 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Nanotechnology-based cancer treatment has received considerable attention, and these treatments generally use drug-loaded nanoparticles (NPs) to target and destroy cancer cells. Nanotechnology combined with photodynamic therapy (PDT) has demonstrated positive outcomes in cancer therapy. Combining nanotechnology and PDT is effective in targeting metastatic cancer cells. Nanotechnology can also increase the effectiveness of PDT by targeting cells at a molecular level. Dendrimer-based nanoconjugates (DBNs) are highly stable and biocompatible, making them suitable for drug delivery applications. Moreover, the hyperbranched structures in DBNs have the capacity to load hydrophobic compounds, such as photosensitizers (PSs) and chemotherapy drugs, and deliver them efficiently to tumour cells. This review primarily focuses on DBNs and their potential applications in cancer treatment. We discuss the chemical design, mechanism of action, and targeting efficiency of DBNs in tumour metastasis, intracellular trafficking in cancer treatment, and DBNs’ biocompatibility, biodegradability and clearance properties. Overall, this study will provide the most recent insights into the application of DBNs and PDT in cancer therapy.
Highlight Points
DBNs’ intracellular journey in cancer-PDT refines targeted therapy, boosting efficacy.
DBN in PDT for tumour metastasis: targeting and drug release mechanisms.
DBNs’ biocompatibility, biodegradability and clearance were explored thoroughly.
Introduction
Cancer has been estimated to have caused the deaths of approximately 10 million individuals by 2020, according to the World Health Organisation [Citation1]. Cancer is a disease in which abnormal cells multiply rapidly and cause tumours that may spread to other organs and tissues, known as tumour metastasis, leading to significant disruption of the body’s functions [Citation2]. It is well known that circulating tumour cells (CTCs) migrate, form colonies, and subsequently cause metastatic growth. CTCs can establish a microenvironment in distant organs by expressing factors derived from primary tumours [Citation3]. There are many treatment options available for cancer, some of them are chemotherapy, surgery, radiotherapy and biological therapy. However, these treatments are time-consuming, expensive, and have the following side effects including nausea, neuropathy, fatigue, alopecia, vomiting, gastrointestinal (GI) toxicity and myelosuppression [Citation4–6]. Combining multiple treatment strategies may reduce side effects and result in highly effective cancer treatment. Combined therapy (nanotechnology and PDT) is a promising alternative to traditional cancer treatments [Citation7,Citation8]. In recent years, nanotechnology has played a significant role in drug development and delivery [Citation6]. Dendrimer-based nanoconjugates (DBNs) can be formed when dendrimers are conjugated with NPs, and these nanoconjugates are more effective and efficient in drug delivery applications [Citation9]. The ability of dendrimers to undergo structural modification makes them compatible with entrapping and delivering high-molecular weight hydrophilic/hydrophobic drugs [Citation10].
Photodynamic therapy (PDT) enhances the efficacy of DBNs by encapsulating photosensitizers (PSs), which are subsequently released and taken up by cells. Upon irradiation with an appropriate laser, these PSs generate reactive oxygen species (ROS), thereby inducing tumour apoptosis [Citation8,Citation11]. While certain cancer treatment options, such as chemotherapy and radiation, may limit other treatment avenues, PDT does not impose such restrictions and allows for alternative treatment modalities [Citation5,Citation12]. The tumour microenvironment (TME) is a diverse population of cells that include both the tumour mass and supportive cells [Citation13]. Compared to healthy cells, tumours have different extracellular pH levels. The extracellular pH of a tumour is normally acidic; however, it can vary from 5.6 to 7.8, according to the type of tumour [Citation14]. Solid tumours generate heat because of metabolic activity and increased vascularization [Citation15]. Many tumours exhibit this characteristic by having a higher temperature than adjacent healthy cells [Citation16]. Tumours are larger than healthy cells, according to research on lymph node cancer, which found that lymph node tumours had a mean size of 5.6 ± 1.9 mm, compared to 3.6 ± 0.8 mm for healthy lymph node cells [Citation17]. Furthermore, internalization and intracellular trafficking of dendrimers can be used to study the essential role that plays in effective drug delivery in a TME [Citation18].
This review article focused on DBNs and their intracellular trafficking in the PDT of tumours and tumour metastasis. Thus, this review article is divided into seven different sections.
Dendrimer-based nanoconjugates in tumour suppression
A class of cancer genes known as tumour suppressor genes (p53 and retinoblastoma protein (pRB)) play essential roles in regulating cell proliferation mechanisms such as inducing apoptosis, initiation, cell-cycle checkpoints and progression of various cancerous tumours [Citation19–21]. There are still bottlenecks when it comes to treating malignant tumours, such as systemic toxicity and tumour heterogeneity [Citation22]. There is a need to develop efficient and safe nanocarriers that are suitable and can cross complex biological barriers. The strategy must involve several synthetic steps and multiple components for affordable nano-enabled therapies [Citation9]
Chemical design, functionalization and mechanism of action of dendrimers-based nanoparticles
Dendrimers are highly branched polymeric polymers made up of three different architectural constituents: branches, terminal functional groups and core. The core is the primary component of the dendrimer scaffold, and it can be a molecule or atom with at least two identical chemical functional groups [Citation23]. Dendrimer branching represents the “dendrimer generation” with the “first generation” dendrimer consisting of one layer of branching points and the “fifth generation” dendrimer consisting of five branches. Higher-generation dendrimers have several functional groups on their surface that allow for interaction with physiologically active moieties [Citation24]. Dendrimers function as NP templates and stabilizers, making them extremely useful in the delivery of drugs [Citation25]. Dendrimers’ structural diversity makes them effective carriers for a variety of active pharmacological compounds. Furthermore, the controllability of dendrimers (shape, size, branch length, surface functionality, liposome blockage in dendrimer structures, and production of tailored dendritic scaffolds) makes these systems suitable carriers in a variety of applications [Citation26]. Dendrimers are promising drug-delivery nanocarriers because of their regulated and variable size, interactions with cell membranes and various active drug molecules, and the features of their internal structures and voids [Citation27]. The internal cavities encapsulate drugs by electrostatic and hydrophobic interactions [Citation28]. Polyvalency depicts the reactive groups’ outward presentation on the dendrimer nanostructure’s exterior. Polyvalency is beneficial since it allows for varied functionalization; it is also critical for producing various interactions with biological receptors, and it enables dendrimers to bind to biological receptors in active targeting [Citation29].
During dendrimer synthesis, the recurrent growth reactions increase the production and degree of branching, finally generating a three-dimensional spherical structure. Dendrimer has a well-defined core-shell architecture and limited polydispersity due to the specific synthesis method. The synthesis technique could additionally adjust the surface charge, size, solubility and peripheral functional groups [Citation30,Citation31]. Dendrimers can be synthesized using various methods: divergent synthesis, convergent synthesis and dendrimer template method. Divergent synthesis involves dendrimers developed from a core that release functional groups to react with monomers. The core molecules interact with the monomer molecules with one reactive group and two unreactive groups to form the first-generation dendrimer. This procedure is repeated to create subsequent generations [Citation32]. Convergent synthesis begins at the dendrimer’s exterior and works its way to the core. The branching units known as dendrons expand and link to the other groupings. When the expanding branches are rich, they link to a core and form a full dendrimer [Citation33]. The dendrimer-template process consists of two steps: complexing metal ions within dendrimers and then chemically reducing the complexed ions to generate NPs. Platinum (Pt) dendrimers are created in two phases using chemical reduction (CR) and galvanic exchange (GE) [Citation25]. An aqueous solution of hydroxyl terminated PAMAM dendrimers is first treated with present amounts of Cu2+ and Pt2+ precursor ions. This phase leads to quick coordination of the additional Cu2+ precursor ions to the dendrimers’ internal tertiary amines, whereas the Pt2+ precursor ions remain uncomplexed due to their delayed coordination. The reducing agent, tetrahydrobiopterin (BH4–) has been added to the resultant solution immediately after mixing the precursor metal ions with the dendrimer solution. As a result, complexed Cu2+ ions are selectively reduced and CuNPs are produced within dendrimers, whereas uncomplexed Pt2+ has been oxidized due to minimal CR of Pt2+ by BH4– in a short time under basic circumstances (pH >9). Interestingly, the CR of Cu2+ ions related to the GE of the reduced CuNPs with neighbouring Pt2+; all the supplied Pt2+ precursor ions develop into PtNPs enclosed within the dendrimers [Citation25,Citation34].
Le et al. synthesized, dendrimer-based nanocarriers to enhance the entrapped efficiency of malloapelta B which then achieved sustained drug release. The DBNs have a larger inner cavity, which improves loading efficiency [Citation35]. Polyamidoamine (PAMAM) dendrimer-coated magnetic NPs of generation (G) 4.5–7.5 were synthesized and coupled with gemcitabine. The magnetic core of NPs is advantageous for selective drug targeting, and these NPs facilitated targeted drug delivery to the tumour site and accumulated in tumour cells utilizing an external magnetic field [Citation36].
Targeted drug delivery is known to reduce side effects and toxicity, which is a necessary component of designing drug nanocarriers. According to Kong et al., targeted drug delivery prevents drug leakage into the circulation during cancer treatment. In this work, a novel multifunctional dendrimer carrier was presented on the surface by combining long hydrophobic C12 alkyl chains, polyethylene glycol (PEG) chains and c(RGDfk) ligands. The combination demonstrated significant anti-cancer activity against 22RV1 cells that overexpress integrin αvβ3; however, it has considerably lower cytotoxicity against MCF-7 cells [Citation37].
The potential role of dendrimers in drug delivery
Nanoparticles (NPs) have a size range from 1 to 100 nm, with surface characteristic, and large surface area-to-volume ratio [Citation38]. The use of NPs enables them to deliver drugs intravenously and treat different parts of body that other delivery systems cannot reach. However, in cancer treatment, high interstitial fluid pressure, strong extracellular pressure matrix, low penetration to deep tumour locations have been highlighted as major barriers to nanomedicine [Citation39,Citation40]. NPs with ultrasmall sizes are naturally advantageous for tumour penetrations since particle size inversely correlates with diffusion [Citation41]. NPs with a diameter of around 100 nm demonstrated high tumour accumulation but had limited tissue penetration; however, a NP smaller than 20 nm, can enter deep tumours but will be promptly eliminated by the systemic circulation [Citation42–44]. Therefore, NPs with sizes ranging from 20 to 100 nm can penetrate tumours more effectively than those with larger sizes. Dendrimers range from 1 to 100 nm; therefore, they have potential applications in the treatment of tumour and tumour metastasis [Citation38].
