seen in the average diameter sizes of the AuNPs before and after loading with ZnPcS4 and GCC mAb, were approximately around 50–60 nm. Our results showed that the aqueous solution contained final conjugates had an average hydrodynamic particle size of 60.91 nm with a mono dispersity index of 0.434. In addition, the zeta potential was used to determine the surface charge and the stability of nanoconjugate aqueous dispersions. The results of the zeta potential measurements revealed that the final nanoconjugates exhibited a low negative zeta potential of 19.8 mV, suggesting the nanoparticles appear fairly stable which is favourable for preventing PS drug leakage prematurely from AuNPs in physiological environment.
Table 1. Mean DLS and Zeta potential.
Photodynamic therapy dose response
Assessment of membrane integrity (LDH assay)
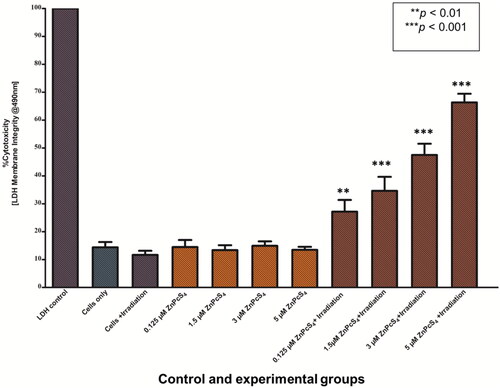
To assess the membrane integrity Caco-2 MCTS 24 h post laser irradiation, LDH assay was performed to measure the LDH release levels. In the absence of laser irradiation and no incubation with ZnPcS4 (cells only group), no significant increase in LDH release content was observed in untreated control cells of spheroids. Similarly, the administration of ZnPcS4 alone (of varying doses 0.125, 1.5, 3 and 5 µM) to cells of spheroids or exposure of cells in the presence of laser irradiation only resulted in no significant increase in LDH release in the supernatant media. These findings suggest that ZnPcS4 alone and laser irradiation have no toxic effect on cancer cells. Notably, after PDT treatment, a statistical significantly increase in LDH release in a dose dependent manner was observed the PDT treated cells of spheroids (p < 0.01** and p < 0.001***). The IC50 concentration of ZnPcS4 in MCTSs was determined by means of a linear regression curve. The IC50 of ZnPcS4 was found to be 3 µM, which was then used throughout the experiment ().
Assessment of uptake of BNC in MCTS using fluorescence imaging
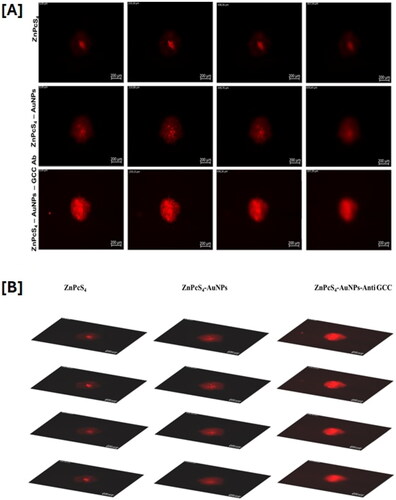
The uptake and distribution of free ZnPcS4 PS and bionanoconjugates in Caco-2 spheroids were assessed by using the 40× oil immersion objective on a Carl Zeiss Axio Z1 microscope (Carl Zeiss MicroImaging GmbH) with the 649Ex/670Em for autofluorescence of the PS. As shown in , the MCTS treated with free ZnPcS4 PS and ZnPcS4- AuNPs all displayed a red autofluorescence which was distributed in the rim and within the depths of the spheroids. Minimal uptake was observed in MCTS incubation with ZnPcS4 alone. Following incubation with ZnPcS4-AuNPs, MCTS exhibited improved uptake patterns, suggesting efficient uptake. However, spheroids incubated with BNC constructs (ZnPcS4-AuNPs – anti-GCC Ab) displayed clear evidence of significantly deeper penetration to the core and higher fluorescence signal in the Z-projection images as shown, compared to the free ZnPcS4 PS images, suggesting higher cellular uptake of ZnPcS4- AuNPs – anti-GCC Ab in spheroids, which may likely reflect that Caco-2 cells expressed levels of GCC receptors which facilitates uptake of BNC nanoconjugates.
