?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Gel casting technology and a two-stage sintering process were used to produce yttria-stabilized tetragonal zirconia (YTZP) ceramics with excellent mechanical properties. A carbonic acid-based polyelectrolyte dispersant was used as a dispersant to prepare a well-dispersed nano-sized (94 nm) zirconia slurry with a high solid content (45 vol%) for gel casting. The functional carboxyl groups of the polyelectrolyte dispersant reacted with epoxy monomer (ethylene glycol diglycidyl ether, EGDGE) as it was added to the slurry, resulting in a decrease in gelling incubation time and an increase in viscosity, resulting in gel casting failure. Adding hydroquinone (HQ) to the slurry can cause polymerization to be delayed and the gel-casting working time to be extended. To control grain growth, a two-stage sintering process was used. The temperature for the first stage of sintering was set at 1300°C, then lowered to 1240°C and soaked for 24 h in the second stage to achieve a relative density of greater than 99%. The two-stage sintered sample has a Vickers hardness of 15.2 GPa, fracture toughness of 7.8 MPa.m1/2, and flexural strength of 771 MPa.
1. Introduction
Yttria-stabilized zirconia (YTZP) is widely used in industry. Its advantages include a high melting point, high chemical stability, high mechanical strength, toughness, and hardness (Mohs hardness 8.5, second only to diamond and corundum). Because of its excellent mechanical properties and wear resistance, YTZP is now widely used in the back cover of mobile phones, thermal barriers, oxygen sensors, dental implants, and knee joint replacements.
With increasing grain size, the resistance to wear and abrasion of YTZP decreases. YTZP ceramics with small grain sizes have received much attention due to their high hardness and fracture toughness [Citation1,Citation2]. As a result, inhibiting grain growth during sintering is a critical factor in the fabrication of nanostructured materials. Densification without significant grain growth requires maintaining grain boundary diffusion while inhibiting grain boundary migration. It is well known that solute drag [Citation3] and two-stage sintering processes [Citation4–8] are effective strategies for controlling grain growth in the final sintering step. However, finding an effective dopant to control the microstructure of newly developed ceramics is difficult. Many researchers [Citation9–12] studied the influence of microwave sintering on Y-TZP microstructure and mechanical characteristics. It has been discovered that microwave heating may be used to sinter Y-TZP commercial ceramics, improving mechanical qualities while significantly reducing time and energy usage. It is still challenging to obtain YTZP ceramics with sintered densities greater than 99% and grain sizes less than 200 nm using the microwave sintering process. Because two-stage sintering is a simple and effective microstructure refinement method, it has been widely used to produce high-density bodies with smaller grain sizes.
The ceramic forming methods can be classified into dry [Citation13] and wet forming processes [Citation14]. Complex-shaped ceramics have been prepared using wet forming processes such as slip casting and gel casting [Citation14–17]. The slip casting method has some drawbacks, such as the need for a plaster mold for dehydration, a long molding time, and low green strength, making it unsuitable for making thick sections. Gel-casting is a wet-forming technology developed by the Oak Ridge National Laboratory (ORNL) that produces high-density ceramics with complex shapes [Citation14]. It has the advantages of having a short molding time, no mold material restrictions, a high green strength, and the ability to apply thick sections. Compared to the solvent system, the aqueous gel casting process is less expensive and more environmentally friendly [Citation18]. For aqueous-based gel-casting, the ceramic powder is first dispersed in water. Then monomer and initiator are added and mixed to form a dispersed slurry, which is poured into the mold and polymerized at high temperatures to create a green body with fewer defects, higher density, and better mechanical properties than other alternatives[Citation19]. Acrylamide (AM) is commonly used as a gelling agent[Citation19]. Due to AM neurotoxicity, gel-casting is not widely employed in the industry. Xie et al. [Citation20] recently reported on the ethylene glycol diglycidyl ether (EGDGE) system as a potential nontoxic monomer for producing a low-viscosity slurry with high green strength. The slurry dispersion affects the green microstructure and, as a result, the microstructure, mechanical strength, and optical properties of sintered ceramics during the wet-forming process. Dolapix CE64 has been identified as the most effective dispersant for aqueous zirconia slurry [Citation21]. However, it was found that Dolapix CE64 was added to the epoxy monomer, tending to induce gelling, leading to a sudden increase in slurry viscosity. To the best of our knowledge, the influence of the dispersant used in this work (Dolapix CE64) on the behavior of the gelling agent (EGDGE) adopted for this research has not been investigated yet.
