 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
We report for the first time the effect of temperature on the mechanical properties of (Ta1/3Ti1/3Zr1/3)C carbide. Flexural strength and fracture toughness were investigated using the three-point bending technique in argon. Using commercially available carbide powders with an equimolar ratio and performing spark plasma consolidation at 1973°C, we obtained a bulk single-phase medium-entropy carbide ceramic with the lattice parameter a= 4.458 Å. The flexural strength and fracture toughness at room temperature reached on average 700 MPa and 3.2 MPa m1/2, respectively. At 1800°C, local decomposition of the medium-entropy carbide took place as a structure with a local chemical gradient was observed after high-temperature tests, which increased fracture toughness by 30% (to 4.4 MPa m1/2).
1. Introduction
Recent progress in high-temperature ceramics based on the solid-solution of transition metal carbides, diborides, and silicides has spurred worldwide research for a new class of ultra-high temperature ceramics (UHTC) [Citation1–4]. Analogous to the recent advances in metallurgy [Citation5], they were defined as high-entropy ceramics [Citation6–10]. This term is generally applied to ceramic compounds with five or more principle metal atoms, while compounds with a fever number of the principle metal atoms can be classified defined as medium-entropy ceramics (their configurational entropy is lower than for high-entropy case). Various methods of development of multi-metal solid-solution high-entropy ceramics have been reported in the last two years [Citation7–10], but due to the slow atomic mobility of transition metals of the IV and V groups, their consolidation or combined consolidation/synthesis [Citation9] requires the temperature range typical for classical UHTCs (>1800°C).
Despite reports of the high-hardness and high Young’s modulus of high-entropy carbides [Citation11–14], studies related to the strength or toughness at room or high temperature are somehow limited [Citation8,Citation10]. A report of the ternary (Ta,Zr,Nb)C [Citation8] showed an interesting high-temperature flexural behavior, suggesting that solid-solution strengthening might lead to further improvement of the high-temperature properties. In this regard, further studies of the ternary or quaternary solid-solution of carbides may clarify the potential development of high-entropy carbides in particular, and the UHTC in general.
Furthermore, for some temperature ranges, binary [Citation17] and ternary [Citation16] solid-solutions based on TiC and ZrC will be a distinctive two-phase ceramic. This is a feature that is known if one uses WC or VC in ternary carbide alloys [Citation18]. In addition, for high-entropy ceramics, the layer-by-layer oxidation behavior of the high-entropy ceramics have been reported [Citation19], suggesting that upon reheating to elevated temperatures, the different diffusion rates for metal and carbon atoms in the pure metals or carbides [Citation4] may lead to localization or decomposition of the complex solid-solution such as medium-entropy or high-entropy ceramics.
The impact of these phenomena on the mechanical properties is still unclear. Thus present investigation will examine the possibility of using ternary solid-solution ceramics based on TiC and ZrC as structural materials at elevated temperatures. In particular, the high-temperature flexural strength and fracture toughness of (Ta,Ti,Zr)C was the main focus of this study.
2. Materials and methods
Commercially available TaC, TiC, and ZrC (Wako Pure Chemicals, Osaka, Japan) powders were used as the starting materials. The raw powders were mixed in ethanol in equimolar ratios of TaC:TiC:ZrC 1:1:1. For simplicity, a ternary equimolar carbide mixture was denoted as TTZ. Based on the previous results for (Ta,Zr,Nb)C [Citation8], a dwell time of 10 min at 1973°C was used.
The spark plasma sintering (SPS) experiments were conducted using the “Dr. Sinter” 1050 (Sumitomo, Japan) unit with a 30-mm die. The schedule for the TTZ carbide specimens prepared in this study had four major steps: (1) heating to 700°C in 4 min following a 5-min dwell, (2) heating to 1500°C in 10 min with a 5-min dwell; (3) 5-min ramp to 1973°C, with the dwell of 10 min. The last step included cooling down to 600°C in 15 min. Steps (1) and (2) were performed in a vacuum; during the dwell at 1500°C, the SPS chamber was backfilled with argon. At the end of the dwell at stage (2), the pressure was increased from 11 to 17 MPa, while reaching 1973°C, the pressure was increased from 17 to 45 MPa. The pressure of 45 MPa was maintained during the consolidation and cooling stages. Argon gas at the flow rate of 2 L/min was used.