PDT effects of active targeted (ZnPcS4-PEG-AuNPs-Anti GCC Ab) BNC
Morphological assessment
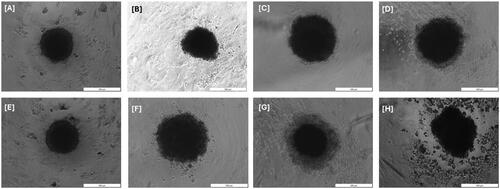
MCTS growth and integrity easily can be visualised by a light inverted microscopy. We observed that untreated MCTS exposed to neither laser nor ZnPcS4 PS showed continuous growth and maintained a dense structure, as shown in . Spheroid damage could be observed 24 h after irradiation in ZnPcS4 PS, ZnPcS4-AuNPs, and BNC. At this point, spheroids disintegrated with dead cells detaching from the outer cell layers and increased debris surrounding the spheroid. This effect was more pronounced in BNC – irradiated spheroids with damage towards the inner layer and abundance in debris on the outer rim suggesting that the cell layers were predominately affected by BNC induced PDT treatment compared to ZnPcS4 or ZnPcS4-AuNPs – irradiated spheroids.
Figure 4. Microscopical images of MCTS cells and their morphological changes before (A–D) and after (E–H) irradiation treatment by 24 h. (A) Non-irradiated MCTS cells, (B) Non-irradiated ZnPcS4, (C) Non-irradiated ZnPcS4-AuNP, (D) Non-irradiated BNC formulation, (E) Irradiated MCTS cells, (F) Irradiated ZnPcS4, (G) Irradiated ZnPcS4-AuNP, and (H) Irradiated BNC formulation.
Assessment of membrane integrity (LDH)
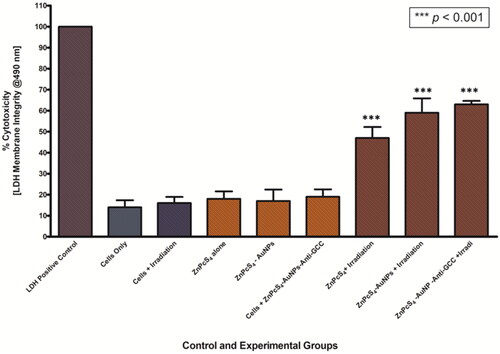
LDH released to the supernatant 24 h after PDT-mediated treatment with ZnPcS4, ZnPcS4-AuNPs and BNC conjugates was measured, to determine membrane integrity of MCTS, and presented in . In the control groups of untreated control cells of spheroids or MCTS exposed to laser irradiation alone or treated with nanoconjugates alone, LDH release activities were not increased. In contrast, the level of LDH contents released was increased in PDT treated with ZnPcS4 and BNC spheroid groups compared to the control group (p < 0.001***), suggesting that the treatment was phototoxic to MCTS. Furthermore, in PDT experimental groups incubated with 3 µM BNC and laser irradation, we found maximum levels of LDH release that show the highest toxicity with 63% LDH release. These findings indicated that the membrane integrity of Caco-2 spheroids was disrupted in the presence of BNC and these effects are closely associated with the highly toxic effects of the BNC-PDT.
Cellular viability
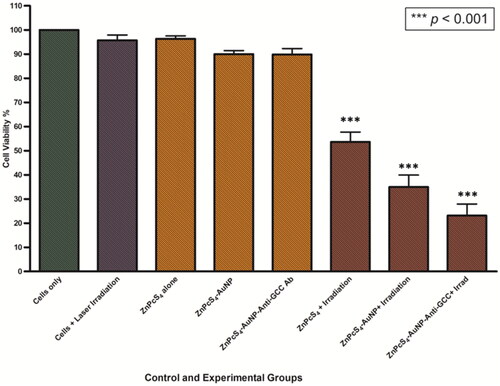
The phototoxic effects of ZnPcS4, ZnPcS4-AuNPs and BNC formulations on the viability of Caco-2 spheroids were measured, 24 h post irradiation exposure (after incubation), using the MTT cellular viability kit. There was no significant reduction in viability observed on spheroids exposed to dark conditions; ZnPcS4 alone or nanoconjugates alone (i.e. ZnPcS4-AuNPs and BNC), as well as in laser irradiation alone, in comparison to the untreated control cells of spheroids. On the opposite, a remarkable decrease in cell viability was observed in Caco-2 spheroids following treatment with ZnPcS4 and nanoformulations (ZnPcS4-AuNPs and BNC) in the presence of laser irradiation at fluency of 10 J/cm2 with 673 nm laser (). ZnPcS4 in the presence of laser irradiation showed a decrease in cell viability, which led to about 55% viable cells. Most notably, PDT-induced treatments with 3 µM ZnPcS4-AuNPs and BNC, showed a significant reduction in cell viability (p < 0.001***) (34.9% and 23.1% viable cells respectively), suggesting that BNC nanoconjugates enhanced the cytotoxicity of ZnPcS4 and more promising in eliminating tumour spheroids.
