?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
MgO was added to the SiO2-Al2O3-CaO-ZrO2-based glaze to produce a high-hardness glaze by forming a composite crystal phase. The effects of MgO addition on the surface properties of the glaze were analyzed through crystal phase and microstructural investigations, and the whiteness, roughness, gloss, wear rate, and hardness were measured. When the amount of added MgO increased by 2.20 wt% or more, the diopside (MgCaSi2O6) crystal phase was observed via X-ray diffraction (XRD) and scanning electron microscopy (SEM), in addition to the zircon (ZrSiO4) crystal phase. All samples exhibited high whiteness and the surface roughness increased with increasing MgO content. At the same time, the gloss decreased significantly, resulting in a matte. The Vickers hardness test results of the glazed surface indicated high hardness values of 6.23–7.07 GPa with increased amounts of MgO.
1. Introduction
A glaze is a glassy coating formed on the surface of ceramic products. The glaze exhibits excellent surface hardness and high chemical and abrasion resistance, and it improves the mechanical properties of ceramic products [Citation1]. Based on the temperature used for sintering the ceramic, glaze is categorized as either high-fire glaze (sintering temperature above 1250°C) or low-fire glaze (sintering temperature below about 1050°C). Glazes are further divided into transparent or opaque and glossy or matte, based on the method of application [Citation2]. Among these, various colors of opaque glaze can be realized by mixing with various coloring oxides. The opaque glaze protects the ceramic body from external contamination and performs various aesthetic functions. Further, it reduces the surface defect and enhances the mechanical properties of the product [Citation3,Citation4].
The opaque glaze exhibits opacity due to diffuse reflection caused by the difference in the refractive index between the crystal phase and glass matrix, where zircon (ZrSiO4) is used as a representative raw material [Citation2,Citation5]. The zircon in the glaze is melted in a glass matrix forming a white opaque crystal phase on the surface, and the crystal phase formed exhibits a high refractive index (1.94), which makes the glaze exhibit high whiteness [Citation5–9]. In addition, it enhances the mechanical properties and chemical resistance of the glazes, but it has the disadvantage of being expensive and emitting trace amounts of radioactive material [Citation10–13].
Currently, studies on glass-ceramic glazes containing anorthite (CaAl2Si2O8) and diopside (MgCaSi2O6) crystal phases with high refractive indices are being actively conducted to replace the zircon component showing opacity property [Citation3,Citation7,Citation14]. The anorthite crystal phase can be opacified in its crystal form, and the CaO component reduces the viscosity of the residual glass, enabling the production of glazes with excellent whiteness [Citation3]. The diopside crystal phase showing a relatively high refractive index (1.67) can be opacified, and with the MgO component acting as a glass network modifier, it is possible to manufacture glossy/matte glazes based on the degree of crystallinity of the glaze [Citation15–17]. In addition, MgO has a relatively high electric field strength; therefore, it is possible to improve the density of the glaze through densification, and it has the advantage of improving the hardness and fracture toughness of the glaze [Citation17,Citation18].
In addition to glass-ceramic glazes with a high refractive index, active research on improving the physical properties of glazes by forming composite crystal phases is being conducted. Cai et al. observed changes in the properties of enstatite crystal glazes with excellent mechanical properties, by forming an additional spinel crystal phase with excellent optical properties through the addition of ZnO [Citation7]. Pei et al. observed improvements in the density and mechanical properties of wollastonite glazes by forming a diopside crystal phase [Citation19]. However, in the case of opaque glazes containing zircon crystal phases used in various fields, there is no research on improving the physical properties of glazes through the formation of complex crystal phases.
Therefore, in this study, the addition of MgO to the existing SiO2-Al2O3-CaO-ZrO2-based opaque sanitary ware glaze, formation of composite crystal phases and microstructure changes of the glaze based on to the added amount was observed, and the changes in the hardness and surface properties of the glaze according to the change in crystallinity, crystalline phase fraction, and microstructure were observed.
2. Experimental procedure
This study employed the glaze used by the Korean sanitary ware company “A”, MgO (Kojundo chemicals, Ltd., Japan) was used to make a composite crystal phase. The mixing ratios for the addition of MgO powder are shown in . Dry milling was performed for 24 h using a ball mill with zirconia balls (diameter = 5 mm, 20 mm). To measure the density and crystallinity of the glaze, a square (20 × 20 mm2) sample was molded using a hydraulic press (3851–0, Carver, USA) under the condition of 6 ton/90s. To analyze the sample excluding crystallinity and density, a slurry was prepared (glaze powder: D.I water, 10:7), after which a glaze with a thickness of 800μm was coated on an alumina substrate using a doctor blade. Each of the prepared samples was sintered in a SiC box electric furnace (heating rate = 0.32°C/min, maximum temperature = 1230°C). The temperature and holding time were applied the same as the heat treatment conditions for the existing sanitary ware manufacturing.