An X-ray diffraction (XRD) analysis (D8 Advance, Bruker, Karlsruhe, Germany) was performed on the polished surfaces of the bars after the flexural tests using Cu-Kα radiation. The intensity data were collected over the 2θ range of 20–130°, in steps of 0.02–0.05° using a sampling time of 10 s for each step. The software used for refinement was TOPAS (TOPAS Ver. 4.0, Bruker AXS, Germany). Instrumental broadening was determined using a NIST 660b LaB6 standard run under the same conditions for each carbide sample [Citation20]. The lattice parameters of the carbides were determined with an accuracy of 0.0001 Å.
The structural characteristics of the TTZ ceramics and were studied using scanning electron microscopy (SEM, JCM-6000, JEOL) with secondary (SE) and backscattered electrons (BSE mode).
The three-point flexural strength was determined using rectangular blocks (2 × 2 × 25 and 2 × 2.5 × 25 mm) and strength testing equipment were previously described in detail [Citation21,Citation22]. A span of 16 mm was used. The fracture toughness of the ceramics was evaluated using specimen bending testing which contained a single edge through-thickness notch following ASTM C1421–10. Details of the testing configuration and the notch profile are presented in ref [Citation23]. Tests were performed using loading rates of 0.05 or 0.5 mm/min. Tests at elevated temperatures were performed in argon. The 10-min dwell to equalize temperature and prevent shock-related behavior was used. Such details are described elsewhere [Citation22]. We evaluated the elastic modulus (Eflex) for the tests at room temperature from the linear portion of the load–displacement curve using the procedure described in ASTM E111–04.
Hardness was determined by an MMT-7 Vickers hardness tester (Matsuzawa MMT-7; Matsuzawa SEIKI Co., Ltd., Tokyo, Japan) using a load of 9.8 N with a dwell time of 15 s following the standard procedure (ASTM C 1327–15).
3. Results and discussion
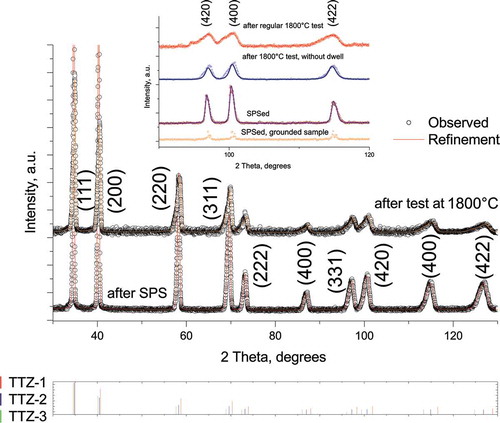
Using X-ray diffraction, we analyzed the SPSed TTZ ceramic specimens and bars following high-temperature flexural tests. After the SPS consolidation, a single-phase ceramic with the lattice parameter a= 4.4583 Å was identified. This remained unchanged for specimens tested at 1000°C or 1600°C. However, after the flexural tests at 1800°C, we noticed the appearance of additional phases. For these specimens, the refinement results can be summarized as follows: the main phase with the lattice parameter a= 4.458(3) Å occupied over 54 vol.%, while two other phases roughly contributed 37 and 8 vol.%. The lattice parameter for these phases was evaluated as a = 4.436(9) Å, and a = 4.493(7) Å. The refinement results are summarized in .
Table 1. Summary of X-ray diffraction analysis and mechanical properties of TTZ ceramics
In conjunction with the XRD analysis (), an SEM investigation of the fractured surfaces after flexural tests () confirmed the existence of at least three phases after the flexural tests at 1800°C (). The fourth phase was found to be free carbon which occupied less than 1 vol% based on the ImageJ analysis. The EDX results suggested that it is possible to estimate that at least 61 vol.% of the examined grains had an equimolar ratio between the Ti, Ta and Zr atoms; 29 vol.% were occupied by the phase with a higher contribution of Ta (Ta, 21–23 mol.%), while roughly 9 vol.% was occupied by the Ti-rich phase (Ti, 19–21 mol.%). This information was used to assign phases resulting from the refinement (see ). The theoretical density of the TTZ bulk was estimated to be 7.617 g/cm3, while the true density measured using the water immersion technique was 7.573 g/cm3.