Enhancing bioavailability of the drugs
Oral administration, injection and transdermal drug delivery (TDD) are the most common drug administration modalities [Citation45]. In addition to its benefits, each approach has its own set of drawbacks [Citation46]. To enhance the efficacy of the treatment and limit any adverse effects, the correct administration strategy must be chosen [Citation47]. Moreover, drug delivery can still be employed to enhance bioavailability at the target site of action. This can be achieved by increasing the rate of absorption and decreasing the rate of metabolism and clearance [Citation42,Citation43]. In cancer therapy, TDD aims to enhance the bioavailability of the drug by achieving maximal therapeutic efficacy and minimizing side effects [Citation48]. During TDD, the drug formulation is directly injected or applied onto the skin. The drug then enters the dermal layer after passing through the stratum corneum. When the drug reaches the dermal layer, it undergoes systemic absorption by dermal microcirculation [Citation49]. Due to the huge surface area of the skin and ease of access, there are several positioning choices for transdermal absorption of the skin [Citation50]. Furthermore, TDD inhibits pre-systematic metabolism, which increases bioavailability [Citation51]. Injectable drug delivery involves introducing drugs into a patient’s bloodstream. In this type of drug administration, the three common injection routes are intramuscular, intravenous and subcutaneous [Citation52]. The injectable technique can provide a rapid response, increased bioavailability and more efficacy while avoiding first-pass metabolism, and active targeting capability [Citation53]. In oral bioavailability, passive membrane permeability is an important component of drug design [Citation54]. Solubility and permeability are the two most important factors determining a drug’s oral bioavailability which are influenced by the GI system. The GI is made up of very heterogeneous cells and tissues, resulting in an incredibly diverse barrier that varies in properties from one region to the next. Thus, more than one uniform technique is required, as that may only apply to a small portion of the entire GI surface [Citation55]. During oral bioavailability, the cell connections tighten as the drug passes from the jejunum to the colon [Citation56]. There are several strategies to loosen the tight junctions in order to improve drug delivery by this route, including permeation enhancers and calcium chelation [Citation57].
Dendrimers have been described as carriers for drug solubility augmentation, therapeutic delivery, drug cytotoxicity reduction, drug stability enhancement and drug bioavailability enhancement in oral administration [Citation58]. Hydrophobic drugs with limited solubility can be physically enclosed inside PAMAM dendrimer internal gaps or pockets. Noncovalent interactions such as: hydrogen bonding, electric interactions, hydrophobic contacts and steric hindrance can be used to modulate the physical interactions with PAMAM and drugs in complexes in an aqueous solution [Citation59]. PAMAM dendrimers have positively charged amino groups that interact with negatively charged phospholipids on the cell membrane, allowing drugs to pass through them. Furthermore, a variety of ligands including cell-penetrating peptides, antibodies and targeting peptides, can modify the surface of PAMAM dendrimers. Due to ligands identifying receptors on cells, modified dendrimers can be taken up by the cells via receptor mediated endocytosis [Citation60].
Targeted drug delivery
Cancer therapy can be improved by using a nanocarrier-based targeted delivery system [Citation61]. The drug administration by nanocarriers is carried out by both active and passive targeted drug delivery. Additionally, NP-based drug delivery can reduce toxicity and increase pharmacological activity of loaded drugs while enhancing their solubility and bioavailability [Citation62]. Schematic representation of both active and passive drug targeting is illustrated in [Citation63]. Active targeting is made possible by a specific ligand being modified on a nanocarrier, which can interact selectively with some unique cell surface receptors that are overexpressed on the target cells [Citation64]. The concept of active and passive targeting uses the enhanced permeation and retention (EPR) effect [Citation65]. Antibodies, nucleic acids or other ligands (carbohydrates, vitamins and peptides) are employed for active targeting and typically bind to a receptor specifically overexpressed by tumour cells. Targeting ligands play an important role in enhancing NPs cellular absorption via endocytosis [Citation66]. Passive drug delivery occurs through nanocarriers entering the tumour cells through the blood vessel [Citation67]. The pathophysiological condition and anatomical changes in tumour tissue and its surroundings generated by tumour angiogenesis give various advantages when delivering nanotherapeutics, which can be adjusted to enable site-specific dispersion via passive administration. Angiogenesis is essential in the progression of cancer because it permits nutrients, growth hormones, oxygen and tumour spread to be transferred to distant organs [Citation68]. It has been reported in various studies that leaky, distended blood capillaries and extensive fenestrations ranging in diameter from 200 nm to 1.2 m characterize cancerous blood arteries [Citation68–71]. A dilated tumour blood vessel allows NPs to escape from filtration traps in the spleen (lymphatic system) and liver, allowing them to enter extravascular areas, resulting in a significant build-up of NPs within tumours. Furthermore, the neutral surface and negative charge of NPs are crucial to establishing longer plasma half-lives than hours spent in circulation [Citation72]
Figure 1. Schematic representation of active and passive targeted drug delivery in anticancer therapy – reprinted from reference [Citation63], copyright 2023, Springer Nature.
![Figure 1. Schematic representation of active and passive targeted drug delivery in anticancer therapy – reprinted from reference [Citation63], copyright 2023, Springer Nature.](/cms/asset/0126e451-74ac-43a7-8fde-095188812a8d/ianb_a_2368033_f0001_c.jpg)
Dendrimer internalization and intracellular tumour trafficking
Nanotechnology-based imaging has the potential to enhance imaging specificity and sensitivity, which could overcome several limitations in the use of imaging agents, particularly for the EPR [Citation73]. Nuclear imaging agents have the advantage of penetrating and accumulating specifically in tumour tissue due to the EPR effect generated by poor lymphatic drainage and vascularization in the TME [Citation74]. Effective treatment and an accurate cancer diagnosis can be made successful by an essential part of molecular imaging techniques [Citation75]. The covalent conjugation of targeted drugs, bioactive and ligands components for imaging purposes and different drug delivery is made possible by unique dendrimer structural features like dendrimers having a core and different generations which are emanated branches with defined molecular weight, terminal functional group and particular size that allow inorganic NPs to be entrapped within their interiors [Citation76,Citation77].
The size, ionic charges and composition of the dendrimers are important not only for the induced internalization mechanism, but also for endocytosis kinetics and intracellular processes [Citation78]. Endocytosis is a technique used by cells to absorb substances from their surroundings by employing vesicles generated by plasma membrane [Citation79]. Dendrimer surface modification increases their selectivity for tumour cells and cellular absorption by this technique. However, in one study, PAMAM dendrimers were unable to penetrate HeLa cells via endocytosis, whereas peptide-modified dendrimers used endocytosis to enhance doxorubicin (DOX) internalization to limit HeLa cells progression [Citation80]. This implies that bioconjugation of dendrimers does improve endocytosis [Citation81]. Dendrimer surface charge is also a major factor in the penetration of tumour cells. Anionic dendrimers were synthesized for oligonucleotide (ON) delivery in cancer treatment. Adsorptive endocytosis, rather than liquid phase endocytosis, transports dendrimers into A431 cells, increasing internalization by 100-fold. The binding of negatively charged dendrimers to positively charged proteins on the cell membrane’s surface mediates adsorptive endocytosis [Citation82]. illustrates more mechanisms by which dendrimer enters tumour cells.
Table 1. Types of mechanisms in which dendrimers penetrate tumour cells.
Dendrimer-based nanoparticles and their imaging in the tumor microenvironment
Bioimaging is crucial for cancer detection and therapy. It tracks progress and responses to treatment. However, imaging specificity and sensitivity remain significant challenges [Citation73]. Traditional tiny molecular contrast agents have intrinsic flaws such as renal toxicity, lack of selectivity, low signal-to-noise ratio and limited imaging duration [Citation86]. Dendrimer-based nanoplatforms for tumour imaging have improved biocompatibility, sensitivity, targeted specificity and accuracy by architectural design, surface modifications and integration of multiple contrast components [Citation86]. A significant amount of contrast agent entities can be loaded at the interior or in the terminals of dendrimers improving image enhancement [Citation87].
Tang et al. used a 9.4 T MR scanner to study the magnetic resonance imaging (MRI) performance of gadolinium-labelled dendrimer nanocluster (GdDN)-loaded graphene oxide nanosheet (GO-GdDN), GdDN and Gd-DTPA in vivo. T1 imaging of an area of interest inside the liver was performed both before and after injection of Gd-DTPA, GdDN and GO-GodDN through the tail vein at a dosage of 2.0 mg (Gd)/kg (body weight). The liver examined in mice treated with GO-GdDN showed a stronger contrast enhancement in T1 imaging from 15 to 90 min after delivery, indicating fast accumulation of GO-GdDN in the liver [Citation88]. However, for Gd-DTPA and GdDN samples, the liver displayed a very moderate contrast increase from 15 to 60 min postinjection, displaying that their contrast capabilities in vivo were inferior to GO-GdDN [Citation88]. In vitro fluorescence or confocal imaging of dendrimer-based NPs in cancer cells are shown in , and in vivo MRI images of dendrimer-based NPs in cancer model are shown in .
Figure 2. In vitro fluorescence or confocal imaging of dendrimer-based nanoparticles in cancer cells. (i) Confocal images of SH-SY5Y cells incubated for 20 min with curcumin/resveratrol/dendrimer NPs – reprinted from reference [Citation89], copyright 2022, ACS publications; (ii) sugar-conjugated dendrimer localizations in glioblastoma – reprinted from reference [Citation90], copyright 2021, Elsevier; and (iii) PEGylated bis-indolyl G2 and G3 polyurethane dendrimer treated cells (A) MDA-MB-231 cells and (B) A549 cells – reprinted from reference [Citation91], copyright 2021, Elsevier.