Table 1. Batch composition of the experimental glaze.
The chemical composition of the glaze was analyzed using X-ray fluorescence XRF (ZSX Primus, Rigaku Co., Japan). An HP-powder XRD (D8 ADVANCE, Bruker Co., Germany) was used to analyze the crystal phase and crystallinity of the glazes with respect to the changes in the mixing ratio, and the analyzed crystal phase was subjected to quantitative phase analysis using Rietveld method. After etching the glaze in a 3 wt% solution of HF for 3 s, the microstructure of the glaze surface was observed using scanning electron microscopy (SEM, Nova Nano SEM450, FEI Company, USA), and the quantitative component analysis of the glaze surface was performed using an energy-dispersive X-ray spectroscopy (EDS, Noran System 7, Thermo Scientific, USA). The surface roughness of the glaze was analyzed using a surface step measurement instrument (Dektak XT Stylus Profiler, BRUKER, USA). The whiteness and gloss of the glaze surface were analyzed using a spectral colorimeter (NR100 Precision Colorimeter, 3NH Technology Co., China) and gloss meter (NOVO-gloss, RHOPOINT, UK), to which the KS L 5113 and ASTM C584–81 standards were applied, respectively. The density of the glazes was measured using Archimedes principle (KS L ISO 18754), and the hardness of glazed surface was measured with a test force of 4.9 N/10 s using a micro-Vickers hardness tester (VH1102, Wilson, USA) by applying the ASTM E384–17 standard. The wear resistance of the glaze surface was measured by ball-on-disk method using a wear tester (TRB3, Anton Paar, Switzerland), according to ASTM G99. The test was conducted using a silicon nitride ball (diameter = 6 mm), at a sliding distance of 25.13 m and the experiment was performed at 200 rpm for 1000 cycles under a load of 6 N. The wear rate and wear volume have the following equations [Citation20,Citation21].
where WR the wear rate (mm3/Nm), WV the wear volume after the test (mm3), FN the applied load (N) and S the sliding distance (m). The thermal expansion coefficient of the glaze was measured using a thermomechanical analyzer (TMA 402 F3, Netzsch, Germany) at a heating rate of 10°C/min in the range of RT to 900°C.
3. Results and discussion
lists the chemical compositions of commercial glazes. Sanitary ware glazes are manufactured by mixing various raw mineral materials. In this sanitary ware glaze, SiO2 (58.48 wt. %), Al2O3 (9.74 wt.%), CaO (8.67 wt.%), and ZrO2 (5.10 wt.%) were observed as the main components, ZnO (2.19 wt.%) and alkali/alkaline earth oxides such as K2O (2.78 wt.%), Na2O (1.87 wt.%), and MgO (1.12 wt.%) were observed in insignificant quantities. In the case of ZrO2, it is to be due to the zircon (ZrSiO4) component included to obtain the chemical and abrasion resistance of the glaze and high whiteness [Citation5,Citation22].
Table 2. Chemical composition of the original glaze (wt%).
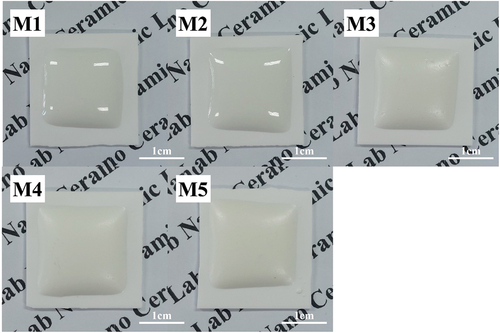
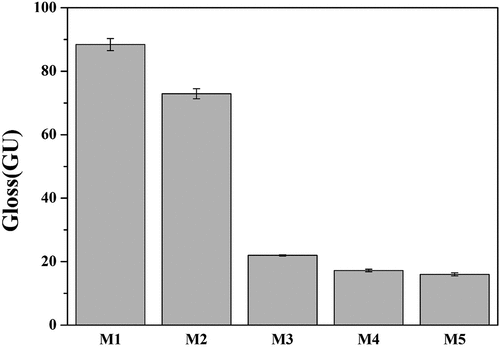
shows the optical photograph of the glaze sample fired at 1230°C after the quantity of MgO was changed. All samples showed a milky white color, but the gloss of the glaze surface decreased with the increase in MgO, resulting in a matte surface from M3.