Figure 1. X-ray diffraction of the TTZ ceramic after SPS consolidation at 1973°C and following the flexural strength test at 1800°C. The inset shows refinement details for deformed specimen at 1800°C for high-angle peaks. The bars indicate the allowed Bragg reflections for the Fm-3 m structure. Observed lattice parameter as SPSed specimen a= 4.458 Å, deformed at 1800°C: a = 4.4583(6) Å, a = 4.4369(2) Å, and a = 4.4937(3) Å (see )
The application of the EDX methods allowed us to observe the local chemical gradient in the metal atoms which becomes quite evident while examining the specimen deformed at 1800°C (). Overall, this may satisfy both the observations [Citation15–17] and the high-entropy alloy concept [Citation7] in which local order–disorder peculiarities may control the macroscopic properties.
Figure 2. Fracture of the TTZ ceramic after flexural strength tests at (a,b) room temperature, (c,d) at 1000°C, and (e,f) at 1600°C. (a,c,e) images acquired using the SE mode, while (b,d,f) used the BSE mode
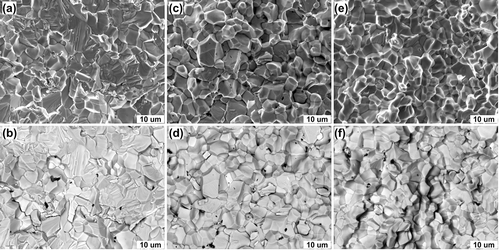
Figure 3. Fracture of the TTZ ceramic after fracture toughness test at 1800°C using two different magnifications (×500, and ×2000). Areas of local carbide decomposition are marked with red (Ti) and green colors (Ta), equimolar TTZ carbide is colored with blue. Uncolored areas are due to EDX’s depth limitation
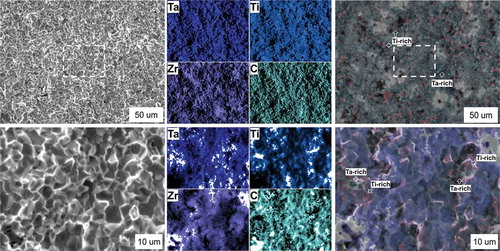
It can be suggested that the appearance of these local gradients is due to the different diffusion rates for metal and carbon atoms in the pure metals or carbides [Citation4]. According to the analysis by Andrievsky [Citation4], the difference in diffusivities becomes rate controlling above 0.7 Tmelting (i.e., >2100°C), thus at lower temperatures the solubility limit [Citation15–17] is a rate controlling factor.
Furthermore, in general, binary systems of group IV and V metal carbides have a two-phase region below 2000°C, and the addition of the WC or VC carbides will expand this multi-phase region [Citation18]. Study [Citation17] suggested that at 1700°C, the pseudo-ternary TiC–ZrC–HfC system had a clear two-phase region. These were explained by the low solubility of ZrC and HfC in TiC. Meanwhile, ref [Citation15] confirmed that for the TiC–ZrC system at 1300°C, the decomposition of the solid-solution will take place following a long-term annealing. In this respect, the layer-by-layer oxidation behavior of the high-entropy ceramics [Citation19] may be triggered by a similar driving force, i.e., low solubility or different diffusivities.
3.1. Mechanical properties
The average hardness lies within the range of 24.4 ± 1.2 GPa. The measured values in the present work were on the same level with the values reported for high-entropy carbides with four and five transition metal atoms () [Citation3,Citation11–14,Citation24–28]. Vickers hardness values can be strongly affected by residual porosity or grain size [Citation29]. Hardness of monolithic titanium carbide reported in ref [Citation27] serves as an example. Hence, it is difficult to speculate if solid-solution hardening affected hardness of ternary carbide in the present study. Such conclusion can be postulated based on results on the monolithic carbides [Citation30] and binary carbides [Citation2,Citation25,Citation26].
Table 2. Hardness data on monolithic UHTC carbides and high-entropy carbides
Data of the temperature dependence for the strength of the individual monolithic TaC, TiC, and ZrC carbides are summarized in [Citation31–37]. Within these monolithic carbides, data for titanium carbide [Citation33,Citation34] show why this monolithic carbide cannot be used for extremely high temperatures. Namely, titanium carbide [Citation33,Citation34] has a slight variation in strength below 800°C, followed by a decrease in strength at elevated temperatures. Below 1400°C, TiC fractured in the brittle manner and load against deflection curves were linear [Citation33,Citation34]. At 1400–1500°C (brittle to ductile transition temperature, BDTT), strength decreased to 90–110 MPa due to activation of the macroscopic plastic deformation [Citation38]. A similar trend can be observed for tantalum carbide in ref [Citation38]. The tendencies for ZrC are fully summarized in ref [Citation2]. For the monolithic zirconium carbide ceramics [Citation2,Citation35,Citation39], the transition between the brittle and ductile fracture is associated with a clear maximum. The peak strength for these ceramics is usually observed in the vicinity of the BDTT, but the position of the peak is quite sensitive to (a) grain size and (b) consolidation conditions, i.e., a higher consolidation temperature allows shifting the peak strength to the higher temperatures.