Colony formation-survival assay
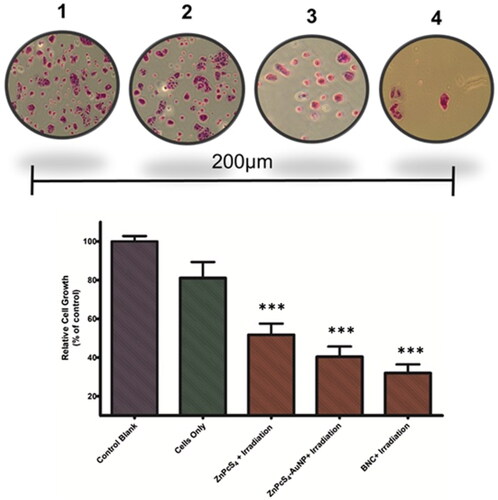
The colony formation assay is used to assess the capability of single cells to survive and grow into colonies post treatment [Citation24]. The in vitro cell survival studies were then conducted to investigate the ability of Caco-2 cells of spheroids to form colonies post PDT treatment with BNC + irradiation. Representative images of the colonies and the quantification of the colony formation assessment are shown in . According to these findings, cells exposed to PDT treatment revealed a significantly decrease in colon formation after 7 days post exposure to PDT treatment. Crystal violet staining of Caco-2 cells of spheroids showed significantly decreased colonies formation in BNC-treated cells compared to control groups which was consistent with the morphological assessment as well as cell viability studies observed within the studies, indicating that the BNC could significantly inhibit Caco-2 cancer cell proliferation.
Figure 7. Cells of spheroids stained with crystal violet, assessing colony formation abilities following PDT treatment (1). Cells controls (2) ZnPcS4 + Irradiation (3) ZnPcS4-AuNP + Irradiation (4) BNC + Irradiation. Data presented as the mean ± standard deviation from three independent experiments, *** p < 0.001, compared with control.
ROS production
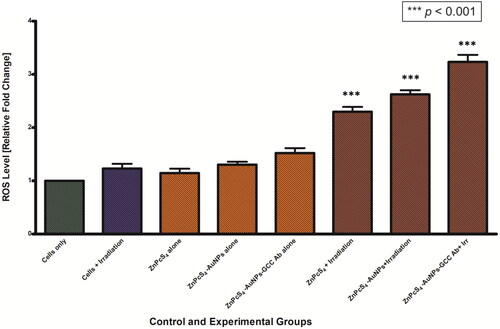
We further assessed whether PDT treatment had any effect on the levels of reactive oxygen species (ROS), which are well known cell damage inducers, by using the DCFH-DA ROS sensitive probe, which has marker for ROS oxidative stress levels. From , it can be observed that there were no significant changes in intracellular ROS levels in all the control groups that were not exposed to laser light (ZnPcS4 alone, ZnPcS4-AuNPs alone, and BNC nanoconjugates alone) compared to cells only control groups. In contrast, upon laser exposure the level of ROS was observed to increase in the experimental groups treated with ZnPcS4, ZnPcS4-AuNPs, and BNC nanoconjugates. Cells of spheroids treated with BNC nanoconjugate showed a statistically significant increase (p < 0.001) of ROS production levels, with a maximum increase of ∼2 fold observed post treatment, compared to cells only control.