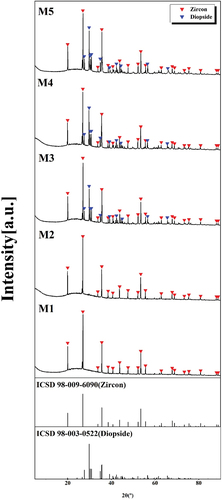
shows the XRD analysis of the crystalline phase of the glaze samples sintered at 1230°C after the quantity of MgO was changed. Zircon (ZrSiO4, ICDS 98-009-6090) crystalline phases were observed in all samples, diopside (MgCaSi2O6, ICDS 98-003-0522) crystal phase was formed in addition to the zircon crystal phase when M3, M4 and M5 composition with MgO addition of 2.20 wt% or more were added. As the MgO content increased, the viscosity of the glass gradually decreased, and the particle migration rate increased, with the enrichment of Ca2+ and Mg2+ around [AlO4] and [SiO4], and the formation of crystals. These factors are believed to contribute to the formation of diopside [Citation17].
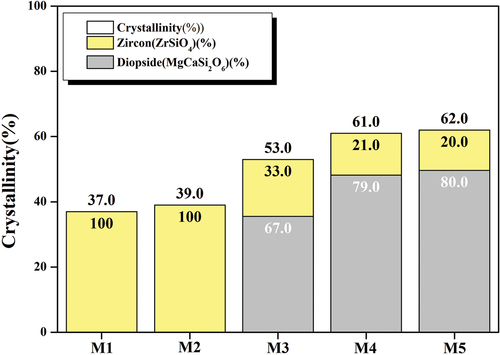
shows the crystallinity and crystal-phase fraction of the glaze samples shown in . The crystal-phase fraction was analyzed through phase identification and Rietveld refinement using X’Pert High Score Plus software [Citation23,Citation24]. The M1 samples without added MgO showed a crystallinity of 37%, and the M2 with 0.81 wt% of MgO added had a slight increase in crystallinity to 39%. However, the M3 with 2.20 wt% of MgO showed a sharp increase in crystallinity (14%), and as the amount of MgO increased, the crystallinity continuously increased, resulting in 62% crystallinity in M5. In addition to the increase in crystallinity, changes in the crystal-phase fraction were observed. In the M5, the crystal-phase fraction of the diopside phase increased to 80%, and the crystal-phase fraction of the zircon phase decreased to 20%. This crystal-phase peak intensity change is consistent with the tendency demonstrated in showing XRD. The decline in the fraction of the zircon crystal phase with an increase in MgO content is thought to decrease the crystal-phase formation ability, which is attributed to the decrease in relative concentration of Zr4+ ions caused by the increasing MgO. Conversely, the increase in the diopside crystal-phase fraction was attributed to the enhanced diopside crystal-phase formation ability owing to the increase in the migration speed of the Mg2+ particles [Citation17,Citation25]. In general, MgO serves as a modifier or intermediate agent in silicate glass systems, and as the amount of MgO added increases, the Tg and Tp decrease, allowing the crystal phase to appear at low temperatures [Citation17,Citation19]. Therefore, it is judged that as the content of MgO increases at the same temperature, the crystallinity increases, and the fraction of the diopside crystal phase related to MgO increases.
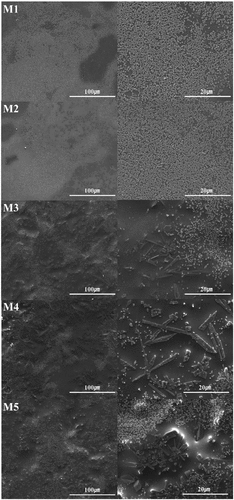
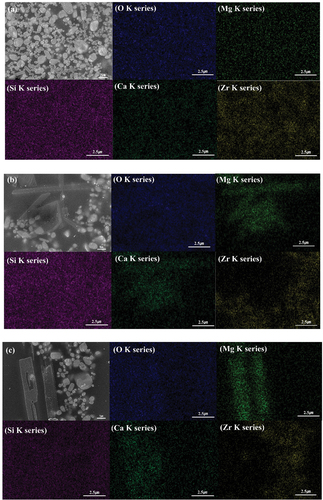
shows the microstructure of the glaze obtained using SEM. In M1 and M2, several micrometer-sized granular particles that partially cohered were observed in the glass matrix. In M3, new needlelike particles were observed in addition to the granular crystal phase. In the case of the M5 glaze with the most MgO added, it was confirmed that the needlelike crystal phase grew in the form of rods and columns. In general, the diopside crystal phase shows a columnar or needle shape, but it may appear in a granular or needle shape depending on the main component contained in the crystal phase [Citation15,Citation26,Citation27].
