ABSTRACT
Owing to an increasing number of infections in adults, Lactococcus (L.) garvieae has gained recognition as an emerging human pathogen, causing bacteraemia and septicaemia. In September 2020, four paediatric onco-hematologic patients received a platelet concentrate from the same adult donor at Bambino Gesù Children’s Hospital IRCCS, Rome. Three of four patients experienced L. garvieae sepsis one day after transfusion. The L. garvieae pediatric isolates and the donor’s platelet concentrates were retrospectively collected for whole-genome sequencing and shot-gun metagenomics, respectively (Illumina HiSeq). By de novo assembly of the L. garvieae genomes, we found that all three pediatric isolates shared a 99.9% identity and were characterized by 440 common SNPs. Plasmid pUC11C (conferring virulence properties) and the temperate prophage Plg-Tb25 were detected in all three strains. Core SNP genome-based maximum likelihood and Bayesian trees confirmed their phylogenetic common origin and revealed their relationship with L. garvieae strains affecting cows and humans (bootstrap values >100 and posterior probabilities = 1.00). Bacterial reads obtained by the donor’s platelet concentrate have been profiled with MetaPhlAn2 (v.2.7.5); among these, 29.9% belonged to Firmicutes, and 5.16% to Streptococcaceae (>97% identity with L. garvieae), confirming the presence of L. garvieae in the platelet concentrate transfusion. These data showed three episodes of sepsis for the first time due to a transfusion-associated transmission of L. garvieae in three pediatric hospitalized hematology patients. This highlights the importance to implement the screening of platelet components with new human-defined pathogens for ensuring the safety of blood supply, and more broadly, for the surveillance of emerging pathogens.
Introduction
Lactococci are Gram-positive, catalase-negative, facultative anaerobic cocci in short chains or pairs traditionally considered to be of low virulence to human beings [Citation1]. Among them, Lactococcus (L.) garvieae species was first described in the 1950s in Japan, when it was discovered in a rainbow trout farm [Citation2,Citation3]. It can be found in a vast variety of environments due to its ability to adapt easily. It has been isolated from aquaculture, rivers, and sewage waters. The host range of L. garvieae is not limited to aquatic species, but is also associated with subclinical mastitis in cows and water buffalos, and pneumonia in pigs [Citation4,Citation5]. Previous studies have reported an association between L. garvieae infection and contaminated food, such as raw milk, cheese, vegetables, cereals, and meat [Citation6,Citation7].
The first case of L. garvieae human infection was observed in 1991 [Citation8]. Since then, the relevance and clinical significance in humans have increased. After the first documented case, more than 30 new cases of L. garvieae infection have been described in literature [Citation6,Citation9–11]. Due to the increasing number of human clinical infections, L. garvieae has gained recognition as an emerging human pathogen. Among the reported cases, majority have been associated with bacteraemia with infective endocarditis among elderly and immunocompromised patients [Citation9]. Other clinical syndromes include spontaneous septicaemia, liver abscess, bone infections, diverticulitis, and secondary peritonitis [Citation3,Citation9,Citation11]. It has also been observed to mainly cause urinary tract infection (UTI) [Citation1]. However, the true incidence of the disease is difficult to assess because of the morphological and biochemical similarities to other Gram-positive cocci like Enterococcus spp. and Streptococci [Citation9,Citation12].
In 2018, the first case of transfusion-transmitted L. garvieae resulting from a platelet transfusion in an adult hospitalized individual was described [Citation13]. Here, we describe a cluster of transmission, with three cases of sepsis in onco-hematologic pediatric patients, derived by transfusion-transmitted L. garvieae from platelet concentrates (PCs) of the same donor.
Material and methods
Study population
On 31 August 2020, blood donation was collected from a healthy 59-year-old Italian female at IRCCS Bambino Gesù Children’s Hospital, in Rome, Italy. PCs were obtained by apheresis collection. The PCs were confirmed to have no abnormalities by visual inspection at the blood centre and again at the bedside just before starting the transfusion. These PCs were transfused to four paediatric patients at different timetable: patient 1 was transfused one day after blood donor’s collection, while patients 2–4 were transfused on day 2 after blood collection (hour:minute: 14:04, 14:32, and 16:36, respectively).
Data and samples collection
Age, sex, data regarding antibiotic therapies, comorbidities, and clinical conditions (body temperature, laboratory tests, and vital parameters) on the day of transfusion were collected for each patient.
At least one blood culture (one bottle for aerobic growth and one for anaerobic growth) was drawn for each patient after the transfusion as part of routine care. The Microbiology Unit of the Hospital processed the samples. According to the standard laboratory operating procedures, the identification of microorganisms was performed by MALDI-TOF mass spectrometry. As no breakpoints for antibiotic susceptibility have been determined for Lactococcus spp., antibiotic susceptibility was analyzed by the Vitek system according to the EUCAST guidelines for Streptococci. Antibiotic susceptibility was analyzed against the glycopeptides, teicoplanin and vancomycin; the penicillins, ampicillin and penicillin G; the cephalosporin, ceftriaxone; the lincosamide, clindamycin; the macrolide, erythromycin; the aminoglycoside, gentamycin; and the fluoroquinolone, levofloxacin.