![Figure 2. In vitro fluorescence or confocal imaging of dendrimer-based nanoparticles in cancer cells. (i) Confocal images of SH-SY5Y cells incubated for 20 min with curcumin/resveratrol/dendrimer NPs – reprinted from reference [Citation89], copyright 2022, ACS publications; (ii) sugar-conjugated dendrimer localizations in glioblastoma – reprinted from reference [Citation90], copyright 2021, Elsevier; and (iii) PEGylated bis-indolyl G2 and G3 polyurethane dendrimer treated cells (A) MDA-MB-231 cells and (B) A549 cells – reprinted from reference [Citation91], copyright 2021, Elsevier.](/cms/asset/eb57cb72-8264-459e-8706-15bab633730e/ianb_a_2368033_f0002_c.jpg)
Figure 3. In vivo MRI images of dendrimer-based NPs in cancer model (i) (a) the coronal MRI images shows the location of U-251 glioma tumour, (b) distribution of G3-curcumin in organs and (c) fluorescence images of kidney (K), spleen (Sp), liver, heart (H), lung (L) and brain and (B) in rat model – reprinted from reference [Citation92], copyright 2016, PubMed Central; and (ii) G5-Gd trastuzumab and G5-Gd NPs injected tumour bearing mice model – reprinted from reference [Citation93], copyright 2020, Springer Nature).
![Figure 3. In vivo MRI images of dendrimer-based NPs in cancer model (i) (a) the coronal MRI images shows the location of U-251 glioma tumour, (b) distribution of G3-curcumin in organs and (c) fluorescence images of kidney (K), spleen (Sp), liver, heart (H), lung (L) and brain and (B) in rat model – reprinted from reference [Citation92], copyright 2016, PubMed Central; and (ii) G5-Gd trastuzumab and G5-Gd NPs injected tumour bearing mice model – reprinted from reference [Citation93], copyright 2020, Springer Nature).](/cms/asset/94ccdda8-03a9-442c-9760-4743a73808b8/ianb_a_2368033_f0003_c.jpg)
Tumour–dendrimer interaction
Surface receptors that promote the growth and survival of cancerous cells are regularly upregulated by different types of receptors. Receptors, particularly those linked to neoplastic progression, are appealing targets [Citation94]. Due to its high affinity for folic acid (FA) receptors, which are overexpressed in many cancer cell lines, FA is one of the most studied targeted ligands for tumour targeting. Dendrimer’s multivalent properties allow for the attachment of multiple diagnostic and therapeutic method payloads. As a result, multivalent FA-modified dendrimers have allowed active targeting of tumour cells [Citation30,Citation31,Citation95,Citation96]. illustrates different kinds of tumours and their specific receptors.
Table 2. Fundamentals of dendrimer-based nanoconjugates for tumour elimination.
Tumour metastasis treatment using PDT
Photodynamic therapy is an innovative, non-invasive therapeutic method for non-oncological disorders and tumours of various forms and areas [Citation103]. The main purpose of nanotechnology-PDT is to deliver a PS to the target area to increase therapeutic effect [Citation104]. This technique eliminates tumours by direct cell destruction, microvascular disruption and inflammation using three crucial components: PSs, oxygen and light [Citation105]. These three components are dependent to each other: the PS needs to be stimulated by light to be active and leading to the harmful production of singlet oxygen; hence, oxygen levels within tumour cells need to be high for this process to succeed [Citation106]. Generally, PDT tumours are eliminated by using ROS produced by PS when activated by light and incidental harm to neighbouring health tissues is reduced [Citation105]. Furthermore, PDT has been found to cause immunogenic cell death and the release of tumour-associated antigens after cancer cell killing, resulting in the activation and proliferation of CD8+ T lymphocytes [Citation107]. An added advantage of PDT involves the PS’s biological action in the cell which varies according to its kind, location within the cell, dosage and method of administration. This may cause changes in the control of cellular signalling pathways. As a result, the cell can respond to PDT in a variety of biological ways, including necrosis, autophagy and apoptosis [Citation108,Citation109].
PDT in cancer therapy
Photodynamic therapy can be coupled with other therapies like immunotherapy, radiotherapy and chemotherapy; nevertheless, issues such as chemotherapeutic drug toxicity and tolerance, radioactive damage caused by radiotherapy, immunotherapy’s poor effectiveness remain a bottleneck for cancer treatment [Citation7,Citation8,Citation110]. Selective use of PSs in PDT can influence biochemical functions, thereby causing oxygen molecules to form ROS that cause necrosis and apoptosis of tumour cells [Citation111].
Mechanism of actions of PDT
The mechanisms of actions of PDT are majorly divided into two types and it has been well reported that both are closely dependent on oxygen molecules present inside the cells [Citation112]. The first mechanism involves the transfer of energy from the surrounding environment to the biomolecules by the PS in the excited triplet state (T1). When the tumour (substrate) meets the PS in the T1 state, an electron or hydrogen is transferred, resulting in the formation of anion and free radicals that can readily interact with oxygen molecules [Citation109]. In response, ROS are being generated, which results in the destruction of cancer cells [Citation113]. In the second mechanism, the energy is transmitted directly to the oxygen molecule in the critical energetic state (the basic triplet state). Since the PS and oxygen have the same spins, direct energy is transferable. This produces excited oxygen particles (singlet oxygen), which have extraordinarily strong oxidizing capabilities [Citation109]. It is common for organic compounds to exist in the basic singlet state. Oxygen molecules, on the other hand, are distinguished by their T1 (as a basis) and excitation into singlet states. As a result, PS particles that have been excited cause no damage to organic cell structures and only interact with oxygen molecules scattered in the cytoplasm. The presence of high ROS causes photodamage to proteins, lipids and other molecules in the area that is photosensitized. Therefore, tumour cells are directly destroyed in the process of apoptosis [Citation114].
Dendrimers as a carrier molecule for photosensitizer delivery in PDT
Photosensitizers are naturally available or synthetic compounds that produce singlet oxygen, which causes apoptosis when activated by the appropriate light source [Citation115]. A PS is encapsulated in nanocarriers, and these nanocarriers penetrate tumour tissue selectively to prevent premature tumour release, thus increasing therapeutic efficacy [Citation116]. However, these coatings on the outer shell may also inhibit singlet oxygen formation and so reduce the harmful impact. Thus, modifying the shell thickness, optimizing the encapsulated structure, and controlling the shell material improve ROS production while also delivering the PS selectively to the TME [Citation117].
Dendrimers are hyperbranched polymers with the ability to regulate and control the amount and size of functional groups that may be changed. This enhances the reliability of drug encapsulation quantities and anti-drug resistance. Thus, pharmacokinetics become more reproducible, allowing dendrimers to become an effective drug delivery method for PDT [Citation118]. The PS may be conjugated in dendrimers in three ways: first, dendrimer structures feature gaps in which the PS is confined; second, the dendrimer and PS are conveniently connected; and third, the PS acts as a frame for the creation of a dendrimer [Citation119].
ALA (aminolaevulinic acid) is a beneficial drug for PDT application; it is critical to increase the efficiency with which it is converted into protoporphyrin IX (PplX) to maximize the phototherapeutic response. A unique approach to ALA-PDT involves dendrimers as carriers for delivering ALA, HPO (3-hydroxy-4-pyridinone), or other synergistic chemicals to tumour cells. A series of ALA–HPO dendrimers with an aromatic core and appropriate spacers were synthesized using convergent technique to allow ALA and HPO co-administration to cancer cell lines (MCF-7 and MCF-7R). The ALA–HPO dendrimers excelled ALA in terms of PplX production and phototoxicity in the cell lines [Citation8]. Xu et al. altered PEG-Cholesterol on the surface of a PAMAM dendrimer to form a dendrimer-based nanocarrier (DCP NPs). DPC NPs were created as drug nanocarriers to encapsulate chlorin (Ce6) resulting in highly effective and stable drug loading and delivery.
PDT limitations for tumour metastasis
Despite its beneficial application, PDT has limited therapeutic efficacy. The efficacy of PDT is restricted by three major factors: inadequate PS accumulation in tumour tissues, a hypoxic core with low oxygen levels in solid tumour, and a restricted depth of light penetration [Citation104]. PSs, like traditional chemotherapeutic drugs, cannot concentrate actively in tumour tissue following in vivo injection, and their therapeutic efficacy is greatly diminished by non-specific absorption by adjacent tissues or cells [Citation120,Citation121]. PDT is a process in which oxygen, a PS, and a certain wavelength interact [Citation122]. Malignant cells need to be closed around the blood vessels to access the blood circulatory system to obtain nutrients and oxygen for the cells to survive and proliferate. Hypoxia TME can be caused by tumour metastasis [Citation48]. Solid tumours located further away from the closest blood capillaries result in a hypoxic microenvironment where solid tumours have a depreciation of nutrients and oxygen [Citation123]. A portion of solid tumours is poorly oxygenated; in this hypoxia region, the hypoxia-inducible factor (HIF-1α) is active. HIF-1α is overexpressed, resulting in an increase in hypoxia predominance in solid tumours, including tumour spread and aggravation [Citation124]. Hypoxia is known to cause drug resistance [Citation125]. Hypoxic TME causes epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) resistance [Citation126]. Zhu et al. designed a trial to overcome EGFR-TKI drug resistance in non-small cell lung cancer (NSCLC). An aptamer-modified fluorinated dendrimer (APF) was used as a drug carrier and APFHG nanocomplexes were prepared by encapsulating haematoporphyrin (Hp) and gefitinib (Gef). APF has a strong oxygen transport capacity, high drug entrapment efficacy, and can release Hp and Gef in response to intracellular pH. Due to the targeting impact of aptamer and the fluorinated dendrimer’s high oxygen-carrying capacity, APF precisely detects EGFR-positive NSCLC cells and significantly alleviates the TME [Citation127]. In another study, PEG-Chol-modified PAMAM dendrimers and pH/H2O2-responsive nanoagents were generated to increase PDT and limit anti-drug resistance effects in vivo and in vitro [Citation11].