shows the results of EDS data for the particles observed at M1, M3, and M5 in . The granular particles were identified as zircon particles composed of Zr and Si, whereas the needlelike particles were identified as diopside particles composed of Mg, Ca, and Si, which is consistent with the data in . It is known that MgO promotes the formation of needlelike crystals, whereas CaO promotes the formation of spherical crystals. J. Ma. et al. reported that when the amount of MgO added to CaO-MgO-SiO2-P2O5-based glass increased, needle-shaped crystals were formed instead of spherical crystals [Citation15,Citation28]. In this study, it was determined that increasing the amount of added promoted the formation of the needle-shaped diopside crystal phase.
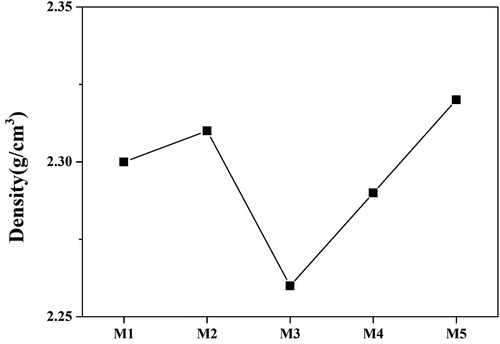
shows the density of the glaze sample sintered at 1230°C after the quantity of MgO was changed using Archimedes principle. M1 and M2 gradually increased to 2.30 g/cm3 and 2.31 g/cm3, respectively. However, the density value decreased to 2.26 g/cm3 in M3, where the zircon and diopside complex crystal phases began to appear. As the amount of MgO increased, the density value gradually increased, showing a density of 2.32 g/cm3 in the M5. M.M.S Wahsh et al. reported that as the amount of dolomite added to the zircon-based glaze increased, the zircon crystal phase decreased, and other crystal phases appeared, resulting in a decrease in the bulk density [Citation29]. K. Das. et al. reported that the type of crystalline phase present in the glass matrix and the chemical composition affected the density of the glaze [Citation30]. In general, the density of zircon (4.65–4.70 g/cm3) is higher than those of diopside (3.38–3.40 g/cm3) [Citation6,Citation29]. Therefore, it was determined that the density decreased and then increased in M3, where the high-density crystal phase (Zircon) decreased, and the low-density crystal phase (Diopside) was expressed in the glass.
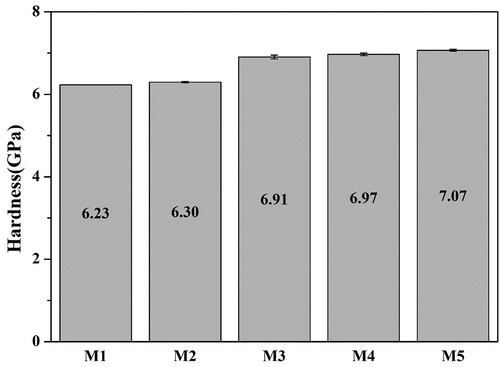
shows the micro-Vickers hardness results of the glaze samples with various amounts of MgO, sintered at 1230°C. M1 showed a hardness value of 6.23 GPa, where an increase in the amount of MgO resulted in an increase in the hardness. M5 showed the highest hardness value of 7.07 GPa. In particular, it was observed that in M3, where crystallinity rapidly increased and a complex crystal phase with a diopside crystal phase in addition to the zircon crystal phase formed, the hardness of the glaze surface increased rapidly. The hardness of the glaze with complex crystal phases was superior to that of commercial zircon-containing glazes of 5.80 to 6.44 GPa [Citation22,Citation25]. F. Pei. et al. reported an increased diopside crystal phase resulting in increased hardness of the glaze surface when MgO was added to the CaO-Al2O3-SiO2-based glaze [Citation19]. S. Banijanali reported that crystallinity increasing, and residual glass matrix affects the enhancing hardness of glaze [Citation31]. Zhu. et al. reported that as TiO2 of nucleating agent addition increased, the crystalline-phase fraction and hardness enhanced [Citation32]. It is determined that the crystallinity of the glaze increases with the increase in the amount of MgO added and the formation of high-hardness zircon (Mohs scale: 7) and diopside (Mohs scale: 6.5) crystal phases contribute to the enhancement of the hardness of glaze [Citation16,Citation19,Citation25,Citation31].