In this study, the YTZP slurry for the gel casting process was prepared. The different dispersants, solid content, and polymerization inhibitor effects on the dispersion and slurry gelling rate were investigated. The sintered YTZP ceramics with a sintered density of 99.4% and a mean grain size of about 195 nm can be successfully prepared using the aqueous gel casting and two-stage sintering processes.
2. Experimental procedure
This study used 3 mol% yttria-stabilized zirconia (YTZP, Aurora Applied Materials, Taiwan) powder with a mean particle size of 94 nm as the raw material to prepare the green body using an aqueous gel casting process. The XRD pattern of the YTZP powder is shown in Figure S1. The YTZP powder contains 38.9 wt% tetragonal phase and 61.1 wt% monoclinic phase. The dispersants were commercial polyelectrolytes Dolapix CE 64 and Darvan 821A obtained from Zschimmer and Schwarz GmbH and Vanderbilt Minerals, respectively. Dolapix CE 64 is a polyelectrolyte based on carbonic acid with a molecular weight of 660 g/mol. Figure S2 (in supplementary material) depicts the chemical structure of Dolapix CE 64. Darvan 821A is a 40 wt% solution of 2-propenoic acid, homopolymer, ammonium salt with a molecular weight of 6000 g/mol. The different amounts of commercial dispersants, 1–2 wt% Darvan 821A (D821A, Vanderbilt Minerals) and 0.15–0.5 wt% Dolapix CE64 (CE64, Zschimmer & Schwarz), were mixed with YTZP powder and deionized water by ball milling for 12 h to prepare the slurry. Monomers and plasticizers are the primary organic vehicles in the slurry. Ethylene glycol diglycidyl ether (EGDGE, Sigma‐Aldrich, Germany), with a molecular weight of 176 g/mol, acted as the monomer, and a polyamine hardener, bis (3-aminopropyl) amine (DPTA, Sigma‐Aldrich, Germany) were used to consolidate the suspensions [Citation20]. EGDGE, in the slurry, plays the role of increasing the green strength through polymerization to become a binder. YTZP suspensions with solid contents of 40, 45, and 50 vol% were prepared by dispersing a calculated amount of YTZP powder and CE64 into the distilled water. After ball-milling for 24 h using Y-TZP balls, 15 wt% EGDGE and glycerol (EGDGE/glycerol = 6/4) and 0.05 mol% Hydroquinone (HQ) relative to EGDGE acted as a polymerization inhibitor were added into the suspension and ball-milled for 24 h using Y-TZP balls. The function of the plasticizer, glycerol, is to allow the green body to be plastic, so the plasticizer can help avoid cracking by promoting plastic deformation. During drying, cracks are thereby reduced. After adding 0.25 mol/eq of DPTA, the suspension was degassed in a vacuum desiccator for 5 minutes and poured into a silicone mold (ϕ = 50 mm). Samples sealed with cling film were polymerized at 30, 45 and 60°C for 6 h. The samples were then dried at 60°C for 24 h before demolding. After demolding, the green body was further compacted using cold isostatic pressing (CIP) at 200 MPa. Afterward, binder burnout was performed at 500°C for 1 h at a heating rate of 1°C/minute. The two-stage sintering method was used to densify and control grain growth. In the first step, the binder-burnout sample was heated to 1300°C at a heating rate of 5°C/minute and soaked for 5 minutes. It was then cooled down to the lower temperatures of 1240°C with a cooling rate of 50°C/min and soaked for 24 h.
The crystalline phase composition was determined using X-ray diffractometry (Dandong Fangyuan, DX-2700, Sandong, China) with CuKα radiation at a voltage of 35 kV, a current of 30 mA, and a scanning rate of 0.01° (2θ)/sec with step size 0.01° (2θ). The volumetric fractions of the m-ZrO2 (Vm) and t-ZrO2 (Vt) phases can be calculated from the intensities of the diffraction peaks ( and (111) of m-ZrO2 and line diffraction (111) t-ZrO2 according to EquationEq. (1
(1)
(1) )-EquationEq. (3
(3)
(3) )[Citation22].
with I the peak intensity, and where the subscripts m and t represent the monoclinic and tetragonal phases, respectively.
The average size and size distribution of YTZP particles in a suspension was measured using a dynamic light scattering analyzer (NanoPlus 3, Micromeritics, USA). The rheological behaviors of the YTZP slurries added with different amounts of dispersants were measured using a rotational viscometer (DV2TLVTJO, Brookfield, USA). The shear rate was raised from 0 to 100 s−1 in 2 min, then maintained at 100 s−1 for 1 min before returning to 0 s−1 in another 2 min.