Figure 4. Effect of temperature on the flexural strength of (Ta1/3Ti1/3Zr1/3)C prepared in this study. (a) provides variation in strength for TTZ ceramic and for selected monolithic carbides [Citation31–37]. The closed symbols indicate that the strength was measured using a four-point setup and the open symbols show the results of the three-point flexural strength tests. (b) shows typical loading curves for TTZ ceramics at 25°C, 1000°C and 1800°C. Dashed lines in (b) visually show the Young’s modulus evolution during the flexural tests at different temperatures. Data for 1600°C are not shown for clarity as they overlap with the data for 1000°C
![Figure 4. Effect of temperature on the flexural strength of (Ta1/3Ti1/3Zr1/3)C prepared in this study. (a) provides variation in strength for TTZ ceramic and for selected monolithic carbides [Citation31–37]. The closed symbols indicate that the strength was measured using a four-point setup and the open symbols show the results of the three-point flexural strength tests. (b) shows typical loading curves for TTZ ceramics at 25°C, 1000°C and 1800°C. Dashed lines in (b) visually show the Young’s modulus evolution during the flexural tests at different temperatures. Data for 1600°C are not shown for clarity as they overlap with the data for 1000°C](/cms/asset/b6abda6f-0a30-47c9-a35c-5c61b650ee8b/tace_a_1840703_f0004_oc.jpg)
The flexural strength data of the binary or ternary carbides are limited by [Citation36] and [Citation8]. In [Citation36], the NbC–ZrC solid-solution follows a trend similar to the individual NbC or ZrC. While the data for the ternary (Ta,Zr,Nb)C suggested that the behavior of the ternary ceramic is comparable to the data for the monolithic NbC [Citation31,Citation32] or NbC–ZrC [Citation36] solid-solution, it also suggests that the magnitude for the solid-solution strengthening contribution.
During cooling of the specimen after the SPS consolidation, the specimen is under external contraption from the graphite die/matrix and experiences stress gradient due to preferential cooling from the surface. This should cause the macroscopic stress inside the specimen. Furthermore, on the lattice level, when the new solid-solution phase is being formed, due to the difference in the metal cation size the local strain will be developed (Hume-Rothery rules are applicable for binary solid-solutions and for high-entropy alloys [Citation5,Citation6]). Hence, these stresses may cause high strength at room temperature (700 MPa) for the (Ta1/3Ti1/3Zr1/3)C carbide. The decrease in strength noticeable at 1000°C can be explained as the effect of the reheating [Citation21] to higher temperatures, where the accumulated thermal stresses should relax. At 1600°C or 1800°C, the flexural strength remained unchanged within 300 ± 25 MPa, which is not typical for other monolithic carbides in this temperature region.
3.2. High-temperature toughness
The high-temperature fracture toughness data for monolithic UHTC carbides were not previously reported, while at room temperature, TaC has a toughness between 3 and 4 MPa m1/2 [Citation40], and below 3 MPa m1/2 [Citation4,Citation41] for the monolithic TiC or ZrC using the indentation method. The room temperature toughness data of the TTZ medium-entropy ceramic favorably agreed with the data for the monolithic carbides (3.2 MPa m1/2) [Citation14].