PDT highly needs oxygen to produce ROS; this will not be possible because of the lack of oxygen in the TME. TMEs lead tumours to create a plethora of physical, biological and chemical barriers to aid tumour cells in evading therapy, and this results in limited light penetration [Citation128,Citation129]. To overcome the limitations of PDT, carbonic anhydrase IX (CAIX) was developed to target the PS, acetazolamide conjugated BODIPY (AZ-BPS). This system was created with two active subunits: (a) an AZ ligand to provide CAIX inhibition as well as efficient tumour targeting and (b) a BODIPY moiety to serve as both a singlet-oxygen producing PS and to enable fluorescence-based monitoring of cellular uptake and distribution. CAIX is a well-known biomarker associated with hypoxia. CAIX inhibition might result in a potent anti-angiogenesis PDT agent. The demonstrated and improved therapeutic effectiveness of AZ-BPS was therefore attributable to the combined effects of angiogenesis and photocytotoxicity suppression via CAIX inhibition [Citation130,Citation131].
Biocompatibility, biodegradability and clearance properties of DBNs
The effects of dendrimer generation (dendrimer architecture, lipid-bearing dendrons) could influence NPs interactions with cellular membranes and organelles, which in turn have an impact on their metabolism, cellular uptake, bioavailability, excretion organ toxicity and tissue accumulation [Citation132,Citation133]. Dendrimers must be able to penetrate biological barriers to be used as excipients. Dendrimer features such as shape, size, surface structure and chemical composition impact on their biodistribution and toxicity in drug delivery [Citation12]. Biodegradation and clearance properties of a dendrimer are shown in .
Biocompatibility of dendrimer-based nanoconjugates
Dendrimer sizes may be adjusted by adjusting the generation numbers or layers inside the dendrimer architecture; enhanced dendrimer size can also be managed by surface modification utilizing non-scaffolding materials such as PEG. PEGylating is a process that links PEG chains to peptides, therapeutic proteins or molecules [Citation134]. PEG has long been used to mask the intrinsic surface charge of PAMAM dendrimers and improve biocompatibility with longer circulation duration. PEG circulation to PAMAM dendrimers can extend biological half-life and systematic exposure via a variety of approaches [Citation135]. PAMAM cytotoxicity is mostly caused by a large positive charge; conjugating PAMAM dendrimers with neutral ONs may reduce their dendrimer’s toxicity [Citation136]. Polypropylene (PPI) dendrimers were electrostatically coupled with gold nanoparticles (AuNPs) to generate excellent transfection vectors [Citation137]. Additionally, combining AuNPs with dendrimers may alleviate issues associated with dendrimer cytotoxicity [Citation138].
Cationic phosphorus dendrimers (G1–3) and surface cycline amine groups were synthesized, characterized and utilized to first compress plasmid DNA-enhanced green fluorescent protein (EGFP) to form polyplexes which will then be transported in cancer cells in vivo and in vitro for studying gene delivery efficacy. Haematoxylin and eosin (H&E) is a technique that distinguishes cellular components by staining the cytoplasm and nucleus with various colours. The technique was used to evaluate in vivo and in vitro biocompatibility of dendrimers. There were no substantial organ abnormalities identified in the liver, heart, lung, spleen or kidney [Citation139]. In an in vitro study, HeLa cells were used, and western blot results reported excellent gene therapy on the cells. This further indicated that the dendrimers were also biocompatibility in vitro [Citation93]. Li et al. studied a novel type of temperature-sensitive dendrimer with adjusted lower critical solution temperature (LCST) by surface modification of PAMAM dendrimers with alkoxy diethylene glycols. At the LCST, these dendrimers’ surface characteristics shifted drastically from hydrophilic to hydrophobic. By adjusting the ratios of various alkoxy diethylene glycols in the dendrimer perimeter, the transition temperature may be altered to be close to the human body temperature. The flow cytometry results confirmed the successful uptake of dendrimers by HeLa cells using endocytosis. Furthermore, the dendrimers were shown to have negligible cellular toxicity both above and below the LCST [Citation140].
Biodegradability of dendrimer-based nanoconjugates
There are various benefits to using biodegradable dendrimers as drug transporters, including the following: the ability to make hydrophobic drugs more soluble while also protecting them against degradation and unwanted interactions with the biological environment [Citation141]. The hydrophobic coating can also obscure the protein and cellular interaction site, lower opsonization and adsorption by the mononuclear phagocytic system. Furthermore, PEG can allow enzymes to access dendrimer structures, protecting the loaded drugs from in vivo degradation [Citation135]. The electrostatic interaction between dendrimers with AuNPs in the biological environment may not be as resistant to variations in temperature, ionic strength and pH. PAMAM dendrimers include amine groups that remain deprotonated when the branches constrict into the central core. This mechanism regulates drugs released in a variety of settings [Citation59]. As a result, PAMAM dendrimer drug release is always pH sensitive, and in acidic environments, it is quicker [Citation142,Citation143]. PAMAM dendrimers also have a high-water solubility; therefore, via renal excretion, they are cleared out of the body [Citation78]. Under physical circumstances, biodegradable dendrimers, which are typically formed of biodegradable units, will disintegrate into tiny fragments. In , tiny fragments can be removed via metabolic pathways [Citation141].
Clearance properties of dendrimer-based nanoconjugates
The size of the nanocarrier can impact NP entrance via endocytosis and other endocytic pathways [Citation144]. NPs circulate throughout the blood vessels in the body and their protein corona has an impact on their biodistribution as well as other nanomaterial biological interactions [Citation145]. When exposed to biological systems, they generate a protein corona which is protein coating that spontaneously absorbs to the surface of nanomaterials [Citation146]. In rare situations, the protein corona arrangement might lead the reticuloendothelial system to sequester most of the NPs. This causes biodistribution to concentrate mostly in the liver and spleen, potentially reducing their therapeutic efficacy [Citation147]. Dendrimers with homogenous and well-defined forms and sizes are useful in biomedical applications because they can penetrate cell membranes and decrease the risk of early clearance from the body [Citation148]. PEG PAMAM dendrimers have a lower tendency for renal clearance because their physical size has increased to the glomerular filtration limit of about 25 kDa [Citation135]. Saw et al. conducted a study that demonstrated a significant amount of TCPP (trischloropropylphosphate) and TCPP-PAMAM-confeito-AuNPs built up in highly vascularized tissues (kidney, liver and lungs). Regardless of size, the liver has been the primary location of the buildup of AuNPs delivered intravenously. At the same time, AuNPs were most likely to be retained in the kidneys by glomerular capillaries during the blood filtering process. NPs with diameters more than 100 nm can be internalized by lung macrophages. Even though the hydrodynamic diameter of TCPP-PAMAM-confeito-AuNPs in water did not exceed the size, prolonged exposure to protein absorption in blood flow may cause the sample to increase and induce delayed accumulation [Citation149]. PAMAM and PPI dendrimers can rapidly be cleared from blood circulation by the mononuclear phagocyte system [Citation150].
Limitations of dendrimers
Dendrimer interaction with biological membranes causes cell death and severe damage and it can be limited due to haematological toxicity and immunoreaction [Citation151]. The toxicity of a dendrimer is influenced by the charge(s) present on its surface; significant haemolysis was seen in PPI (G4–5) with terminal amine groups [Citation26]. When dendrimers enter the systemic circulation, their nanosize allows it to easily interact with numerous blood components such as red blood cells (RBCs), and its positive charge leads to the haemolysis of negatively charged RBCs [Citation152,Citation153]. Biodistribution and toxicity are closely related to dendrimers’ surface and size chemistry [Citation154]. The 5th or lower generation of PAMAM is eliminated via glomerular filtration in the renal excretion pathway, and the clearance of the 6th generation of PAMAM dendrimers and higher is highly dependent on the hepatic clearance pathway [Citation30,Citation31]. Accelerated elimination of cationic dendrimers by the reticuloendothelial system and non-specific adsorptions of plasma proteins are often encountered by cationic dendrimers. Cell lysis due to cationic dendrimers is caused by the interaction of negatively charged cell membranes, which destabilizes biological membranes. Compared to anionic or neutral dendrimers, cationic dendrimers at higher doses display higher toxicity [Citation155,Citation156]. The alternatives for reducing dendrimer toxicity are to synthesize biodegradable dendrimers and surface engineer cationic dendrimers with anionic, biodegradable and neutral functional groups [Citation157]. Luong et al. successfully PEGylated PAMAM dendrimers ligands to target tumour cells, increasing anticancer efficacy while decreasing antidrug toxicity to normal cells. System circulation time increased [Citation135]. Dendrimers having neutral or negatively charged terminal surface groups are not toxic to cells and may be safely administered. As a result, converting these cationic terminal surface groups of dendrimers to neutral or anionic groups reduces their toxicity [Citation157].
The intratumour efficacy and anticancer effects of tiny NPs were examined utilizing G3, G5 and G7-PAMAM dendrimers as models. The mechanism investigation found that G3-PAMAM had a very low particle–cell interaction and therefore eliminated the tumour swiftly, but G7-PAMAM was unable to penetrate effectively due to its relatively big size and too high particle-cell contact. G5-PAMAM, on the other hand, was of intermediate size and particle-cell contact, allowing it to perfuse tumour tissue efficiently and be absorbed by tumour cells [Citation42,Citation43].
There have been several reports on dendrimer synthesis. Only a few have made it to the market. This is supported by the enormous amount of reaction steps, which not only increase the chemical loss of important starting materials but also raise the likelihood of introducing structural defects in the dendritic form. As a result, dendrimer manufacturing slows and becomes more expensive. To address these issues, accelerated techniques have been developed with the goal of minimizing the number of reaction stages, reaction time, starting materials and manufacturing costs [Citation158]. The stability of G4-PAMAM dendrimers with different functionalities (PEG and NH2) was examined at different temperatures (0 °C, room temperature (RT) and 60 °C) after being stored in the dark (in amber-coloured vials) and under light (in colourless vials). The obtained results indicated that dendrimer-based systems are stable at increased temperatures of up to 60 °C when stored in the dark. Formulations with hydroxyl and amine surface were found to be most stable in the dark, and at 0 °C, whereas in the dark, RT was the most acceptable storage conditions for formulation with PEGylated dendrimers [Citation159].