Discussion
Despite the availability of existing mainstream treatment approaches, CRC still presents with high mortality rates and poor survival rate, especially in late-stage diagnoses [Citation1,Citation3]. PDT emerges as a promising minimally therapeutic approach with minimal toxicity [Citation4]. In recent years, there has been growing interest in utilising nanoparticles such as AuNPs to improve the delivery of PS to cancerous tissue [Citation12]. Currently, 2D cell culture models are widely used to evaluate cellular uptake and cytotoxicity effects of photosensitizers and newly developed PS- nanoparticles- delivery platforms in in vitro photodynamic studies [Citation25–27]. Our recent in vitro research focused on conjugating core/shell Ag@mSiO2 NPs with ZnPcS4 PS, modified with folic acid, to investigate their efficacy against colorectal cancer [Citation7]. Results indicated that these active nanoconjugates exhibited improved cellular localisation and improved anticancer effects in 2D Caco-2 tumour cell cultures [Citation7]. Therefore, the development of site-specific PS-nanoparticle delivery systems offers promise in overcoming challenges associated with conventional photosensitizers [Citation3,Citation5]. However, the investigation of actively targeted PS delivery nanoconjugates, particularly AuNP functionalization with tumour-targeting molecules like antibodies, has not yet be fully investigated in in vitro 3D multicellular Caco-2 CRC tumour spheroids for PDT applications [Citation18,Citation26]. Evidence suggests that 3D multicellular spheroids culture models closely mirror in vivo cellular microenvironment, making them valuable models compared to 2D cell cultures models [Citation20,Citation22]. Therefore, 3D in vitro models could be indispensable for further understanding the efficacy of actively targeted PS-NPS delivery systems in antitumour activity within CRC PDT treatment investigations, potentially facilitating the translation of in vitro research findings into clinical applications [Citation18].
In this work, we synthesised, and characterised ZnPcS4-AuNPs delivery system that was functionalised with anti GCC antibodies and we determined the photodynamic activities of the nanosystem in 3D spheroids [Citation18].
The FT-IR spectroscopic analysis of BNC and its components were displayed comparatively (Figure S1) and indicated successful integration into the nanocarrier system. Characteristic peaks of C = O functional groups and S = O stretching pegylated AuNPs and ZnPcS4 PS were observed, respectively, alongside evidence of characteristic peaks of (C = O) and the weaking NH bending vibration indicating functionalization with anti GCC mAb, confirming successful conjugation via EDC-NHS reaction. Several studies have highlighted the significance of AuNPs ranging from 1 to 100 nm in size, for their effective accumulation in tumour tissues, penetration through cellular membranes and enhancement of PDT [Citation3,Citation11]. The TEM image confirmed the mono-dispersed and spherical morphology of the nanoparticles, indicating minimal aggregation. This characteristic contributes to increased internalisation of photosensitizers into cancerous cells through the enhanced permeability and retention effect (EPR), along with the presence of anti GCC Ab which interacts with GCC receptors on CRC cell membranes [Citation5,Citation28]. EDX spectrum analysis revealed the presence of zinc and Au peaks, as the primary elements constituting the resulting BNC nanoformulations. Additionally, the artificial signals of copper observed in the EDX spectral analysis, were likely from the mesh copper grid residue used during analysis, consistent with findings from previous research [Citation29]. DLS measurements also further confirmed monodispersed nature of the nanoparticles. Intriguingly, the DLS analysis showed a size distribution between 50 and 60 nm, compared to 13 nm observed in TEM. This discrepancy maybe due to their interactions influenced by the Brownian motions effect [Citation30] and entrapment of the bio-compounds on the surface of the NPs, increasing their thickness. The effects of nanoparticle size and shape has been reported in studies that synthesised spherical shaped nanoparticles of < 60 nm and reported their effectiveness in CRC anticancer activities [Citation16,Citation31]. Studies have reported that nanoparticles with a zeta potential more negative than −30 mV are considered stable [Citation32]. Our BNC nanoconjugates, with a zeta potential of −19.8 mV, exhibited stability, which could potentially enhance PS accumulation and delivery to CRC cells.