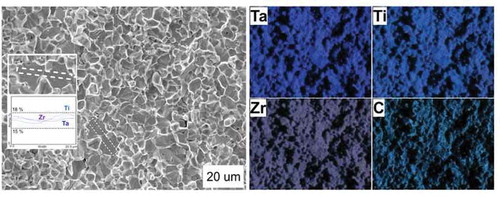
Despite the fact that reheating above 1000°C causes a decrease in strength, the toughness remains unchanged at 1000°C, and rises to 3.4 MPa m1/2 at 1600°C. Interestingly, despite an elastic loading behavior at 1800°C, the fracture toughness increases up to 4.4 MPa m1/2. In the absence of the toughness data for these temperatures, we can speculate that the local “decomposition” of carbide into several carbide phases observed in () may contribute to the increase in toughness. To confirm our observation, an additional KIC test was performed at 1800°C. During this test, the specimen was immediately loaded after 1800°C was reached (10-min dwell was used otherwise). This test provides the fracture toughness of 2.9 MPa m1/2, but essentially, a local chemical gradient was not observed using EDX (see inset, ). The toughness value itself could not be fully understood or used for engineering purposes as it is believed that thermal shock [Citation2,Citation4] may contribute when using immediate loading (see ). Other dwell such as 1 or 5 min were attempted. The latter result was consistent with 10 min dwell observations, while 1 min dwell did not result in the formation of the gradient using EDX. With some uncertainty, one can predict that the observed phenomenon is kinetically controlled, but further testing is required.
Figure 5. Fracture of the TTZ ceramic after flexural strength tests at 1800°C using an immediate loading procedure. Insets show a linear mapping for Ta, Ti and Zr performed on the flat neighboring grains. Dotted lines show a tolerance limit for equimolar composition 16.6 at.% (assuming that C occupies 50 at.%)
Although one expects the sufficient contribution of plastic deformation at 1800°C, neither of the loading curves recorded within this study had a visible plastic part. Thus, it is thought that an ~30% increase in toughness cannot be fully explained by contribution of the local plastic deformation [Citation38]. For the ZrB2–ZrC ceramics the increase in toughness coincided with the increase in strength at the elevated temperatures [Citation42] attributable to blunting of the crack tip due to plastic flow.
For the TTZ ceramics, the fracture stress slight changed at test between 1000°C and 1600°C (86 to 91 N, for KIC tests), suggesting the absence of crack tip blunting. Furthermore, at the temperatures of 1000°C and higher, from the observation of fracture surface, a subcritical crack growth is considered to occur. The extent of slow crack growth increases with increasing temperature and is intergranular in nature (see ).
Mechanistically, crack blunting can be expected as incipient crack-branching or slippage along the grain boundaries in proportion to the local resolved shear stress within the stress field of the crack tip. These agree with the crack propagation observed for the bars at 1800°C ().
Figure 6. Possible toughening mechanism due to local chemical gradient at ambient temperature and at 1800°C. Microcracks and related force fields are being generated as a result of the local carbide decomposition into several phases, initial medium-entropy or high-entropy carbide acts as a matrix under residual compressive stresses. These microcracks may shield crack tip during fracture, or directly pin the crack. Note, that microcracks after the flexural strength tests at 2000°C were observed in ref [Citation8], Scr Mat. Right image shows crack propagation behavior at the lateral surface of the test bar after fracture toughness test following three-point flexure at 1800°C (SE mode)
![Figure 6. Possible toughening mechanism due to local chemical gradient at ambient temperature and at 1800°C. Microcracks and related force fields are being generated as a result of the local carbide decomposition into several phases, initial medium-entropy or high-entropy carbide acts as a matrix under residual compressive stresses. These microcracks may shield crack tip during fracture, or directly pin the crack. Note, that microcracks after the flexural strength tests at 2000°C were observed in ref [Citation8], Scr Mat. Right image shows crack propagation behavior at the lateral surface of the test bar after fracture toughness test following three-point flexure at 1800°C (SE mode)](/cms/asset/e319b015-fe1f-4d24-a9e8-fa84151884ff/tace_a_1840703_f0006_oc.jpg)
Figure 7. Change in fracture toughness during cooling (a,b) and (d,c) reheating using different relation in CTE between matrix and inclusion
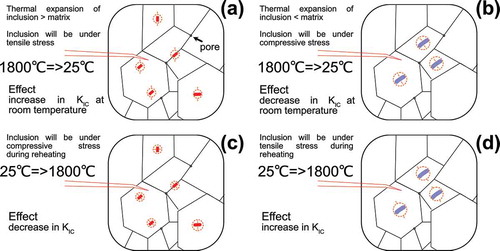
suggest that the local chemical gradient in TTZ carbide is only one of the factors that contribute to the fracture toughness increase, and its contribution is expected to be similar to that of the transformation induced toughening, i.e., via generation of the local microcracks. This is in agreement with observation in ref [Citation8], Scr Mat. at elevated temperatures. Nevertheless, local decomposition was not observed in [Citation8], Scr Mat. for (Ta,Zr,Nb)C. Judging from results presented in [Citation15–17] and considering solubility of ZrC in TiC [Citation17] one can anticipate that complex solid-solution involving both TiC and ZrC (i.e., HfC-ZrC-TiC or TaC-ZrC-NbC-TiC) can also experience the decomposition during reheating.