The functional groups of the YTZP slurries obtained by mixing EGDGE and DPTA for various reaction times (immediate mixing (0 min) to 30 min) were characterized by ATR (attenuated total reflection)-FTIR (EQUINO 55, Bruker, Germany). Every spectrum was the average of 32 scans in the range of 650–4000 cm−1.
The sintering temperature was determined using a dilatometer (DIL 402C, NETZSCH, Germany) in an air atmosphere between 30 and 1400°C at a heating rate of 5°C/minute. The cylindrical specimens with 8 mm diameters and 5 mm heights were prepared for dilatometry measurement. The densities of the green and sintered bodies were determined using Archimedes’ method, with the theoretical density of YTZP set at 6.08 g/cm3. The green and sintered microstructures were observed using scanning electron microscopy (SEM, SU‐5000, Hitachi, Japan). The grain size was calculated using the linear intercept method based on SEM images (average value multiplied by 1.54 to account for off-center intersections). The hardness and fracture toughness evaluations were performed using Vickers indentation (MVK‐H1, Akashi, Japan) at a 10 N load. The fracture toughness ( was calculated according to EquationEq.(4)
(4)
(4) reported by Antis [Citation23].
Where c is the crack length from the center of the impression to the crack tip; E is Young’s modulus (210 GPa for YTZP); H is Vickers hardness; P is applied load.
Flexural strength was determined with the 3‐point bending test (support span: 30 mm; thickness: 3 mm; width: 4 mm) on bar specimens. Ten bar specimens with the dimension of 3 mm×4 mm×40 mm were prepared for flexural strength measurement.
3. Results and discussion
depicts the rheological properties of YTZP slurries with varying amounts of dispersant. Shear thinning was observed in the rheological behaviors of the slurries treated with Darvan 821A and CE64. The slurry viscosity was higher at low shear rates, which could be attributed to the network structure formed by Van der Waals attraction between nano-sized YTZP particles. As the shear rate increased, the shearing force broke up the network structure, decreasing viscosity.
Figure 1. Rheological behaviors of the YTZP slurries added with different amounts of dispersants (a) D-821A; (b) CE64.
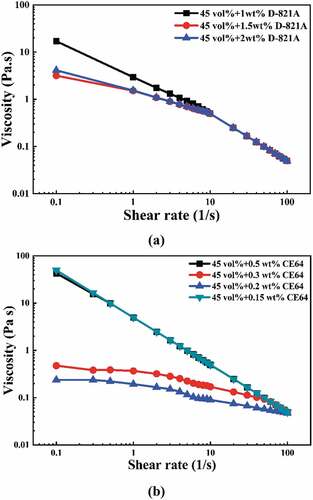
The viscosity of the slurry added with 1 wt% Darvan 821A exceeded 10 Pas at a shear rate of 0.1 s−1, indicating insufficient dispersant adsorbed on the surface of YTZP powder. The viscosity decreased significantly when the Darvan 821A addition was increased to 1.5 wt%. The viscosity increased again when the addition amount was increased to 2 wt%. This means that the adsorbed dispersant has become saturated; too much dispersant causes the particles to flocculate. For the slurry added with 0.2 wt% CE64, the slurry rheological behavior became nearly Newtonian, indicating that the YTZP slurry was completely dispersed. The slurry rheology increased slightly as the dispersant concentration was increased to 0.3 wt%. It suggests that the optimal dispersant content for the YTZP slurry is about 0.2 wt%. CE64 demonstrated better dispersion capability than Darvan 821A due to lower optimal addition and viscosity. compares the slurry viscosity of this study with the results reported in the literature [Citation21,Citation24,Citation25]. The YTZP slurry has better dispersion at higher alkaline conditions (pH 10) compared to the pH range of 7–8 reported in the literature. This is because despite its small molecular size, CE64 can be completely dissociated at pH 10 and then adsorbed on the powder surface via the reaction of the carboxylic groups with the hydroxyl groups of YTZP [Citation26], which increases electrostatic repulsion and decreases viscosity [Citation27].
Table 1. Comparison of the slurry viscosity of the result with those reported in the literature.