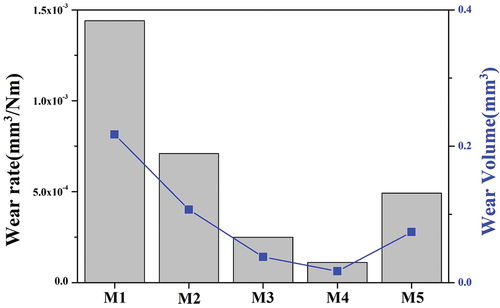
shows the wear rate and wear volume result of the glaze sample sintered at 1230°C after the quantity of MgO was changed. The M1 without MgO had the largest wear volume of 0.217 mm3 and the highest wear rate of 1.44 × 10−3 mm3/Nm. As the amount of MgO increased, the wear rate decreased to 0.11 × 10−3 mm3/Nm in M4 but increased to 0.49 × 10−3 mm3/Nm in M5 with the highest amount of MgO. In general, the wear resistance of a glaze is proportional to its hardness; however, it is affected by many factors, including surface roughness. F. Svahn. et al. reported that the wear rate and friction characteristics are affected by the surface roughness [Citation33]. M. Gajek. et al. reported different hardness values at the same wear rate owing to the differences in surface roughness, porosity, and residual glass composition [Citation16]. K.S. Kanaga Karuppiah et al. confirmed that an increase in crystallinity increases scratch resistance and abrasion resistance [Citation34], and Zhang et al. reported that the wear rate and roughness are related to each other, and at the same time, increased roughness worsens the wear of ceramics [Citation35]. As shown in , the decrease in the wear rate of the glaze surface with increasing MgO content is attributed to the increase in the hardness of the glaze, and the increase in the wear rate in the M5 with the highest hardness value is attributed to the surface roughness.
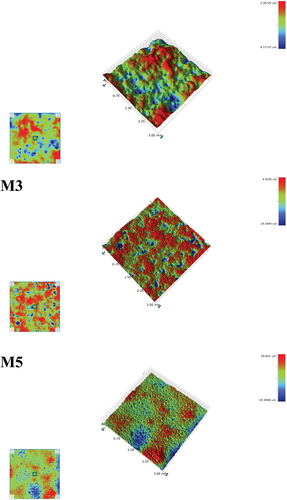
and show the 3D images of the glaze surface and surface roughness (Ra, Rt, Rq) results of the glaze sample sintered at 1230°C after the amount of MgO was changed. The average surface roughness (Ra) of the glaze is in the 0.666–1.893 µm range and the maximum roughness (Rt) is in the 7.081–35.306 µm range, and the roughness of the glaze surface increased as the amount of MgO increased. General ZrO2 and TiO2 based opaque glazes show surface roughness (Ra) of 0.040 µm and 0.016 µm, respectively, and CaO- and ZnO-based matte glazes show a relatively high surface roughness of 0.277 µm [Citation36]. In this study, it was confirmed that the developed glaze exhibited a higher surface roughness value than that of a commercial opaque glaze and a surface roughness similar to that of a commercial matte glaze. The surface roughness of the glaze depends on the shape of the crystal phases, and it is known that the needle-shaped crystal phase increases the surface roughness of the glaze compared to the plate-shaped crystal phase [Citation36]. In this study, as shown in , it is determined that the surface roughness of the glaze increased due to the additional formation of needle-shaped diopside crystal phase by the addition of MgO and the increase of the crystal-phase fraction of diopside. In addition, it is believed that the addition of MgO reduced the Tg and Tp values and promoted the crystal formation, thereby increasing in the surface roughness of the glaze [Citation17]. It is determined that in surface roughness of the glaze increases with the increase in the amount of MgO affected the change in wear resistance, as in .
Table 3. Surface roughness of the glaze samples heat treated at 1230°C.
shows the results of analyzing the gloss of the glaze sintered at 1230°C after the amount of MgO was changed using “ASTM C584–81”. In the case of gloss, if the 60° measured value is 70 GU or more, it is classified as high gloss, and if it is 10 GU or less, it is classified as a matte glaze [Citation37]. The M1 without MgO had a high gloss of 88 GU. The increase in the amount of MgO resulted in a rapid decrease in the gloss, and M3 changed the matte glaze to 22 GU. This is the same as the image observation results for the glazed surface shown in . In the case of glazing, as the roughness of the surface increases, the light scatters, and the gloss decreases. As shown in , as the amount of MgO increased, the surface roughness increased and the gloss of the glaze decreased [Citation3,Citation37].