Furthermore, without reliable data on the coefficient of thermal expansion (CTE) or elastic moduli of the binary or ternary carbides, it is not possible to make an estimation for toughness behavior using models proposed in [Citation43,Citation44]. For example, data on the CTE for HfC–TaC ceramic reported in [Citation25,Citation45] had different trends: (i) monotonic growth following a rule of mixtures [Citation25], (ii) a curve with a clear maximum for equimolar concentration but with low CTE for other non-equimolar [Citation45]. Therefore, using different CTE data and a model proposed in [Citation43] may lead to controversial results. Let us consider the following scenario: a precipitation process of the TaC from HfC–TaC, where a solid-solution carbide acts as a matrix. Using data of ref [Citation25] () and assuming 20 vol.% of the TaC precipitates, and a temperature gradient of 1800°C, one can estimate [Citation43], that at room temperature matrix will under tensile stress of 52 MPa, while TaC will be under a compressive stress of 208 MPa. If one assumes that the size of the precipitates is 1 µm, the toughness of the “composite” should decrease (i.e., positive ) by 0.09 MPa m1/2 (0.03 MPa m1/2 for 100 nm, 0.31 MPa m1/2 for 10 µm). This suggests that upon the reheating, ignoring the plasticity, toughness of the composite should increase by the same amount. Similar estimation can be made if a matrix is a 1:1 solid-solution, while precipitates are 4:1 and 1:4 solid-solutions (). provides a visual illustration how the toughness would change for the solid-solution carbide if a solid-solution phase with (a) higher or (b) lower CTE compared with matrix is being precipitated during cooling. Any change in CTE ratio between newly precipitated phase, its volume or size may lead to enhancement of this effect. As underlined above, due to the absence of the CTE data on ternary (Ta,Ti,Zr)C it is unwise to bold predictions using approach described in [Citation43]. or clearly illustrate conditions for increase in toughness due to mismatch in thermal expansion for the multiphase carbide ceramics, nevertheless this scenario is valid for the matrix–inclusion configuration existing during cooling-heating or heating-cooling cycles, whereas we observe phases that form in situ at 1800°C.
Table 3. Data on thermal expansion and Young moduli for TaC – HfC ceramic based on ref [Citation25]
Table 4. Evaluation of the thermal stresses and change in toughness for different matrix – inclusion configurations based on data of ref [Citation25]
Analysis of ref [Citation44] suggests that the fracture toughness should decrease with the increase in temperature based on the decay of elastic modulus. Using data in as a source for a first approximation (see for values), one should expect a 20% decrease in toughness at 1800°C, but the opposite observation was made.
This suggests that further work involving, for example, evaluation of the thermal expansion for solid-solution carbides and their elastic moduli at elevated temperatures, which are beyond scope of this work, is needed to clarify details on high-temperature toughening in ultra-high temperature carbides.
The observation of the crack propagation on the bar surface after 1800°C suggests that additional mechanisms can be involved, such as crack branching and crack deflection, which are among the mechanisms involved in the high-temperature fracture. The latter observations may suggest contribution of the crack blunting via plasticity, as it was observed in [Citation46]. Overall, the observed decomposition process and data in refs. [Citation15–17] at lower temperatures indicate that this process can be tailored, if required. However, the exact temperature range for this de facto self-toughening phenomenon cannot be currently predicted as further experimental confirmation is required.
4. Conclusions
Bulk solid-solution medium-entropy carbide ceramics have been obtained using the spark plasma sintering method. Phase analysis via lattice parameter measurements by X-ray diffraction showed that after subsequent flexural or fracture toughness tests at 1800°C, the (Ta,Ti,Zr) carbide undergoes local decomposition and changes to the three-phase ceramic.
High-temperature fracture toughness for these compounds was reported for the first time. It was noted that the toughness remains unchanged up to 1600°C. During the tests at 1800°C, the fracture toughness reaches 4.4 MPa m1/2. The increase in the fracture toughness can be attributed to the observed local carbide decomposition; however, a fracture analysis suggests that multiple mechanisms may also contribute to the improvement.
Supplemental Material
Download MS Word (2.5 MB)Acknowledgments
D.D. was supported by World Premier International Research Center Initiative (WPI), MEXT, Japan.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary materials
Supplemental data for this article can be accessed here.