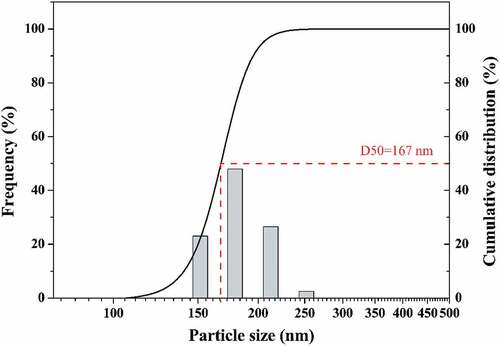
shows the particle size distribution of YTZP slurry added with 0.2 wt% CE64. The mean particle size (d50) was approximately 165 nm, which was close to the primary particle size of 94 nm. This confirms that a well-dispersed YTZP slurry can be obtained after adding 0.2 wt% CE64.
) shows the effect of solid content on the rheological properties of YTZP slurries containing 0.2 wt% CE64. Shear-thinning behavior was observed in all YTZP slurries, and viscosity increased significantly at low shear rates as the YTZP powder content was increased to 50%. The particles have distance-dependent interactions that can be repulsive or attractive due to interparticle forces such as Van der Waals forces, electrostatic forces, and steric effects. When the solid content reached 50%, the sum of interparticle forces acting on the CE64-adsorbed YTZP particles formed a balanced network structure. The network structure controls dissipation at low shear rates. The strong network structure and high yield stress of the YTZP slurry do not meet the shear requirements for the gel-casting process and easily result in bridging flocculation. As a result, the appropriate YTZP slurry solid content with 0.2 wt% CE64 is 45 vol%.
Figure 3. (a) Effect of the solid content on the rheological behaviors of YTZP slurries with 0.2 wt% CE64; (b) Effect of adding different organic components on the viscosity of the YTZP slurry.
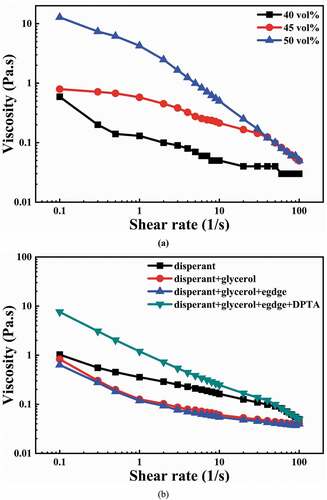
After making the dispersed YTZP slurry, epoxy monomer (EGDGE), plasticizer (glycerol), and polymerization initiator (DPTA) are added for gel casting. ) shows the effect of different organic components on the YTZP slurry viscosity. After adding glycerol and EGDGE, the viscosity remained unchanged at low shear rates and decreased significantly at high shear rates, indicating that the slurry was well-dispersed [Citation28]. However, when DPTA was added, the viscosity at a low shear rate increased nearly tenfold. This suggests that the well-dispersed slurry became gelled simultaneously upon the addition of DPTA. This prevents the slurry from being poured into the mold.
The reaction time between the monomer, EGDGE, and the initiator, DPTA, can be investigated using FTIR spectroscopy, as shown in (a). Figure S3 (the supplementary material) depicts the structures of EGDGE and DPTA and the polymerization reaction mechanism. When EGDGE reacts with DPTA, its glycidyl and ether groups are reduced, but a new functional hydroxyl (-OH) group is formed [Citation20]. There was no significant difference in the intensity of the peaks at 1250 cm−1 and 1120 cm−1 when compared to pure EGDGE, and the OH functional group did not appear after mixing EGDGE and DPTA for 0–20 minutes. As the mixing time was increased to 30 min, the glycidyl group peak weakened significantly, and the hydroxyl group appeared, indicating that polymerization had started. Based on the above results, the polymerization incubation time is greater than 20 minutes, which is sufficient for gel casting.
Figure 4. (a) FTIR spectroscopies of the products obtained by mixing EGDGE and DPTA for various reaction time (0 min to 30 min); (b) Effect of CE64 addition on the polymerization rate measured by FTIR spectroscopy; (c) Effect of HQ addition on the polymerization rate measured by FTIR spectroscopy.