shows the results of the whiteness of glaze sintered at 1230°C after the amounts of MgO was changed using the CIE Lab parameters and “KS L 5113”. In general, the lightness value (L*) indicates a proportional relationship with whiteness, while the yellowness index (b*) indicates an inversely proportional relationship with whiteness [Citation7,Citation37]. All samples showed high lightness values (L*) of approximately 93–94. The yellowness index (b*) increased from 2.47 to 3.32 as the amount of MgO increased, and the whiteness tended to decrease starting from the M3. Yanmei et al. improved the bleaching effect by reducing the extinction coefficient when a small amount of MgO was added; however, it was reported that the extinction coefficient increased as the amount of MgO increased, which affected the bleaching effect [Citation38]. In addition, it is judged that the added MgO interferes with the formation of the zircon crystal phase and forms diopside with a relatively low refractive index (Zircon: 1.94, Diopside: 1.67), resulting in a decrease in whiteness. This result is consistent with those shown in [Citation6,Citation38,Citation39]. The whiteness value () of all glazed samples was 93 ± 1, which were similar to those of commercial glazes (including 7.5–12.5 wt% of ZrO2) [Citation3,Citation10,Citation40].
Table 4. CIE Lab of glaze sample heat treated at 1230°C.
shows the results of the thermal expansion coefficient of glaze sintered at 1230°C after the amounts of MgO was changed using TMA. The thermal expansion coefficient (α) of the glaze ranged from 5.52 to 6.56 × 10−6/K, and M5 showed the highest value of 6.56 × 10−6/K. This is judged to be influenced by the diopside crystal phase (50 ~ 150×10−7) with a high thermal expansion coefficient [Citation41]. The thermal expansion coefficient of the general sanitary ware glaze was 5.8 ~ 6.95 ×10−6/K, similar to that of the glaze in this study [Citation1,Citation42].
Table 5. Thermal expansion coefficient of glaze sample heat treated at 1230°C.
4. Conclusion
In this study, the surface and mechanical properties of the glaze were observed with respect to the formation of the composite crystal phase to add MgO to the SiO2-Al2O3-CaO-ZrO2-based glaze. Through XRD and SEM analyses, a granular zircon crystal phase affecting the high hardness and opalescent color was observed in the glaze. Further, the crystallinity of the glaze tended to increase as the diopside crystal phase was additionally expressed when the amount of added MgO increased. With the formation of a composite crystal phase and increasing roughness, it is possible to manufacture a glaze that has a hardness value of 7.07 GPa and wear rate of 0.11 × 10−3 mm3/Nm. Although the gloss of the glaze decreased by more than 60 GU, due to the increase in roughness as the formation of a needle-shaped diopside crystal phase. The whiteness of the glaze was 93 ± 1, showing characteristics similar to the existing commercial opaque glaze. Adding MgO to a SiO2-Al2O3-CaO-ZrO2-based glaze can lead to the formation of a composite crystal phase of zircon and diopside, resulting in an opacified glaze with better mechanical properties.
Acknowledgments
This work was supported by the Technology Innovation Program (20010483, Key technology development of porcelain ceramic with high energy efficiency) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Boudeghdegh K, Diella V, Bernasconi A, et al. Composition effects on the whiteness and physical-mechanical properties of traditional sanitary-ware glaze. J Eur Ceram Soc. 2015;35(13):3735–3741. DOI:10.1016/j.jeurceramsoc.2015.05.003
- Rho Y, Kang S, Kim J, et al. Study on crystallization of the glaze for tiles by substitution of TiO 2 , Al 2 O 3 and ZrO 2 nucleating agents. J Nanosci Nanotechnol. 2020;20(1):557–563. DOI:10.1166/jnn.2020.17292
- Gajek M, Partyka J, Rapacz-Kmita A, et al. Development of anorthite based white porcelain glaze without ZrSiO4 content. Ceram Int. 2017;43(2):1703–1709. DOI:10.1016/j.ceramint.2016.08.140
- Gol F, Saritas ZG, Cıbuk S, et al. Coloring effect of iron oxide content on ceramic glazes and their comparison with the similar waste containing materials. Ceram Int. 2022;48(2):2241–2249. DOI:10.1016/j.ceramint.2021.10.001
- Yu Y, Su H, Peng C, et al. Submicro-zirconia crystal-intergrown zircon opaque glaze. J Eur Ceram Soc. 2019;39(2–3):652–659. DOI:10.1016/j.jeurceramsoc.2018.09.044
- Casasola R, Rincón JM, Romero M. Glass-ceramic glazes for ceramic tiles: a review. J Mater Sci. 2012;47(2):553–582. DOI:10.1007/s10853-011-5981-y.