Future perspectives and conclusions
In this review article, we discussed the different types of DBNs and their intracellular trafficking in PDT of tumours and tumour metastasis. The use of biocompatible DBNs in cancer therapy has demonstrated their importance and efficiency, as well as their functional applications in imaging, diagnosis and targeting tumours. Nanoconjugates based on dendrimers enable the delivery of therapeutic PSs to target cancer cells using active and passive targeting mechanisms. Many studies have been conducted that demonstrate the positive results of DBNs both in vitro and in vivo. A combination of PDT and nanotechnology has indeed provided an effective treatment for cancer. The effectiveness of dendrimer-based imaging for intracellular trafficking is well reported in the literature. There are several drawbacks to utilizing dendrimers and PDT to treat cancer. These drawbacks include hypoxia, low PS solubility, ineffective drug encapsulation and cellular absorption, and low tumour cell selectivity. Microneedles have been proven in recent studies to be an effective transdermal delivery system for NPs in cancer therapy. PSs can be doped with g-C3N4 to improve PDT by boosting ROS through water splitting. In the future, it is anticipated that combined therapies will offer a synergistic and effective approach to cancer treatment.
Author contributions
L. Nemakhavhani: methodology, data curation, formal analysis, investigation, writing-original draft, visualization and software. H. Abrahamse: co-supervision, writing – review and editing, funding acquisition and resources. S.S. Dhilip Kumar: conceptualization, project administration, supervision, visualization, software, writing-review and editing and funding acquisition. All authors read and approved the final manuscript.
Acknowledgements
The graphical abstract and Figure 4 were “Created with BioRender.com.”
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data are openly available in a public repository that issues datasets with DOIs.
Additional information
Funding
References
- World Health Organization. Cancer; 2022 [cited 2023 Mar 17]. Available from: https://www.who.int/news-room/fact-sheets/detail/cancer
- Hobbs H. Cancer: types, cause, prevention and more. In: Healthline cancer: types, causes, treatment, and prevention; 2022 [cited 2022 May 22]. Available from: healthline.com
- Peinado H, Zhang H, Matei IR, et al. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer. 2017;17(5):302–317. doi: 10.1038/nrc.2017.6.
- Luke JJ, Schwartz GK. Chemotherapy in the management of advanced cutaneous malignant melanoma. Clin Dermatol. 2013;31(3):290–297. doi: 10.1016/j.clindermatol.2012.08.016.
- Naidoo C, Kruger CA, Abrahamse H. Photodynamic therapy for metastatic melanoma treatment: a review. Technol Cancer Res Treat. 2018;17:1533033818791795. doi: 10.1177/1533033818791795.
- Nasir A, Khan A, Li J, et al. Nanotechnology, a tool for diagnostics and treatment of cancer. Curr Top Med Chem. 2021;21(15):1360–1376. doi: 10.2174/1568026621666210701144124.
- Sztandera K, Gorzkiewicz M, Dias Martins AS, et al. Noncovalent interactions with PAMAM and PPI dendrimers promote the cellular uptake and photodynamic activity of rose bengal: the role of the dendrimer structure. J Med Chem. 2021;64(21):15758–15771. doi: 10.1021/acs.jmedchem.1c01080.
- Zhou T, Battah S, Mazzacuva F, et al. Design of bifunctional dendritic 5-aminolevulinic acid and hydroxypyridinone conjugates for photodynamic therapy. Bioconjug Chem. 2018;29(10):3411–3428. doi: 10.1021/acs.bioconjchem.8b00574.
- Patil S, Mishra VS, Yadav N, et al. Dendrimer-functionalized nanodiamonds as safe and efficient drug carriers for cancer therapy: nucleus penetrating nanoparticles. ACS Appl Biomater. 2022;5(7):3438–3451.
- Singh V, Sahebkar A, Kesharwani P. Poly(propylene imine) dendrimer as an emerging polymeric nanocarrier for anticancer drug and gene delivery. Eur Polym J. 2021;158:110683. doi: 10.1016/j.eurpolymj.2021.110683.
- Xu K, Jia H, Zhu Y, et al. Cholesterol-modified dendrimers for constructing a tumor microenvironment-responsive drug delivery system. ACS Biomater Sci Eng. 2019;5(11):6072–6081. doi: 10.1021/acsbiomaterials.9b01386.
- Santos A, Veiga F, Figueiras A. Dendrimers as pharmaceutical excipients: synthesis, properties, toxicity, and biomedical applications. Materials. 2019;13(1):65. doi: 10.3390/ma13010065.
- Bussard KM, Mutkus L, Stumpf K, et al. Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res. 2016;18(1):84. doi: 10.1186/s13058-016-0740-2.
- Pershina AG, Brikunova OY, Demin AM, et al. Variation in tumor pH affects pH-triggered delivery of peptide-modified magnetic nanoparticles. Nanomedicine. 2021;32:102317. doi: 10.1016/j.nano.2020.102317.
- Alyssa Owens, Manasi Godbole, Donnette Dabydeen, Lori Medeiros, Pradyumna Phatak, Satish Kandlikar. Conference Proceedings of the Xth Conference on Nanochannels, Microchannels, and Minichannels. American Society of Mechanical Engineers; Conference date: 13-15 July 2020. United States of America (Virtual, online): New York City. 2020.
- Sella T, Sklair-Levy M, Cohen M, et al. A novel functional infrared imaging system coupled with multiparametric computerized analysis for risk assessment of breast cancer. Eur Radiol. 2013;23(5):1191–1198. doi: 10.1007/s00330-012-2724-7.
- Rössler, O., Betge, J., Harbaum, L., Mrak, K., Tschmelitsch, J. and Langner, C., 2017. Tumor size, tumor location, and antitumor inflammatory response are associated with lymph node size in colorectal cancer patients. Modern Pathology, 30(6):897–904. doi: 10.1038/modpathol.2016.227.
- Avaritt BR, Swaan PW. Internalization and subcellular trafficking of poly-l-lysine dendrimers are impacted by the site of fluorophore conjugation. Mol Pharm. 2015;12(6):1961–1969. doi: 10.1021/mp500765e.
- Hilgendorf KI, Leshchiner ES, Nedelcu S, et al. The retinoblastoma protein induces apoptosis directly at the mitochondria. Genes Dev. 2013;27(9):1003–1015. doi: 10.1101/gad.211326.112.
- Wang H, Guo M, Wei H, et al. Targeting p53 pathways: mechanisms, structures, and advances in therapy. Signal Transduct Target Ther. 2023;8(1):92. doi: 10.1038/s41392-023-01347-1.
- Zhao M, Sun J, Zhao Z. TSGene: a web resource for tumor suppressor genes. Nucleic Acids Res. 2013;41:D970–D976. doi: 10.1093/nar/gks937.
- Fang J, Feng Y, Zhang Y, et al. Alkaline phosphatase-controllable and red light-activated RNA modification approach for precise tumor suppression. J Am Chem Soc. 2022;144(50):23061–23072. doi: 10.1021/jacs.2c10409.
- Umoren SA, Solomon MM, Saji VS. Polymeric materials in corrosion inhibition: fundamentals and applications. San Diego: Elsevier; 2022.
- Karandikar S, Mirani A, Waybhase V, et al. Nanovaccines for oral delivery—formulation strategies and challenges. In: Ecaterina Andronescu, Alexandru Grumezesc (editors), Nanostructures for oral medicine. Amsterdam: Elsevier; 2017. p. 263–293.
- Cho T, Yoon CW, Kim J. Repetitively coupled chemical reduction and galvanic exchange as a synthesis strategy for expanding applicable number of Pt atoms in dendrimer-encapsulated Pt nanoparticles. Langmuir. 2018;34(25):7436–7444. doi: 10.1021/acs.langmuir.8b01169.
- Chis AA, Dobrea C, Morgovan C, et al. Applications and limitations of dendrimers in biomedicine. Molecules. 2020;25(17):3982. doi: 10.3390/molecules25173982.
- Cojocaru F, Botezat D, Gardikiotis I, et al. Nanomaterials designed for antiviral drug delivery transport across biological barriers. Pharmaceutics. 2020;12(2):171. doi: 10.3390/pharmaceutics12020171.
- Palmerston Mendes, L., Pan, J. & Torchilin, V.P. 2017. Dendrimers as nanocarriers for nucleic acid and drug delivery in cancer therapy. Molecules, 22(9):1401. doi: 10.3390/molecules22091401.
- Abbasi E, Aval SF, Akbarzadeh A, et al. Dendrimers: synthesis, applications, and properties. Nanoscale Res Lett. 2014;9(1):247. doi: 10.1186/1556-276X-9-247.
- Wang H, Lin S, Wang S, et al. Folic acid enables targeting delivery of lipodiscs by circumventing IgM-mediated opsonization. Nano Lett. 2022;22(16):6516–6522. doi: 10.1021/acs.nanolett.2c01509.
- Wang J, Li B, Qiu L, et al. Dendrimer-based drug delivery systems: history, challenges, and latest developments. J Biol Eng. 2022;16(1):18. doi: 10.1186/s13036-022-00298-5.
- Bacha K, Chemotti C, Mbakidi J, et al. Dendrimers: synthesis, encapsulation applications and specific interaction with the stratum corneum—a review. Macromol. 2023;3(2):343–370. doi: 10.3390/macromol3020022.
- Nikzamir M, Hanifehpour Y, Akbarzadeh A, et al. Applications of dendrimers in nanomedicine and drug delivery: a review. J Inorg Organomet Polym. 2021;31(6):2246–2261. doi: 10.1007/s10904-021-01925-2.
- Anderson RM, Yancey DF, Loussaert JA, et al. Multistep galvanic exchange synthesis yielding fully reduced Pt dendrimer-encapsulated nanoparticles. Langmuir. 2014;30(49):15009–15015. doi: 10.1021/la503956h.
- Le, P.N., Pham, D.C., Nguyen, D.H., Tran, N.Q., Dimitrov, V., Ivanov, P., Xuan, C.N., Nguyen, H.N. & Nguyen, C.K. 2017. Poly (N-isopropylacrylamide)-functionalized dendrimer as a thermosensitive nanoplatform for delivering malloapelta B against HepG2 cancer cell proliferation. Advances in natural sciences: Nanoscience and nanotechnology, 8(2):025014. doi: 10.1088/2043-6254/aa5e32
- Parsian M, Mutlu P, Yalcin S, et al. Half generations magnetic PAMAM dendrimers as an effective system for targeted gemcitabine delivery. Int J Pharm. 2016;515(1–2):104–113. doi: 10.1016/j.ijpharm.2016.10.015.