References
- Fahrenholtz WG, Wuchina EJ, Lee WE, et al. editor. Ultra-high temperature ceramics. Hoboken (NJ): Wiley; 2014.
- Zubarev PV. High-temperature strength of the interstitial phases. Moscow (USSR): Metallurgiya; 1985. ( [in Russian]).
- Mroz C. Processing TiZrC and TiZrB2. Am Ceram Soc Bull. 1994;73:78–81.
- Andrievski RA, Spivak II. Strength of refractory compounds. Chelyabinsk (USSR): Metallurgiya; 1989. ( [in Russian]).
- Oses C, Toher C, Curtarolo S. High-entropy ceramics. Nat Rev Mater. 2020;5:295–309.
- Castle E, Csanadi T, Grasso S, et al. Processing and properties of high-entropy ultra-high temperature carbides. Sci Rep. 2018;8(1):8609.
- Sarker P, Harrington T, Toher C, et al. High-entropy high-hardness metal carbides discovered by entropy descriptors. Nat Commun. 2018;9:4980.
- Demirskyi D, Borodianska H, Suzuki TS, et al. High-temperature flexural strength performance of ternary high-entropy carbide consolidated via spark plasma sintering of TaC, ZrC and NbC. Scr Mater. 2019;164:12–16.
- Wei X-F, Liu J-X, Li F, et al. High entropy carbide ceramics from different starting materials. J Eur Ceram Soc. 2019;39(10):2989–2994.
- Wang K, Chen L, Xu C, et al. Microstructure and mechanical properties of (TiZrNbTaMo)C high-entropy ceramic. J Mater Sci Technol. 2020;39(15):99–105.
- Sedegov A, Vorotilo S, Tsybulin V, et al. Synthesis and study of high-entropy ceramics based on the carbides of refractory metals. IOP Conf Series. 2019;558:012043.
- Feng L, Fahrenholtz WG, Hilmas GE. Low‐temperature sintering of single‐phase, high‐entropy carbide ceramics. J Am Ceram Soc. 2019;102(12):7217–7224.
- Yan XL, Contantin L, Lu YF, et al. (Hf0.2Zr0.2Ta0.2Nb0.2Ti0.2)C high‐entropy ceramics with low thermal conductivity. J Am Ceram Soc. 2018;101(10):4486–4491.
- Ye BL, Wen TQ, Huang KH, et al. First‐principles study, fabrication, and characterization of (Hf0.2Zr0.2Ta0.2Nb0.2Ti0.2)C high‐entropy ceramic. J Am Ceram Soc. 2019;102:4344–4352.
- Borgh I, Hedström P, Blomqvist A, et al. Synthesis and phase separation of (Ti,Zr)C. Acta Mater. 2014;66:209–218.
- Li Y, Katsui H, Goto T. Microstructure evolution of (Ti,Zr)C solid solution at the initial stage of phase decomposition. Mater Today Proc. 2017;4:11449–11512.
- Murray P, Weston JE. The 1700 °C isothermal section of the pseudoternary system TiC-ZrC-HfC. J Less Common Met. 1981;81(1):173–179.
- Kieffer R, Benesovsky F. Hartstoffe. Wien: Springer; 1963. ( [in German]).
- Ye B, Wen T, Liu D, et al. Oxidation behavior of (Hf0.2Zr0.2Ta0.2Nb0.2Ti0.2)C high-entropy ceramics at 1073-1473 K in air. Corros Sci. 2019;153:327–332.
- Cullity BD. Elements of X-Ray Diffraction. 2nd ed. Reading (MA): Addison-Wesley Publishing Inc; 1978.
- Demirskyi D, Vasylkiv O. Analysis of the high-temperature flexural strength behavior of B4C–TaB2 eutectic composites produced by in situ spark plasma sintering. Mater Sci Eng A. 2017;697:71–78.
- Demirskyi D, Solodkyi I, Nishimura T, et al. High‐temperature strength and plastic deformation behavior of niobium diboride consolidated by spark plasma sintering. J Am Ceram Soc. 2017;100(11):5295–5305.
- Demirskyi D, Vaslykiv O. Spark plasma sintering and high-temperature strength of B6O–TaB2 ceramics. J Eur Ceram Soc. 2017;37(8):3009–3014.