- Cai J, Lu M, Guan K, et al. Effect of ZnO/MgO ratio on the crystallization and optical properties of spinel opaque glazes. J Am Ceram Soc. 2018;101(4):1754–1764. DOI:10.1111/jace.15321
- Melchiades FG, Rego BT, Higa SM, et al. Factors affecting glaze transparency of ceramic tiles manufactured by the single firing technique. J Eur Ceram Soc. 2010;30(12):2443–2449. DOI:10.1016/j.jeurceramsoc.2010.04.030
- Gajek M, Partyka J, Leśniak M, et al. Gahnite white colour glazes in ZnO–R2O–RO–Al2O3–SiO2 system. Ceram Int. 2018;44(13):15845–15850. DOI:10.1016/j.ceramint.2018.05.265
- Wang S, Peng C, Huang Z, et al. Clustering of zircon in raw glaze and its influence on optical properties of opaque glaze. J Eur Ceram Soc. 2014;34(2):541–547. DOI:10.1016/j.jeurceramsoc.2013.08.018
- Selby JH, Strydom R. The effect of manufacturing variables on radiation doses from porcelain tiles. Health Phys. 2008;94(6):539–547. DOI:10.1097/01.HP.0000308503.41839.34.
- Muzakky HP, Taftazani A, Taftazani A, et al. Reduction of UO2 and ThO2 content in zircon mineral from Bangka Region using calcium borate and hydrochloric acid. J Indian Chem Soc. 2022;99(3):100371. DOI:10.1016/j.jics.2022.100371
- Abo-Elmagd M. Radon exhalation rates corrected for leakage and back diffusion – Evaluation of radon chambers and radon sources with application to ceramic tile. J Radiat Res Appl Sci. 2014;7(4):390–398. DOI:10.1016/j.jrras.2014.07.001.
- Tarhan M. Whiteness improvement of porcelain tiles incorporated with anorthite and diopside phases. J Therm Anal Calorim. 2019;138(1):929–936. DOI:10.1007/s10973-019-08268-8.
- Suvaci E, Yildiz B. Roles of CaO, MgO and SiO2 on crystallization and microstructure development in diopside-based glass-ceramic glazes under industrial fast-firing condition. J Aust Ceram Soc. 2019;53(1):75–81. DOI:10.1007/s41779-016-0011-9.
- Gajek M, Rapacz-Kmita A, Stodolak-Zych E, et al. Microstructure and mechanical properties of diopside and anorthite glazes with high abrasion resistance. Ceram Int. 2022;48(5):6792–6798. DOI:10.1016/j.ceramint.2021.11.230
- Li B, Guo Y, Fang J. Effect of MgO addition on crystallization, microstructure and properties of glass-ceramics prepared from solid wastes. J Alloys Compd. 2021;881(10):159821. DOI:10.1016/j.jallcom.2021.159821.
- Eppler RA, Obstler M. Understanding Glazes. USA: Wiley-American ceramic society; 2005.
- Pei F, Guo H, Li P, et al. Influence of low magnesia content on the CaO-Al2O3-SiO2 glass-ceramics: its crystallization behaviour, microstructure and physical properties. Ceram Int. 2018;44(16):20132–20139. DOI:10.1016/j.ceramint.2018.07.306
- Pina R, Esteban A, Bartolome JF, et al. High wear resistance white ceramic glaze containing needle like zircon single crystals by the addition of sepiolite n-ZrO2. J Eur Ceram Soc. 2013;33(15–16):3379–3385. DOI:10.1016/j.jeurceramsoc.2013.05.033
- Fuertes V, Cabrera MJ, Seores J, et al. Enhanced wear resistance of engineered glass-ceramic by nanostructured self-lubrication. Mater Des. 2019;168(15):106723. DOI:10.1016/j.matdes.2019.107623
- Topateş G, Alici B, Tarhan B, et al. The effect of zircon particle size on the surface properties of sanitaryware glaze. Mater Res Express. 2020;7(1):015203. DOI:10.1088/2053-1591/ab657d
- Bajpai S, Gupta A, Pradhan SK, et al. Crack propagation resistance of α-Al2O3 reinforced pulsed laser-deposited hydroxyapatite coating on 316 stainless steel. JOM. 2014;66(10):2095–2107. DOI:10.1007/s11837-014-1152-3
- Low ZH, Chen SK, Ismail I, et al. Structural transformations of mechanically induced top-down approach BaFe12O19 nanoparticles synthesized from high crystallinity bulk materials. J Magn Magn Mater. 2017;429(1):192–202. DOI:10.1016/j.jmmm.2017.01.036
- Yekta BE, Alizadeh P, Rezazadeh L. Floor tile glass-ceramic glaze for improvement of glaze surface properties. J Eur Ceram Soc. 2006;26(16):3809–3812. DOI:10.1016/j.jeurceramsoc.2005.12.016.