- Kong L, Alves CS, Hou W, et al. RGD peptide-modified dendrimer-entrapped gold nanoparticles enable highly efficient and specific gene delivery to stem cells. ACS Appl Mater Interfaces. 2015;7(8):4833–4843. doi: 10.1021/am508760w.
- Carvalho MR, Reis RL, Oliveira JM. Dendrimer nanoparticles for colorectal cancer applications. J Mater Chem B. 2020;8(6):1128–1138. doi: 10.1039/C9TB02289A.
- Anselmo AC, Mitragotri S. Nanoparticles in the clinic. Bioeng Transl Med. 2016;1(1):10–29. doi: 10.1002/btm2.10003.
- Lei Q, Wang S, Hu J, et al. Stimuli-responsive “Cluster bomb” for programmed tumor therapy. ACS Nano. 2017;11(7):7201–7214. doi: 10.1021/acsnano.7b03088.
- Sykes EA, Chen J, Zheng G, et al. Investigating the impact of nanoparticle size on active and passive tumor targeting efficiency. ACS Nano. 2014;8(6):5696–5706. doi: 10.1021/nn500299p.
- Li C, Wang J, Wang Y, et al. Recent progress in drug delivery. Acta Pharm Sin B. 2019;9(6):1145–1162. doi: 10.1016/j.apsb.2019.08.003.
- Li H, Liu J, Luo Y, et al. Intratumor performance and therapeutic efficacy of PAMAM dendrimers carried by clustered nanoparticles. Nano Lett. 2019;19(12):8947–8955. doi: 10.1021/acs.nanolett.9b03913.
- Wang J, Mao W, Lock LL, et al. The role of micelle size in tumor accumulation, penetration, and treatment. ACS Nano. 2015;9(7):7195–7206. doi: 10.1021/acsnano.5b02017.
- Jeong WY, Kwon M, Choi HE, et al. Recent advances in transdermal drug delivery systems: a review. Biomater Res. 2021;25(1):24. doi: 10.1186/s40824-021-00226-6.
- Jain KK. An overview of drug delivery systems. In: Kewal K. Jain (editor), Drug delivery systems; 2020. Springer nature: New York. p. 1–54.
- Wen H, Jung H, Li X. Drug delivery approaches in addressing clinical pharmacology-related issues: opportunities and challenges. AAPS J. 2015;17(6):1327–1340. doi: 10.1208/s12248-015-9814-9.
- Li X, Chen L, Huang M, et al. Innovative strategies for photodynamic therapy against hypoxic tumor. Asian J Pharm Sci. 2023;18(1):100775. doi: 10.1016/j.ajps.2023.100775.
- Zaid Alkilani A, McCrudden MT, Donnelly RF. Transdermal drug delivery: innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics. 2015;7(4):438–470. doi: 10.3390/pharmaceutics7040438.
- Schoellhammer CM, Blankschtein D, Langer R. Skin permeabilization for transdermal drug delivery: recent advances and future prospects. Expert Opin Drug Deliv. 2014;11(3):393–407. doi: 10.1517/17425247.2014.875528.
- Ita KB. Transdermal drug delivery: progress and challenges. J Drug Deliv Sci Technol. 2014;24(3):245–250. doi: 10.1016/S1773-2247(14)50041-X.
- Jin JF, Zhu LL, Chen M, et al. The optimal choice of medication administration route regarding intravenous, intramuscular, and subcutaneous injection. Patient Prefer Adherence. 2015;9:923–942. doi: 10.2147/PPA.S87271.
- Kafle, U., Agrawal, S. and Dash, A.K., 2022. Injectable Nano Drug Delivery Systems for the Treatment of Breast Cancer. Pharmaceutics 2022, 14(12):2783. doi: 10.3390/pharmaceutics14122783
- Linker SM, Schellhaas C, Kamenik AS, et al. Lessons for oral bioavailability: how conformationally flexible cyclic peptides enter and cross lipid membranes. J Med Chem. 2023;66(4):2773–2788. doi: 10.1021/acs.jmedchem.2c01837.
- Padhye T, Maravajjala KS, Swetha KL, et al. A comprehensive review of the strategies to improve oral drug absorption with special emphasis on the cellular and molecular mechanisms. J Drug Deliv Sci Technol. 2021;61:102178. doi: 10.1016/j.jddst.2020.102178.
- Chelakkot C, Ghim J, Ryu SH. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp Mol Med. 2018;50(8):1–9. doi: 10.1038/s12276-018-0126-x.
- Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci. 2013;70(4):631–659. doi: 10.1007/s00018-012-1070-x.
- Singh AK, Sharma AK, Khan I, et al. Oral drug delivery potential of dendrimers. In: Ecaterina Andronescu & Alexandru Mihai Grumezescu (editors), Nanostructures for oral medicine. Amsterdam: Elsevier; 2017. p. 231–261.
- Abedi-Gaballu F, Dehghan G, Ghaffari M, et al. PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl Mater Today. 2018;12:177–190. doi: 10.1016/j.apmt.2018.05.002.
- Opitz AW, Czymmek KJ, Wickstrom E, et al. Uptake, efflux, and mass transfer coefficient of fluorescent PAMAM dendrimers into pancreatic cancer cells. Biochim Biophys Acta. 2013;1828(2):294–301. doi: 10.1016/j.bbamem.2012.09.016.
- Bhatia R, Sharma A, Narang RK, et al. Recent nanocarrier approaches for targeted drug delivery in cancer therapy. Curr Mol Pharmacol. 2021;14(3):350–366. doi: 10.2174/1874467213666200730114943.
- Yafout M, Ousaid A, Khayati Y, et al. Gold nanoparticles as a drug delivery system for standard chemotherapeutics: a new lead for targeted pharmacological cancer treatments. Sci Afr. 2021;11:e00685. doi: 10.1016/j.sciaf.2020.e00685.
- Shi P, Cheng Z, Zhao K, et al. Active targeting schemes for nano-drug delivery systems in osteosarcoma therapeutics. J Nanobiotechnology. 2023;21(1):103. doi: 10.1186/s12951-023-01826-1.
- Bertrand N, Wu J, Xu X, et al. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev. 2014;66:2–25. doi: 10.1016/j.addr.2013.11.009.
- Attia, M.F., Anton, N., Wallyn, J., Omran, Z. & Vandamme, T.F. 2019. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. Journal of pharmacy and pharmacology, 71(8):1185–1198. doi: 10.1111/jphp.13098.
- Ahmad A, Khan F, Mishra RK, et al. Precision cancer nanotherapy: evolving role of multifunctional nanoparticles for cancer active targeting. J Med Chem. 2019;62(23):10475–10496. doi: 10.1021/acs.jmedchem.9b00511.
- Stanislawska I, Liwinska W, Lyp M, et al. Recent advances in degradable hybrids of biomolecules and NGs for targeted delivery. Molecules. 2019;24(10):1873. doi: 10.3390/molecules24101873.
- Behera A, Padhi S. Passive and active targeting strategies for the delivery of the camptothecin anticancer drug: a review. Environ Chem Lett. 2020;18(5):1557–1567. doi: 10.1007/s10311-020-01022-9.
- Huang D, Sun L, Huang L, et al. Nanodrug delivery systems modulate tumor vessels to increase the enhanced permeability and retention effect. J Pers Med. 2021;11(2):124. doi: 10.3390/jpm11020124.
- Steichen SD, Caldorera-Moore M, Peppas NA. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur J Pharm Sci. 2013;48(3):416–427. doi: 10.1016/j.ejps.2012.12.006.
- Yazdani S, Bansal R, Prakash J. Drug targeting to myofibroblasts: implications for fibrosis and cancer. Adv Drug Deliv Rev. 2017;121:101–116. doi: 10.1016/j.addr.2017.07.010.
- Zhang, M., Gao, S., Yang, D., Fang, Y., Lin, X., Jin, X., Liu, Y., Liu, X., Su, K. and Shi, K., 2021. Influencing factors and strategies of enhancing nanoparticles into tumors in vivo. Acta Pharmaceutica Sinica B, 11(8):2265–2285. doi: 10.1016/j.apsb.2021.03.033.
- Garrigue P, Tang J, Ding L, et al. Self-assembling supramolecular dendrimer nanosystem for PET imaging of tumors. Proc Natl Acad Sci U S A. 2018;115(45):11454–11459. doi: 10.1073/pnas.1812938115.
- Shi J, Kantoff PW, Wooster R, et al. Cancer nanomedicine: progress, challenges, and opportunities. Nat Rev Cancer. 2017;17(1):20–37. doi: 10.1038/nrc.2016.108.
- Zhao N, Yan L, Zhao X, et al. Versatile types of organic/inorganic nanohybrids: from strategic design to biomedical applications. Chem Rev. 2018;119(3):1666–1762. doi: 10.1021/acs.chemrev.8b00401.
- Fan Y, Tu W, Shen M, et al. Targeted tumor hypoxia dual-mode CT/MR imaging and enhanced radiation therapy using dendrimer-based nanosensitizers. Adv Funct Mater. 2020;30(13):1909285. doi: 10.1002/adfm.201909285.
- Xiong Z, Shen M, Shi X. Dendrimer-based strategies for cancer therapy: recent advances and future perspectives. Sci China Mater. 2018;61(11):1387–1403.
- Vidal F, Guzman L. Dendrimer nanocarriers drug action: perspective for neuronal pharmacology. Neural Regen Res. 2015;10(7):1029–1031. doi: 10.4103/1673-5374.160063.
- Wang X, Qiu Y, Wang M, et al. Endocytosis and organelle targeting of nanomedicines in cancer therapy. Int J Nanomedicine. 2020;15:9447–9467. doi: 10.2147/IJN.S274289.
- Burns KE, Delehanty JB. Cellular delivery of doxorubicin mediated by disulfide reduction of a peptide–dendrimer bioconjugate. Int J Pharm. 2018;545(1–2):64–73. doi: 10.1016/j.ijpharm.2018.04.027.
- Fu F, Wu Y, Zhu J, et al. Multifunctional lactobionic acid-modified dendrimers for targeted drug delivery to liver cancer cells: investigating the role played by PEG spacer. ACS Appl Mater Interfaces. 2014;6(18):16416–16425. doi: 10.1021/am504849x.