In view of the remarkable physicochemical characteristics of the BNC nanoparticles, we then evaluated the in vitro PDT potential of BNC nanoparticles against Caco-2 MCTS cultures. LDH release post- treatment with free ZnPcS4 to evaluate membrane integrity was measured using the LDH assay, as LDH assay is deemed more accurate for spheroids [Citation33]. Incremental LDH levels with increasing concentration of ZnPcS4 showed an IC50 value of 3 µM, suggesting that higher ZnPcS4 concentration was required for spheroids treatment compared to Caco-2 monolayers [Citation7], reported elsewhere, consistent with previous observations of MCTS resistance to PDT compared to monolayers [Citation22,Citation34]. To ensure that the BNC effectively exert phototoxic activities on spheroids, it is necessary to investigate the penetration and uptake studies of the nanoconjugates [Citation35]. Enhanced fluorescence signals in spheroids incubated with ZnPcS4-BNC nanoconjugates in comparison to the free ZnPcS4 was observed, suggestive of improved uptake facilitated by anti-GCC mAb targeting GCC receptors within CRC cells (). Surface functionalisation, therefore of nanoparticles could have played a role in efficient delivery of PS drug within 3D spheroids, in agreement with existing literature [Citation22,Citation36,Citation37]. As expected, Caco-2 spheroids treated with BNC nanoconjugates and PDT displayed more pronounced cell damage observed as dead cells detaching from the outer cell layers and increased debris surrounding the spheroid, compared to non- treated groups or free PS, as evidenced by morphological assessment analysis. The increased LDH release post BNC -mediated PDT was also suggestive that it was more likely caused by the improved actively targeting of the PS, which was supported by the significant reduction in cell viability relative to controls. In addition, the survival rate was greatly reduced in BNC -mediated PDT relative to control groups, 7 days post treatment. Overall, our in vitro results supported that post exposure to laser irradiation, the BNC + irradiation significantly increased LDH release levels, promoted a decrease in cell viability, inhibition of cell growth and reduction in ATP levels relative to control groups ( and Figure S3, respectively). Studies have reported that cell death mechanism induced by PDT,such as apoptosis and necrosis, are primarily triggered by reactive oxygen species (ROS) generation in the cancerous cells [Citation13]. So, lastly, we investigated the intracellular ROS levels induced by BNC nanoconjugates using the DCFH-DA ROS sensitive probe assay. Significantly increased intracellular ROS levels, up to two folds were observed in BNC treated groups, likely due to enhanced accumulation and uptake of BNC in spheroids facilitated by antibody-decorated AuNPs. This finding is in accordance with previous reports for targeted PDT treatment in multicellular spheroids, suggesting that active targeted PS delivery using nanoconjugates can potentially result in high ROS generation [Citation22,Citation36].
Conclusions
In conclusion, the present study assessed the potential of ZnPcS4-PEG-AuNPs-Anti GCC Ab targeted photodynamic therapy in colorectal cancer, against 3D multicellular tumour spheroid models. The most pronounced PDT effects were observed in BNC actively targeted treated population. This feasibility study demonstrates the potential of active targeting PS with nanoparticles in PDT-based treatment. However, further in vitro and in vivo studies are still required.
Authors contributions
Conceptualisation, N.W.N.S and H.A; Methodology, N.W.N.S; Investigation N.W.N.S; Writing original draft preparation, N.W.N.S; Writing-review and editing, N.W.N.S, H.A; Supervision, H.A.; Funding acquisition. H.A.
Supplemental Material
Download MS Word (435.6 KB)Acknowledgements
The authors gratefully acknowledge the support from the Organization for Women in Science for the Developing World (OWSD) and the Swedish International Development Cooperation Agency (SIDA) for the fellowship received by N.W.N.S.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the results are available in this article. Additional data are available from the corresponding author, upon reasonable request.
Additional information
Funding
References
- Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. 2021;14(10):101174. doi: 10.1016/j.tranon.2021.101174.
- Gunaydin G, Gedik ME, Ayan S. Photodynamic therapy—current limitations and novel approaches. Front Chem. 2021;9:691697. doi: 10.3389/fchem.2021.691697.
- Simelane NWN, Kruger CA, Abrahamse H. Targeted nanoparticle photodynamic diagnosis and therapy of colorectal cancer. Int J Mol Sci. 2021;22(18):9779. doi: 10.3390/ijms22189779.
- Correia JH, Rodrigues JA, Pimenta S, et al. Photodynamic therapy review: principles, photosensitizers, applications, and future directions. Pharmaceutics. 2021;13(9):1332. doi: 10.3390/pharmaceutics13091332.