- Sciti D, Guicciardi S, Nygren M. Densification and mechanical behavior of HfC and HfB2 fabricated by spark plasma sintering. J Am Ceram Soc. 2008;91:1433–1440.
- Cedillos-Barraza O, Grasso S, Al Nasiri N, et al. Sintering behaviour, solid solution formation and characterisation of TaC, HfC and TaC–HfC fabricated by spark plasma sintering. J Eur Ceram Soc. 2016;36(7):1539–1548.
- Acicbe RB, Goller G. Densification behavior and mechanical properties of spark plasma‐sintered ZrC–TiC and ZrC–TiC–CNT composites. J Mater Sci. 2013;48(6):2388–2393.
- Cheng L, Xie Z, Liu G, et al. Densification and mechanical properties of TiC by SPS‐effects of holding time, sintering temperature and pressure condition. J Eur Ceram Soc. 2012;32(12):3399–33406.
- Demirskyi D, Sakka Y. High-temperature reaction consolidation of TaC–TiB2 ceramic composites by spark-plasma sintering. J Eur Ceram Soc. 2015;35(1):405–410.
- Rice RW. Mechanical properties of ceramics and composites: grain and particle effects. New York (NY): CRC Press; 2000.
- Gillman JJ. Hardnesses of carbides and other refractory hard metals. J Appl Phys. 1970;41(4):1664–1666.
- Ordan’yan SS, Stepanenko EK, Sokolov IV. Strength of NbC-NbB2 sintered composites. Izv Vyssh Uchebn Zaved Khim. 1984;27(10):1201–1203.
- Kelly A, Rowcliffe DJ. Deformation of polycrystalline transition metal carbides. J Am Ceram Soc. 1967;50(5):253–256.
- Katz AP, Lipsitt HA, Mah T, et al. Mechanical behaviour of polycrystalline TiC. J Mater Sci. 1983;18(7):1983–1992.
- Miracle DB, Lipsitt HA. Mechanical properties of fine‐grained substoichiometric titanium carbide. J Am Ceram Soc. 1983;66(8):592–597.
- Zubarev PV, Kuraev AB. Stress relaxation in zirconium carbide. 2. Mechanisms of stress relaxation. The relationship of the processes of creep and relaxation. Strength Mater. 1994;26(2):132–136.
- Lanin AG, Zubarev PV, Vlasov KP. Mechanical and thermophysical properties of materials in HTGR fuel bundles. Atomic Energy. 1993;74(1):40–44.
- Demirskyi D, Nishimura T, Sakka Y, et al. High-strength TiB2–TaC ceramic composites prepared using reactive spark plasma consolidation. Ceram Int. 2016;42(1):1298–1306.
- Demirskyi D, Vasylkiv O. Mechanical properties of SiC–NbB2 eutectic composites by in situ spark plasma sintering. Ceram Int. 2016;42(16):19372–19385.
- Zhao J, Zou J, Man Z-Y, et al. Dopant effects on high temperature mechanical properties of zirconium carbide ceramics. Adv Appl Ceram. 2015;114(6):338–343.
- Silvestroni L, Pienti L, Guicciardi S, et al. Strength and toughness: the challenging case of TaC-based composites. Compos B. 2015;72:10–20.
- Maerky C, Guillou M-O, Henshall JL, et al. Indentation hardness and fracture toughness in single crystal TiC0.96. Mater Sci Eng A. 1996;209(1–2):329–336.
- Neuman EW, Hilmas GE, Fahrneholtz WG. Ultra‐high temperature mechanical properties of a zirconium diboride–zirconium carbide ceramic. J Am Ceram Soc. 2016;99(2):597–603.
- Taya M, Hayashi S, Kobayashi AS, et al. Toughening of a particulate-reinforced ceramics-matrix composite by thermal residual stress. J Am Ceram Soc. 1990;73(5):1382–1391.
- Stewart RL, Bradt RC. Fracture of polycrystalline MgAl2O4. J Am Ceram Soc. 1980;63(11–12):619–623.
- Barantseva IG, Paderno VN. Thermal expansion of solid solutions in thesystems ZrC–NbC and HfC–TaC. In: Samsonov GV, editor. Refractory Carbides. London (UK): Consultants Bureau; 1974. p. 283–285.
- Maloy S, Heuer AH, Lewandowski J, et al. Carbon addition to molybdenum disilicide: improved high-temperature mechanical properties. J Am Ceram Soc. 1991;74(10):2704–2706.