- Dey, A.D., Bigham, A., Esmaeili, Y., Ashrafizadeh, M., Moghaddam, F.D., Tan, S.C., Yousefiasl, S., Sharma, S., Maleki, A., Rabiee, N., Kumar, A.P., Thakur, V.K., Orive, G., Sharifi, E., Kumar, A. & Makvandi, P. 2022. Dendrimers as nanoscale vectors: Unlocking the bars of cancer therapy. Seminars in cancer biology, 86396-419.
- Zhang J, Liu D, Zhang M, et al. The cellular uptake mechanism, intracellular transportation, and exocytosis of polyamidoamine dendrimers in multidrug-resistant breast cancer cells. Int J Nanomedicine. 2016;11:3677–3690. doi: 10.2147/IJN.S106418.
- Oliveira JP, Prado AR, Keijok WJ, et al. Impact of conjugation strategies for targeting of antibodies in gold nanoparticles for ultrasensitive detection of 17β-estradiol. Sci Rep. 2019;9(1):13859. doi: 10.1038/s41598-019-50424-5.
- Matai I, Sachdev A, Gopinath P. Self-assembled hybrids of fluorescent carbon dots and PAMAM dendrimers for epirubicin delivery and intracellular imaging. ACS Appl Mater Interfaces. 2015;7(21):11423–11435. doi: 10.1021/acsami.5b02095.
- Xiao Y, Shi X. Improved tumor imaging using dendrimer-based nanoplatforms. Nanomedicine. 2019;14(19):2515–2518. doi: 10.2217/nnm-2019-0288.
- Ding L, Lyu Z, Dhumal D, et al. Dendrimer-based magnetic resonance imaging agents for brain cancer. Sci China Mater. 2018;61(11):1420–1443. doi: 10.1007/s40843-018-9323-6.
- Tang T, Ma X, Bian Y, et al. Composite of gadolinium-labeled dendrimer nanocluster and graphene oxide nanosheet for highly efficient liver T1-weighted imaging probe. ACS Biomater Sci Eng. 2019;5(4):1978–1986. doi: 10.1021/acsbiomaterials.8b01641.
- Ben-Zichri S, Meltzer M, Lacham-Hartman S, et al. Synergistic activity of anticancer polyphenols embedded in amphiphilic dendrimer nanoparticles. ACS Appl Polym Mater. 2022;4(12):8913–8925. doi: 10.1021/acsapm.2c01316.
- Sharma R, Liaw K, Sharma A, et al. Glycosylation of PAMAM dendrimers significantly improves tumor macrophage targeting and specificity in glioblastoma. J Control Release. 2021;337:179–192. doi: 10.1016/j.jconrel.2021.07.018.
- Bargathulla I, Ashwaq A, Sathiyaraj S, et al. Pegylated bis-indolyl polyurethane dendrimer: empty drug carrier with prominent anticancer activity. Eur Polym J. 2021;153:110491. doi: 10.1016/j.eurpolymj.2021.110491.
- Gamage NH, Jing L, Worsham MJ, et al. Targeted theranostic approach for glioma using dendrimer-based curcumin nanoparticle. J Nanomed Nanotechnol. 2016;7(4):393. doi: 10.4172/2157-7439.1000393.
- Chen JS, Chen J, Bhattacharjee S, et al. Functionalized nanoparticles with targeted antibody to enhance imaging of breast cancer in vivo. J Nanobiotechnology. 2020;18(1):135. doi: 10.1186/s12951-020-00695-2.
- Antignani A, Ho ECH, Bilotta MT, et al. Targeting receptors on cancer cells with protein toxins. Biomolecules. 2020;10(9):1331. doi: 10.3390/biom10091331.
- Liu Y, Hui Y, Ran R, et al. Synergetic combinations of dual-targeting ligands for enhanced in vitro and in vivo tumor targeting. Adv Healthc Mater. 2018;7(15):e1800106. doi: 10.1002/adhm.201800106.
- Ouyang Z, Li D, Shen M, et al. Dendrimer-based tumor-targeted systems. In: Rongqin Huang & Yi Wang (editors), New nanomaterials and techniques for tumor-targeted systems; Springer: Singapore, 2020. p. 337–369.
- Wei P, Chen J, Hu Y, et al. Dendrimer-stabilized gold nanostars as a multifunctional theranostic nanoplatform for CT imaging, photothermal therapy, and gene silencing of tumors. Adv Healthc Mater. 2016;5(24):3203–3213. doi: 10.1002/adhm.201600923.
- Li Y, He H, Lu W, et al. A poly(amidoamine) dendrimer-based drug carrier for delivering DOX to gliomas cells. RSC Adv. 2017;7(25):15475–15481. doi: 10.1039/C7RA00713B.
- Xu L, Yeudall WA, Yang H. Folic acid-decorated polyamidoamine dendrimer exhibits high tumor uptake and sustained highly localized retention in solid tumors: its utility for local siRNA delivery. Acta Biomater. 2017;57:251–261. doi: 10.1016/j.actbio.2017.04.023.
- Ouyang Z, Li D, Xiong Z, et al. Antifouling dendrimer-entrapped copper sulfide nanoparticles enable photoacoustic imaging-guided targeted combination therapy of tumors and tumor metastasis. ACS Appl Mater Interfaces. 2021;13(5):6069–6080. doi: 10.1021/acsami.0c21620.
- Liu H, Wang H, Xu Y, et al. Lactobionic acid-modified dendrimer-entrapped gold nanoparticles for targeted computed tomography imaging of human hepatocellular carcinoma. ACS Appl Mater Interfaces. 2014;6(9):6944–6953. doi: 10.1021/am500761x.
- Zhou Z, Wang Y, Yan Y, et al. Dendrimer-templated ultrasmall and multifunctional photothermal agents for efficient tumor ablation. ACS Nano. 2016;10(4):4863–4872. doi: 10.1021/acsnano.6b02058.
- Luo D, Carter KA, Miranda D, et al. Chemophototherapy: an emerging treatment option for solid tumors. Adv Sci. 2017;4(1):1600106. doi: 10.1002/advs.201600106.
- Itoo AM, Paul M, Padaga SG, et al. Nanotherapeutic intervention in photodynamic therapy for cancer. ACS Omega. 2022;7(50):45882–45909. doi: 10.1021/acsomega.2c05852.
- Liu Z, Xie Z, Li W, et al. Photodynamic immunotherapy of cancers based on nanotechnology: recent advances and future challenges. J Nanobiotechnology. 2021;19(1):160. doi: 10.1186/s12951-021-00903-7.
- Gunaydin G, Gedik ME, Ayan S. Photodynamic therapy—current limitations and novel approaches. Front Chem. 2021;9:691697. doi: 10.3389/fchem.2021.691697.
- Chen Z, Liu L, Liang R, et al. Bioinspired hybrid protein oxygen nanocarrier amplified photodynamic therapy for eliciting anti-tumor immunity and abscopal effect. ACS Nano. 2018;12(8):8633–8645. doi: 10.1021/acsnano.8b04371.
- Chilakamarthi U, Giribabu L. Photodynamic therapy: past, present, and future. Chem Rec. 2017;17(8):775–802. doi: 10.1002/tcr.201600121.
- Kwiatkowski S, Knap B, Przystupski D, et al. Photodynamic therapy – mechanisms, photosensitizers, and combinations. Biomed Pharmacother. 2018;106:1098–1107. doi: 10.1016/j.biopha.2018.07.049.
- Zhang Q, Li L. Photodynamic combinational therapy in cancer treatment. J BUON. 2018;23(3):561–567.
- Moosavi MA, Sharifi M, Ghafary SM, et al. Photodynamic N-TiO2 nanoparticle treatment induces controlled ROS-mediated autophagy and terminal differentiation of leukemia cells. Sci Rep. 2016;6(1):34413. doi: 10.1038/srep34413.
- Sun Y, Zhao D, Wang G, et al. Recent progress of hypoxia-modulated multifunctional nanomedicines to enhance photodynamic therapy: opportunities, challenges, and future development. Acta Pharm Sin B. 2020;10(8):1382–1396. doi: 10.1016/j.apsb.2020.01.004.
- Nowak-Stepniowska A, Pergoł P, Padzik-Graczyk A. Photodynamic method of cancer diagnosis and therapy – mechanisms and applications. Postepy Biochem. 2013;59(1):53–63.
- Baptista MS, Cadet J, Di Mascio P, et al. Type I and type II photosensitized oxidation reactions: guidelines and mechanistic pathways. Photochem Photobiol. 2017;93(4):912–919. doi: 10.1111/php.12716.
- Bhatt H, Kiran Rompicharla SV, Ghosh B, et al. Α-tocopherol succinate-anchored PEGylated poly(amidoamine) dendrimer for the delivery of paclitaxel: assessment of in vitro and in vivo therapeutic efficacy. Mol Pharm. 2019;16(4):1541–1554. doi: 10.1021/acs.molpharmaceut.8b01232.
- Guo W, Wang F, Ding D, et al. TiO2–x based nanoplatform for bimodal cancer imaging and NIR-triggered chem/photodynamic/photothermal combination therapy. Chem Mater. 2017;29(21):9262–9274. doi: 10.1021/acs.chemmater.7b03241.
- Zhou, Z., Song, J., Nie, L., Chen, X., 2016. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem Soc Rev, 45 (23):6597–6626. doi: 10.1039/c6cs00271d.
- Tomalia DA, Khanna SN. A systematic framework and nanoperiodic concept for unifying nanoscience: hard/soft nanoelements, superatoms, meta-atoms, new emerging properties, periodic property patterns, and predictive Mendeleev-like nanoperiodic tables. Chem Rev. 2016;116(4):2705–2774. doi: 10.1021/acs.chemrev.5b00367.
- Caminade A, Turrin C, Majoral J. Biological properties of water-soluble phosphorhydrazone dendrimers. Braz J Pharm Sci. 2013;49:33–44. doi: 10.1590/S1984-82502013000700004.
- Liu Y, Ma K, Jiao T, et al. Water-insoluble photosensitizer nanocolloids stabilized by supramolecular interfacial assembly towards photodynamic therapy. Sci Rep. 2017;7(1):42978. doi: 10.1038/srep42978.