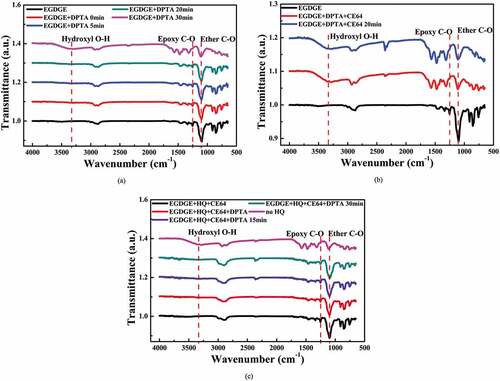
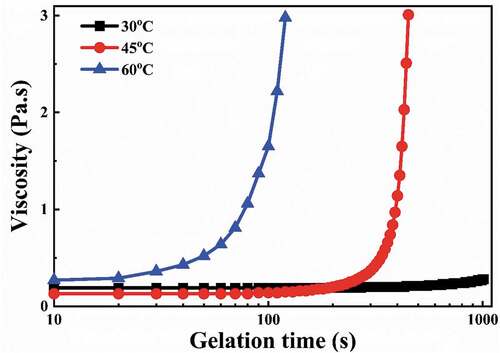
(b) shows the effect of CE64 addition on the polymerization rate as measured by FTIR spectroscopy. After mixing EGDGE, DPTA, and CE64, a prominent hydroxyl peak appeared, and the intensity of the glycidyl group peak decreased significantly. The FTIR spectrum of the sample mixed for 20 minutes is similar to that of the immediately mixed sample. It implies that the polymerization reaction was completed instantly after adding CE64. CE64, a dispersant, contains many carboxyl groups [Citation21]. The glycidyl group base catalysis reaction with the carboxyl groups occurs at a low temperature [Citation29], resulting in a slurry with limited stability at room temperature. (c) shows the effect of HQ addition on the polymerization rate. After 30 min from the addition of HQ to a mixture of EGDGE, DPTA, and CE64, there was no significant change in the intensity of the peaks at 1250 cm−1 and 1120 cm−1, and the OH functional group did not appear, indicating that polymerization had not occurred. This means that HQ can improve slurry stability and extend the gel casting working time to more than 30 minutes, which is beneficial for the subsequent molding process. HQ is an aromatic organic compound that is a type of phenol. Phenol derivatives can be oxidized to benzoquinone and used as free radical scavengers by reacting with polymeric radicals [Citation30,Citation31]. This reaction lowers the concentration of free radicals, and polymerization is delayed or even prevented. As a result, the polymerization time can be postponed and the idle time prolonged until the additional phenol is depleted [Citation32]. depicts the effects of various curing temperatures and gelation times on the viscosity (at the shear rate of 10s−1) of the slurry with 45 vol% solid content (the mixture of EGDGE, DPTA, HQ, and CE64). At 30°C, the slurry viscosity at the shear rate of 10s−1 remained nearly constant for an extended period, indicating that gelation did not occur. When the temperature was raised to 45°C and 60°C, there was a significant increase in viscosity after 200s and 20s, respectively, indicating that gelation occurred.
Figure 5. Effects of different curing temperatures and gelation times on the viscosity (at the shear rate of 10s−1) of the slurry with 45 vol% solid content (the mixture of EGDGE, DPTA, HQ, and 0.2 wt% CE64).
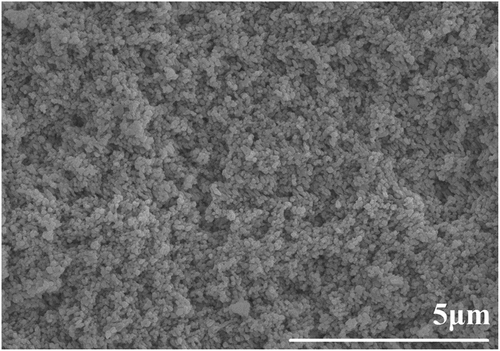
The relative green densities of the samples prepared by gel casting can reach about 60.7 ± 0.6%. After binder burnout, the relative green density can reach 58.4 ± 0.5%. shows the SEM microstructure of the green body prepared through gel casting. It can be seen that no large inter-agglomerate pores were observed, indicating the green body exhibited a homogeneous green microstructure. It reveals that the green body with a high density can be obtained by gel casting of a well-dispersed slurry.
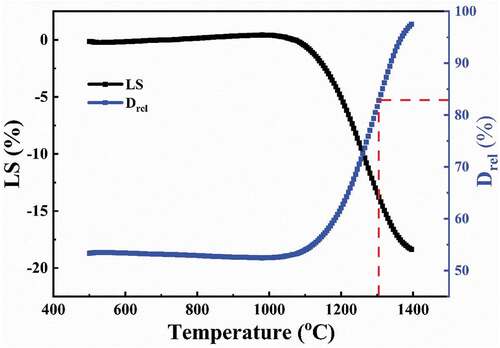
The two-stage sintering method was used to control grain growth. According to the previous reports [Citation6,Citation33,Citation34], in two-stage sintering, a critical relative density of about 83% needs to be reached in the first step. shows the variation in shrinkage and instantaneous relative density with temperature for the YTZP green body. The instantaneous relative density of sintered specimen was calculated from specimen height variation as follows:
Figure 7. Variation of the shrinkage and instantaneous relative density with the temperature for the YTZP green body.