- Hodgkinson N, Kruger CA, Abrahamse H. Targeted photodynamic therapy as potential treatment modality for the eradication of colon cancer and colon cancer stem cells. Tumour Biol. 2017;39(10):1010428317734691. doi: 10.1177/1010428317734691.
- Razlog R, Kruger CA, Abrahamse H. Cytotoxic effects of combinative ZnPcS4 photosensitizer photodynamic therapy (PDT) and cannabidiol (CBD) on a cervical cancer cell line. Int J Mol Sci. 2023;24(7):6151. doi: 10.3390/ijms24076151.
- Montaseri H, Simelane NWN, Abrahamse H. Zinc phthalocyanine tetrasulfonate-loaded Ag@mSiO2 nanoparticles for active targeted photodynamic therapy of colorectal cancer. Front Nanotechnol. 2022;4:928010. doi: 10.3389/fnano.2022.928010.
- Brozek-Pluska B, Jarota A, Kania R, et al. Zinc phthalocyanine photochemistry by Raman imaging, fluorescence spectroscopy and femtosecond spectroscopy in normal and cancerous human colon tissues and single cells. Molecules. 2020;25(11):2688. doi: 10.3390/molecules25112688.
- Niculescu A-G, Grumezescu AM. Photodynamic therapy—an up-to-date review. Applied Sciences. 2021;11(8):3626. doi: 10.3390/app11083626.
- Kawczyk-Krupka A, Bugaj AM, Latos W, et al. Photodynamic therapy in colorectal cancer treatment—the state of the art in preclinical research. Photodiagnosis Photodyn Ther. 2016;13:158–174. doi: 10.1016/j.pdpdt.2015.07.175.
- Hu X, Zhang Y, Ding T, et al. Multifunctional gold nanoparticles: a novel nanomaterial for various medical applications and biological activities. Front Bioeng Biotechnol. 2020;8:990. doi: 10.3389/fbioe.2020.00990.
- Montaseri H, Kruger CA, Abrahamse H. Inorganic nanoparticles applied for active targeted photodynamic therapy of breast cancer. Pharmaceutics. 2021;13(3):3. doi: 10.3390/pharmaceutics13030296.
- Simelane NWN, Matlou GG, Abrahamse H. Photodynamic therapy of aluminum phthalocyanine tetra sodium 2-mercaptoacetate linked to PEGylated copper–gold bimetallic nanoparticles on colon cancer cells. Int J Mol Sci. 2023;24(3):1902. doi: 10.3390/ijms24031902.
- Naidoo C, Kruger CA, Abrahamse H. Targeted photodynamic therapy treatment of in vitro A375 metastatic melanoma cells. Oncotarget. 2019;10(58):6079–6095. doi: 10.18632/oncotarget.27221.
- Danaee H, Kalebic T, Wyant T, et al. Consistent expression of guanylyl cyclase-C in primary and metastatic gastrointestinal cancers. PLoS One. 2017;12(12):e0189953. doi: 10.1371/journal.pone.0189953.
- Obaid G, Chambrier I, Cook MJ, et al. Cancer targeting with biomolecules: a comparative study of photodynamic therapy efficacy using antibody or lectin conjugated phthalocyanine-PEG gold nanoparticles. Photochem Photobiol Sci. 2015;14(4):737–747. doi: 10.1039/c4pp00312h.
- Pinto B, Pacheco C, Silva P, et al. Nanomedicine internalization and penetration: why should we use spheroids? Sci Lett. 2022;1(1):1.
- Winifred Nompumelelo Simelane N, Abrahamse H. Nanoparticle-mediated delivery systems in photodynamic therapy of colorectal cancer. Int J Mol Sci. 2021;22(22):12405. doi: 10.3390/ijms222212405.
- Mohammad-Hadi L, MacRobert AJ, Loizidou M, et al. Photodynamic therapy in 3D cancer models and the utilisation of nanodelivery systems. Nanoscale. 2018;10(4):1570–1581. doi: 10.1039/c7nr07739d.
- Han SJ, Kwon S, Kim KS. Challenges of applying multicellular tumor spheroids in preclinical phase. Cancer Cell Int. 2021;21(1):152. doi: 10.1186/s12935-021-01853-8.
- Nkune NW, Simelane NWN, Montaseri H, et al. Photodynamic therapy-mediated immune responses in three-dimensional tumor models. Int J Mol Sci. 2021;22(23):12618. doi: 10.3390/ijms222312618.