- Dongfeng H, Chong G, Jiangtao P, et al. Preparation of glass-ceramics with diopside as the main crystalline phase from low and medium titanium-bearing blast furnace slag. Ceram Int. 2018;44(2):1384–1393. DOI:10.1016/j.ceramint.2017.09.019
- Ashouri Rad B, Alizadeh P. Pressureless sintering and mechanical properties of SiO2-Al2O3-MgO-K2O-TiO2-F(CaO-Na2O) machinable glass-ceramics. Ceram Int. 2009;35(7):2775–2780. DOI:10.1016/j.ceramint.2009.03.027.
- Ma J, Chen CZ, Wang DG, et al. Effect of MgO addition on the crystallization and in vitro bioactivity of glass ceramics in the CaO-MgO-SiO2-P2O5 system. Ceram Int. 2012;38(8):6677–6684. DOI:10.1016/j.ceramint.2012.05.056
- Wahsh MMS, Bakare SB, Bakr IM, et al. Physico-mechanical properties and microstructure of multi-phase ceramic composites based on zircon and dolomite mixtures. Main Gr Chem. 2020;19(4):305–314. DOI:10.3233/MGC-200966
- Das K, Raha S, Chakraborty D, et al. Effect of nucleating agents on the crystallization and microstructural characteristics of blast furnace slag derived glass-ceramics. Trans Indian Ceram Soc. 2012;71(3):137–142. DOI:10.1080/0371750X.2012.738482
- Banijamali S. Preparation of glass-ceramic glazes for fast firing applications by CaF2 substitution with B2O3 in the CaO-CaF2-Al2O3-SiO2 system. Ceram Int. 2013;39(8):8815–8822. DOI:10.1016/j.ceramint.2013.04.069.
- Weiwei Z, Haifeng J, He Z, et al. Effect of TiO2 and CaF2 on the crystallization behavior of Y2O3-Al2O3-SiO2 glass ceramics. Ceram Int. 2018;44(6):6653–6658. DOI:10.1016/j.ceramint.2018.01.076
- Svahn F, Kassman-Rudolphi Å, Wallén E. The influence of surface roughness on friction and wear of machine element coatings. Wear. 2003;254(11):1092–1098. DOI:10.1016/S0043-1648(03)00341-7.
- Kanaga KS, Bruck AL, Sriram S, et al. Friction and wear behavior of ultra-high molecular weight polyethylene as a function of polymer crystallinity. Acta Biomater. 2008;4(5):1401–1410. DOI:10.1016/j.actbio.2008.02.022
- Zhenzhen Z, Jiawen G, Yali S, et al. Effects of crystal refining on wear behaviors and mechanical properties of lithium disilicate glass-ceramics. J Mech Behav Biomed Mater. 2018;81:52–60. DOI:10.1016/j.jmbbm.2018.02.023
- Sheikhattar M, Attar H, Sharafi S, et al. Influence of surface crystallinity on the surface roughness of different ceramic glazes. Mater Charact. 2016;118:570–574. DOI:10.1016/j.matchar.2016.07.003
- Platova RA, Platov YT. Whiteness and gloss evaluation of porcelain. Glas Ceram. 2017;74(3–4):91–94. DOI:10.1007/s10717-017-9935-y.
- Yanmei L, Jinliang T, Jiao S, et al. Removing polysaccharides-and saccharides-related coloring impurities in alkyl polyglycosides by bleaching with the H2O2/TAED/NaHCO3 system. Carbohydr Polym. 2014;112(4):416–421. DOI:10.1016/j.carbpol.2014.05.065
- Pekkan K, Karasu B. Zircon-free frits suitable for single fast-firing opaque wall tile glazes and their industrial productions. J Eur Ceram Soc. 2009;29(9):1571–1578. DOI:10.1016/j.jeurceramsoc.2008.10.010.
- Atkinson I, Smith ME, Zaharescu M. Examining correlations between composition, structure and properties in zircon-containing raw glazes. Ceram Int. 2012;38(3):1827–1833. DOI:10.1016/j.ceramint.2011.10.007.
- Hu AM, Li M, Mao DL. Growth behavior, morphology and properties of lithium aluminosilicate glass ceramics with different amount of CaO, MgO and TiO2 additive. Ceram Int. 2008;34(6):1393–1397. DOI:10.1016/j.ceramint.2007.03.032.
- Benkacem S, Boudeghdegh K, Zehani F, et al. Effect of ZrSiO4/ZnO ratio on the properties of opaque glazes used in ceramic sanitary ware industry. Iran J Mater Sci Eng. 2020;17(2):104–115.