Where represents the instantaneous relative density at time t,
is the initial green density, LS is the height at time t divided by the initial specimen height.
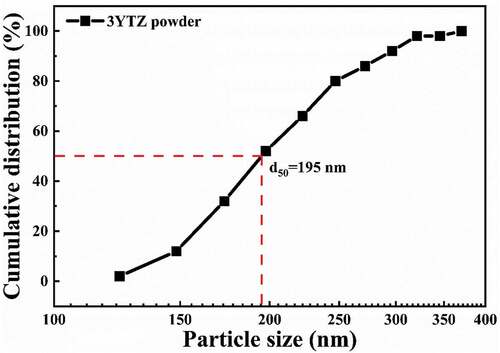
Based on the dilatometry results, the first stage sintering temperature was set at 1300°C, where the relative density reached 83%, indicating that YTZP has reached the final sintering stage. The sintering temperature in the first stage was 1300°C, the same as that of Y-TZP reported by Mazaheri et al. [Citation6]. In the second sintering stage, the temperature was lowered to 1240°C and soaked for 24 h to inhibit the grain growth and continue densification. shows the SEM microstructure of the polished surface of a two-stage sintered YTZP ceramic after thermal etching (1200°C for 30 min). The sample after two-stage sintering had a relative sintered density of 99.4 ± 0.2%, a mean grain size of 195 nm (), and almost no intergranular pores. This suggests that two-stage sintering can simultaneously inhibit grain growth and promote densification.
Figure 8. SEM microstructure of the polished surface following thermal etching (1200°C for 30 min) of a two-stage sintered (first stage sintering temperature: 1300°C, second stage sintering temperature: 1240°C for 24 hours) YTZP ceramic.
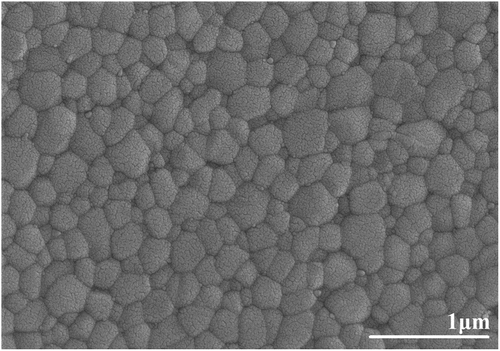
Figure 9. Cumulative grain size distribution of YTZP ceramics after two-stage sintering (first stage sintering temperature:1300°C, the second stage sintering: 1240°C for 24 h).
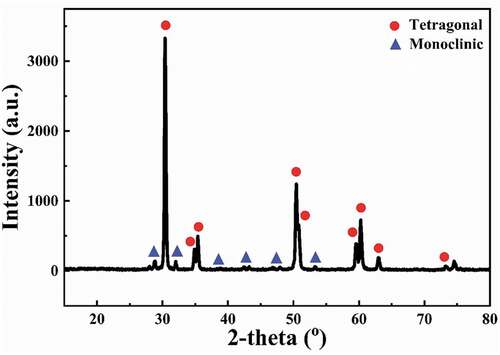
shows the XRD pattern of the YTZP ceramic after two-stage sintering. It indicates that many metastable tetragonal phases were retained at room temperature after sintering. After two-stage sintering, the sintered material contains 92.3 wt% tetragonal phase and 7.7 wt% monoclinic phase.
After two-stage sintering, the average flexural strength, Vickers hardness, and fracture toughness (KIC) of the YTZP ceramics can reach 771 ± 210 MPa, 15.2 ± 0.3GPa, and 7.80 ± 1.63 MPa.m1/2, respectively. These results are better than the mechanical properties of many conventional sintered bodies with YTZP (flexural strength: 600–700 MPa, Vickers hardness: 13–14 GPa, and the KIC values: 5.5–6.0 MPa m1/2) in the literature [Citation9,Citation34–37]. The Vickers hardness and flexural strength strongly depend on porosity, grain size and flaws [Citation38–40] for dense ceramics with high densities (> 99%). It has been reported that grain boundaries can serve as the main obstacles for dislocation motion during deformation, increasing the hardness with decreasing grain size due to increasing grain boundary density [Citation41]. Therefore, the YTZP ceramics prepared by the gel casting and two-stage sintering process exhibited superior hardness and fracture strength due to the high sintered density, small grain size, and homogeneous microstructure. The flexural strength of 771 MPa for the two-step sintering is slightly lower than that reported by Kastyl et al. [Citation19] for TZP ceramics (1000 MPa). This may be due to the presence of a small number of microstructural defects in our samples. In the future, countermeasures and minimization of microstructural defects will be further developed.