- Pereira PMR, Berisha N, Bhupathiraju NVSDK, et al. Cancer cell spheroids are a better screen for the photodynamic efficiency of glycosylated photosensitizers. PLoS One. 2017;12(5):e0177737. doi: 10.1371/journal.pone.0177737.
- Zafari J, Zadehmodarres S, Javani Jouni F, et al. Investigation into the effect of photodynamic therapy and cisplatin on the cervical cancer cell line (A2780). J Lasers Med Sci. 2020;11(Suppl 1):S85–S91. doi: 10.34172/jlms.2020.S14.
- Feoktistova M, Geserick P, Leverkus M. Crystal violet assay for determining viability of cultured cells. Cold Spring Harb Protoc. 2016;2016(4):pdb.prot087379. doi: 10.1101/pdb.prot087379.
- Sardoiwala MN, Kushwaha AC, Dev A, et al. Hypericin-loaded transferrin nanoparticles induce PP2A-regulated BMI1 degradation in colorectal cancer-specific chemo-photodynamic therapy. ACS Biomater Sci Eng. 2020;6(5):3139–3153. doi: 10.1021/acsbiomaterials.9b01844.
- Nkune NW, Kruger CA, Abrahamse H. Synthesis of a novel nanobioconjugate for targeted photodynamic therapy of colon cancer enhanced with cannabidiol. Oncotarget. 2022;13(1):156–172. doi: 10.18632/oncotarget.28171.
- Gierlich P, Mata AI, Donohoe C, et al. Ligand-targeted delivery of photosensitizers for cancer treatment. Molecules. 2020;25(22):5317. doi: 10.3390/molecules25225317.
- Shirasu N, Nam SO, Kuroki M. Tumor-targeted photodynamic therapy. Anticancer Res. 2013;33(7):2823–2831.
- Chizenga EP, Abrahamse H. Design and assembly of a nanoparticle, antibody, phthalocyanine scaffold for intracellular delivery of photosensitizer to human papillomavirus-transformed cancer cells. Artif Cells Nanomed Biotechnol. 2023;51(1):205–216. doi: 10.1080/21691401.2023.2199037.
- Almatroudi A. Silver nanoparticles: synthesis, characterisation and biomedical applications. Open Life Sci. 2020;15(1):819–839. doi: 10.1515/biol-2020-0094.
- Kruger CA, Abrahamse H. Utilisation of targeted nanoparticle photosensitiser drug delivery systems for the enhancement of photodynamic therapy. Molecules. 2018;23(10):2628. doi: 10.3390/molecules23102628.
- Saeb ATM, Alshammari AS, Al-Brahim H, et al. Production of silver nanoparticles with strong and stable antimicrobial activity against highly pathogenic and multidrug resistant bacteria. Scientific World Journal. 2014;2014:e704708–9. doi: 10.1155/2014/704708.
- Cox MC, Mendes R, Silva F, et al. Application of LDH assay for therapeutic efficacy evaluation of ex vivo tumor models. Sci Rep. 2021;11(1):18571. doi: 10.1038/s41598-021-97894-0.
- Manoto SL, Houreld NN, Abrahamse H. Phototoxic effect of photodynamic therapy on lung cancer cells grown as a monolayer and three dimensional multicellular spheroids. Lasers Surg Med. 2013;45(3):186–194. doi: 10.1002/lsm.22121.
- Yakavets I, Francois A, Lamy L, et al. Effect of stroma on the behavior of temoporfin-loaded lipid nanovesicles inside the stroma-rich head and neck carcinoma spheroids. J Nanobiotechnology. 2021;19(1):3. doi: 10.1186/s12951-020-00743-x.
- Bonelli J, Ortega-Forte E, Rovira A, et al. Improving photodynamic therapy anticancer activity of a mitochondria-targeted coumarin photosensitizer using a polyurethane–polyurea hybrid nanocarrier. Biomacromolecules. 2022;23(7):2900–2913. doi: 10.1021/acs.biomac.2c00361.
- Carver K, Ming X, Juliano RL. Multicellular tumor spheroids as a model for assessing delivery of oligonucleotides in three dimensions. Mol Ther Nucleic Acids. 2014;3:e153. doi: 10.1038/mtna.2014.5.