- Mokwena MG, Kruger CA, Ivan M, et al. A review of nanoparticle photosensitizer drug delivery uptake systems for photodynamic treatment of lung cancer. Photodiagn Photodyn Ther. 2018;22:147–154. doi: 10.1016/j.pdpdt.2018.03.006.
- Rkein AM, Ozog DM. Photodynamic therapy. Dermatol Clin. 2014;32(3):415–425, x. doi: 10.1016/j.det.2014.03.009.
- Forster JC, Harriss-Phillips WM, Douglass MJ, et al. A review of the development of tumor vasculature and its effects on the tumor microenvironment. Hypoxia. 2017;5:21–32.
- Zhao Y, Biswas S, Chen Q, et al. Direct readout hypoxia tumor suppression in vivo through NIR-theranostic activation. ACS Appl Biomater. 2021;4(7):5686–5694.
- Tan Z, Xu J, Zhang B, et al. Hypoxia: a barricade to conquer the pancreatic cancer. Cell Mol Life Sci. 2020;77(16):3077–3083. doi: 10.1007/s00018-019-03444-3.
- Rotow J, Bivona TG. Understanding and targeting resistance mechanisms in NSCLC. Nat Rev Cancer. 2017;17(11):637–658. doi: 10.1038/nrc.2017.84.
- Zhu F, Xu L, Li X, et al. Co-delivery of gefitinib and hematoporphyrin by aptamer-modified fluorinated dendrimer for hypoxia alleviation and enhanced synergistic chemo-photodynamic therapy of NSCLC. Eur J Pharm Sci. 2021;167:106004. doi: 10.1016/j.ejps.2021.106004.
- Wang L, Huo M, Chen Y, et al. Tumor microenvironment-enabled nanotherapy. Adv Healthc Mater. 2018;7(8):1701156. doi: 10.1002/adhm.201701156.
- Zhang D, Cai Z, Liao N, et al. pH/hypoxia programmable triggered cancer photo-chemotherapy based on a semiconducting polymer dot hybridized mesoporous silica framework. Chem Sci. 2018;9(37):7390–7399. doi: 10.1039/c8sc02408a.
- Huizing FJ, Hoeben BA, Franssen GM, et al. Quantitative imaging of the hypoxia-related marker CAIX in head and neck squamous cell carcinoma xenograft models. Mol Pharm. 2018;16(2):701–708. doi: 10.1021/acs.molpharmaceut.8b00950.
- Jung HS, Han J, Shi H, et al. Overcoming the limits of hypoxia in photodynamic therapy: a carbonic anhydrase IX-targeted approach. J Am Chem Soc. 2017;139(22):7595–7602. doi: 10.1021/jacs.7b02396.
- Arima H, Motoyama K, Higashi T. Sugar-appended polyamidoamine dendrimer conjugates with cyclodextrins as cell-specific non-viral vectors. Adv Drug Deliv Rev. 2013;65(9):1204–1214. doi: 10.1016/j.addr.2013.04.001.
- Yang J, Zhang Q, Chang H, et al. Surface-engineered dendrimers in gene delivery. Chem Rev. 2015;115(11):5274–5300. doi: 10.1021/cr500542t.
- Alam K, Rahman M, Beg S, et al. Advancement in protein-based nanocarriers in targeted anticancer therapy. In: Mahfoozur Rahman, Waleed H. Almalki, Hani Choudhry, Nabil A. Alhakamy, & Sarwar Beg (editors), Nanotherapeutics in cancer vaccination and challenges. Amsterdam: Elsevier; 2022. p. 95–102.
- Luong D, Kesharwani P, Deshmukh R, et al. PEGylated PAMAM dendrimers: enhancing efficacy and mitigating toxicity for effective anticancer drug and gene delivery. Acta Biomater. 2016;43:14–29. doi: 10.1016/j.actbio.2016.07.015.
- Ming X, Wu L, Carver K, et al. Dendritic nanoconjugates for intracellular delivery of neutral oligonucleotides. Nanoscale. 2015;7(29):12302–12306. doi: 10.1039/c5nr01665g.
- Figueroa ER, Lin AY, Yan J, et al. Optimization of PAMAM–gold nanoparticle conjugation for gene therapy. Biomaterials. 2014;35(5):1725–1734. doi: 10.1016/j.biomaterials.2013.11.026.
- Kasturirangan V, Nair BM, Kariapper MT, et al. In vivo toxicity evaluation of gold–dendrimer composite nanodevices with different surface charges. Nanotoxicology. 2013;7(4):441–451. doi: 10.3109/17435390.2012.668570.
- Feldman AT, Wolfe D. Tissue processing and hematoxylin and eosin staining. In: Christina E. Day (editors), Histopathology: methods and protocols; Springer: New York . 2014. p. 31–43.
- Li X, Haba Y, Ochi K, et al. PAMAM dendrimers with an oxyethylene unit-enriched surface as biocompatible temperature-sensitive dendrimers. Bioconjug Chem. 2013;24(2):282–290. doi: 10.1021/bc300190v.
- Huang D, Wu D. Biodegradable dendrimers for drug delivery. Mater Sci Eng C. 2018;90:713–727. doi: 10.1016/j.msec.2018.03.002.
- Mignani S, Rodrigues J, Tomas H, et al. Dendrimers in combination with natural products and analogues as anti-cancer agents. Chem Soc Rev. 2018;47(2):514–532. doi: 10.1039/c7cs00550d.
- Zhu J, Xiong Z, Shen M, et al. Encapsulation of doxorubicin within multifunctional gadolinium-loaded dendrimer nanocomplexes for targeted theranostics of cancer cells. RSC Adv. 2015;5(38):30286–30296. doi: 10.1039/C5RA01215E.
- Chakraborty S, Dhakshinamurthy GS, Misra SK. Tailoring of physicochemical properties of nanocarriers for effective anti-cancer applications. J Biomed Mater Res. 2017;105(10):2906–2928. doi: 10.1002/jbm.a.36141.
- Zhang A, Meng K, Liu Y, et al. Absorption, distribution, metabolism, and excretion of nanocarriers in vivo and their influences. Adv Colloid Interface Sci. 2020;284:102261. doi: 10.1016/j.cis.2020.102261.
- Wheeler KE, Chetwynd AJ, Fahy KM, et al. Environmental dimensions of the protein corona. Nat Nanotechnol. 2021;16(6):617–629. doi: 10.1038/s41565-021-00924-1.
- Tekie FSM, Hajiramezanali M, Geramifar P, et al. Controlling evolution of protein corona: a prosperous approach to improve chitosan-based nanoparticle biodistribution and half-life. Sci Rep. 2020;10(1):9664. doi: 10.1038/s41598-020-66572-y.
- Kesharwani P, Jain K, Jain NK. Dendrimer as nanocarrier for drug delivery. Prog Polym Sci. 2014;39(2):268–307. doi: 10.1016/j.progpolymsci.2013.07.005.
- Saw WS, Anasamy T, Do TTA, et al. Nanoscaled PAMAM dendrimer spacer improved the photothermal–photodynamic treatment efficiency of photosensitizer-decorated confeito-like gold nanoparticles for cancer therapy. Macromol Biosci. 2022;22(8):e2200130. doi: 10.1002/mabi.202200130.
- Zhu Y, Liu C, Pang Z. Dendrimer-based drug delivery systems for brain targeting. Biomolecules. 2019;9(12):790. doi: 10.3390/biom9120790.
- Beddoes CM, Case CP, Briscoe WH. Understanding nanoparticle cellular entry: a physicochemical perspective. Adv Colloid Interface Sci. 2015;218:48–68. doi: 10.1016/j.cis.2015.01.007.
- Kesharwani P, Mishra V, Jain NK. Generation dependent hemolytic profile of folate engineered poly(propyleneimine) dendrimer. J Drug Deliv Sci Technol. 2015;28:1–6. doi: 10.1016/j.jddst.2015.04.006.
- Soni N, Tekade M, Kesharwani P, et al. Recent advances in oncological submissions of dendrimer. Curr Pharm Des. 2017;23(21):3084–3098. doi: 10.2174/1381612823666170329150201.
- Kannan, R.M., Nance, E., Kannan, S. and Tomalia, D.A., 2014. Emerging concepts in dendrimer-based nanomedicine: from design principles to clinical applications. Journal of internal medicine, 276(6):579-617. doi: 10.1111/joim.12280.
- Araújo R, Santos S, Igne Ferreira E, et al. New advances in general biomedical applications of PAMAM dendrimers. Molecules. 2018;23(11):2849. doi: 10.3390/molecules23112849.
- Thiagarajan G, Greish K, Ghandehari H. Charge affects the oral toxicity of poly(amidoamine) dendrimers. Eur J Pharm Biopharm. 2013;84(2):330–334. doi: 10.1016/j.ejpb.2013.01.019.
- Kumbhar SA, Gorain B, Choudhury H, et al. Safety and toxicity issues of dendrimers. In: Prashant Kesharwani (editors), Dendrimer-based nanotherapeutics. Amsterdam: Elsevier; 2021. p. 143–162.
- Felder-Flesch D. Dendrimers in nanomedicine. New York : Jenny Stanford Publishing; 2016.
- Kulhari H, Pooja D, Prajapati SK, et al. Performance evaluation of PAMAM dendrimer based simvastatin formulations. Int J Pharm. 2011;405(1–2):203–209. doi: 10.1016/j.ijpharm.2010.12.002.

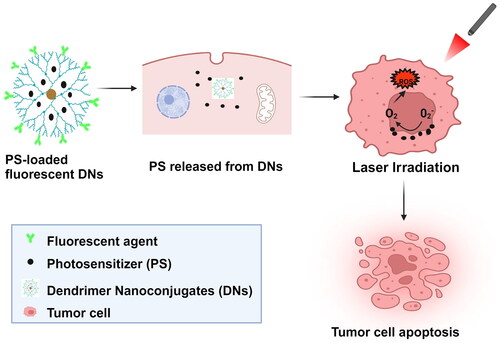
![Figure 4. [a] Biodegradation and [b] Clearance properties of a dendrimer.](/cms/asset/f72c6242-30d6-4f39-be45-8088fddf786a/ianb_a_2368033_f0004_c.jpg)