4. Conclusions
After adding 0.2 wt % CE64, a well-dispersed and high solid content (45 vol %) YTZP slurry can be obtained. The glycidyl group base catalysis reaction with CE64 carboxyl groups occurs at a low temperature, resulting in a slurry with limited room temperature stability. When HQ was added to the EGDGE, DPTA, and CE64 mixture, slurry stability improved, and the gel casting working time was increased to more than 30 minutes, which was beneficial for the subsequent molding process. YTZP ceramics with a relative sintered density of 99.4% and a mean grain size of 195 nm can be produced using two-stage sintering. After two-stage sintering, the average flexural strength, Vickers hardness, and fracture toughness (KIC) of the YTZP ceramics can reach 771 ± 210 MPa, 15.2 ± 0.3GPa, and 7.80 ± 1.63 MPa.m1/2, respectively. This shows that combining the gel casting technique with a two-stage sintering method might be an excellent way to make YTZP ceramics with outstanding mechanical characteristics.
Supplemental Material
Download MS Word (330.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/21870764.2022.2163962
Additional information
Funding
References
- Evans AG, Charles EA. Fracture toughness determinations by indentation. J Am Ceram Soc. 1976;59(7–8):371–372.
- Swain MV. Grain-size dependence of toughness and transformability of 2mol% Y-TZP ceramics. J Mater Sci Lett. 1986;5(11):1159–1162.
- Matsui K, Yoshida H, Ikuhara Y. Review: microstructure-development mechanism during sintering in polycrystalline zirconia. Inter Mater Rev. 2018;63(6):375–406.
- Chen IW, Wang XH. Sintering dense nanocrystalline ceramics without final-stage grain growth. Nature. 2000;404(6774):168.
- Wang XH, Chen PL, Chen IW. Two‐step sintering of ceramics with constant grain‐size, I. Y2O3. J Am Ceram Soc. 2006;89(2):431–437.
- Mazaheri M, Simchi A, Golestani-Fard F. Densification and grain growth of nanocrystalline 3Y-TZP during two-step sintering. J Europ Ceram Soc. 2008;28(15):2933–2939.
- Lourenc MA, Cunto GG, Figueiredo FM, et al. Model of two-step sintering conditions for yttria-substituted zirconia powders. Mater Chem Phys. 2011;126(1–2):262–271.
- Grech J, Antunes E. Optimization of two-step sintering conditions of zirconia blanks for dental restorations. Ceram Int. 2020;46(16):24792–24798.
- Presenda Á, Salvadora MD, Peñaranda-Foixb FL, et al. Effect of microwave sintering on microstructure and mechanical properties in Y-TZP materials used for dental applications. Ceram Int. 2015;41(5):7125–7132.
- Ramesh S, Zulkifli N, Tan CY, et al. Comparison between microwave and conventional sintering on the properties and microstructural evolution of tetragonal zirconia. Ceram Int. 2018;44(8):8922–8927.
- Charmond S, Carry CP, Bouvard D. Densification and microstructure evolution of Y-Tetragonal Zirconia Polycrystal powder during direct and hybrid microwave sintering in a single-mode cavity. J Europ Ceram Soc. 2010;30(6):1211–1221.
- Nogueir J, Marina L, Kaizer R, et al. Novel speed sintered zirconia by microwave technology. Dental Mater. 2021;37(5):875–881.
- Drazin JW, Wollmershauser JA, Ryou H, et al. Pressureless low temperature sintering of nanocrystalline zirconia ceramics via dry powder processing. J Am Ceram Soc. 2020;103(1):60–69.
- Young AC, Omatete OO, Janney MA, et al. Gelcasting of alumina. J Am Ceram Soc. 1991;74(3):612–618.
- Tulliani JM, Bartuli C, Bemporad E, et al. Preparation and mechanical characterization of dense and porous zirconia produced by gel casting with gelatin as a gelling agent. Ceram Int. 2009;35:2481–2491.
- Zhao H, Ye C, Fan Z. A simple and effective method for gel casting of zirconia green bodies using phenolic resin as a binder. J Europ Ceram Soc. 2014;34(5):1457–1463.
- Lee KY, Chen CC, Hsiang HI, et al. Fully sintered alumina with a higher Vickers hardness prepared using a gel-casting process. Int J Appl Ceram Technol. 2019;16(4):1493–1500.
- Liu XG, Li GJ, Tong JF, et al. Low-cost fabrication for ZrO2-based electrolyte thin-substrate by aqueous gel-casting. J Rare Earths. 2004;22:514–516.
- Kastyl J, Stastny P, Chlup Z, et al. Gelcast zirconia ceramics for dental applications combining high strength and high translucency. J Am Ceram Soc. 2022;105(6):3909–3924.
- Xie R, Zhou K, Gan X, et al. Effects of epoxy resin on gelcasting process and mechanical properties of alumina ceramics. J Am Ceram Soc. 2013;96:1107–1112.
- Rao SP, Tripathy SS, Raichur AM. Dispersion studies of sub-micron zirconia using Dolapix CE 64. Colloids Surf A Physicochem Eng Asp. 2007;302(1–3):553–558.
- Toraya H, Yoshimura M, Somiya S. Calibration curve for quantitative analysis of the monoclinic tetragonal ZrO2 system by X-ray diffraction. J Am Ceram Soc. 1984;67:C119–121.
- Anstis GR, Chantikul P, Lawn BR, et al. A critical evaluation of indentation techniques for measuring fracture toughness: i, direct crack measurements. Journal of the American Ceramic Society. 1981;64(9):533–538.
- Liu DM. Rheology of aqueous suspensions containing highly concentrated nano-sized zirconia powders. J Mater Sci Lett. 1998;17(22):1883–1885.
- Xie Z, Ma J, Xu Q, et al. Effects of dispersants and soluble counter-ions on aqueous dispersibility of nano-sized zirconia powder. Ceram Int. 2004;30(2):219–224.
- Tang F, Huang X, Zhang Y, et al. Effect of dispersants on surface chemical properties of nano-zirconia suspensions. Ceram Int. 2000;26(1):93–97.
- Sarraf H, Sabet A, Herbig R, et al. Advanced colloidal techniques for characterization of the effect of electrosteric dispersant on the colloidal stability of nanocrystalline ZrO2 suspension. J Ceram Soc Jap. 2009;117(1363):302–307
- Lee KY, Chen CC, Hsiang HI, et al. Effects of glycerol addition on the slurry dispersion and mechanical properties of alumina ceramics prepared by gel-casting process. Ceram Int. 2021;47(14):20260–20267.
- Blank WJ, He ZA, Picci M. Catalysis of the epoxy-carboxyl reaction. J Coat Technol. 2002;74(3):33–41.
- Kadoma Y, Fujisawa S. Kinetic evaluation of reactivity of bisphenol A derivatives as radical scavengers for methacrylate polymerization. Biomaterials. 2000;21(21):2125–2130.
- Fujisawa S, Kadoma Y. Action of eugenol as a retarder against polymerization of methyl methacrylate by benzoyl peroxide. Biomaterials. 1997;18(9):701–703.
- Batch GL, Macosk CW. Oxygen inhibition in differential scanning calorimetry of free radical polymerization. Thermochim Acta. 1990;166:185–198.
- Lóh NJ, Simão L, Jiusti J, et al. Effect of temperature and holding time on the densification of alumina obtained by two-step sintering. Ceram Int. 2017;43(11):8269–8275.
- Yang H, Li L, Li Y, et al. Unveiling exceptional sinterability of ultrafine α-Al2O3 nanopowders. J Materiomics. 2021;7:837–844.
- Soylemez B, Sener E, Yurdakul A, et al. Fracture toughness enhancement of yttria-stabilized tetragonal zirconia polycrystalline ceramics through magnesia-partially stabilized zirconia addition. J Sci Adv Mater Devices. 2020;5:527–534.
- Gupta TK, Lange FF, Bechtold JH. Effect of stress induced phase transformation on the properties of polycrystalline zirconia containing metastable tetragonal phase. J Mat Sci. 1978;13(7):1464–1470.
- Chevalier J, Gremillard L. Ceramics for medical applications: a picture for the next 20 years. J Eur Ceram Soc. 2009;29(7):1245–1255.
- Zhang X, Liang S, Li H, et al. Mechanical and optical properties of transparent alumina obtained by rapid vacuum sintering. Ceram Int. 2017;43(1):420–426.
- Krell A, Blank P. Grain size dependence of hardness in dense submicrometer alumina. J Am Ceram Soc. 1995;78(4):1118–1120.
- Pande CS, Cooper KP. Nanomechanics of Hall–Petch relationship in nanocrystalline materials. Prog Mat Sci. 2009;54(6):689–706.
- Rice RW, Wu CC, Borchel F. Hardness-grain-size relations in ceramics. J Am Ceram Soc. 1994;77(10):3539–3553.