Sepsis disease is defined as a suspected or proven infection with at least two of the following criteria: abnormal temperature (>38.5°C or <36°C), abnormal white-blood-cell count (elevated [>20,000 × 10⁹ per L] or decreased [<4000 × 10⁹ per L] for age), tachycardia or bradycardia, or tachypnoea [Citation14], C-reactive protein greater than 15 mg/L, and serum procalcitonin increase above 0.05 ng/mL [Citation15].
Ethics approval
This study was conducted with respect to the Helsinki Declaration, and all the participants (parents) signed an informed consent to allow the use of clinical data for research purposes. Ethical approval was obtained from the Ethics Committee of Bambino Gesù Children’s Hospital in Rome, Italy (reference ID 2602/2021).
Whole-genome sequencing and metagenomic analysis of donor’s platelet concentrates
Extraction and sequencing
Microbial DNA was extracted from the positive cultures and the donor’s blood samples using the QIAamp DNA Microbiome Kit (Qiagen, Germany) according to the manufacturer’s instructions. DNA was quantified using the Qubit fluorometer. Libraries were obtained by QIAseq FX Single Cell DNA Library kit and DNAs were paired-end sequenced (2 × 150 bp) using Illumina HiSeq (Illumina, USA). All bacterial sequence data obtained were screened for the evidence of baseline quality control for short and low sequences. The quality check was done with FastQC v0.72.14. Adaptors were clipped and quality trimmed using FASTP 0.20.1 using default parameters (Q > 30). Quality trimmed paired reads were assembled into contigs using ABYSS v2.0, a de-novo assembler based on de Bruijn graph path reconstruction [Citation16]. K-mer size optimization has been reached by comparing QUAST indices [Citation17]obtained by assembling bacterial genomes with different k-mer sizes and by selecting the one returning the best score.
Phylogenetic analysis
To explore a possible clonal origin of the three L. garvieae strains isolated from paediatric patients, the L. garvieae genomes were compared to 20 publicly available L. garvieae sequences by both maximum likelihood and Bayesian approaches. Four publicly available additional Lactococcus (L.) lactis sequences were used as an outgroup. The characteristics of the 24 reference genomes are described in Table S1. PhaME software was used to perform the core single nucleotide polymorphism (SNP) genome typing [Citation18]. The core SNP alignment was indeed composed of 27 sequences 62,990 nucleotide long. Phylogenetic relatedness was first analyzed by the maximum likelihood (ML) method using iqTree2 [Citation19], under the nucleotide substitution GTR+I+G4 model [Citation20] and 1000 bootstrap replicates. The results were confirmed by a Bayesian inference analysis through BEAST v.1.10.4 [Citation21] by setting a chain length of 100 million of states under a strict molecular clock model and the GTR+I+G4 substitution model. The core SNP multiple alignment composed of the three L. garvieae strains isolated from paediatric patients plus 24 publicly available L. garvieae and L. lactis strains used for phylogenetic analysis is available at d oi: 10.5281/zenodo.6473676. To better appreciate the conserved residues between Enterococcus, Streptococcus, and Lactococcus species, the core SNP multiple alignments composed of (i) the three L. garvieae strains isolated from paediatric patients plus 24 publicly available L. garvieae, L. lactis, and Streptococcus (S.) pneumoniae strains, (ii) the three L. garvieae strains isolated from paediatric patients plus three publicly available L. lactis, S. canis, and Enterococcus (E.) raffinosus strains are available at the same d oi: 10.5281/zenodo.6473676.
Functional analysis
Resistance genes, plasmids, virulence factors, insertion sequence elements (IS), and phages were annotated using BLASTN e BLASTX against several specific databases, including CARD, ARDB, PlasmidFinder, ResFinder, VirulenceFinder, ICEberg, VFDB, ISFinder, phySPY, PHASTER, and PLSDB [Citation7,Citation22–26]. Outputs have been parsed to discard all the hits with a BIT score <300, coverage <65%, and similarity <70%. The remaining hits have been ordered by position, and in case of overlapping matches, the ones with the highest BIT score have been selected.
Metagenomics
Sequencing data pre-processing of the donor’s blood sample was performed by FASTP 0.20.1. Human reads were mapped against the human reference genome hg19 using bowtie2 and removed by samtools tools [Citation27]. The host-filtered microbial reads were taxonomically profiled using MetaPhlAn2 (version 2.7.5) [Citation28,Citation29].
Results
Patients’ characteristics
In September 2020, four paediatric onco-hematologic patients received PCs from the same irradiated sample, prepared by a single-donor (a healthy 59-year-old Italian female) plateletpheresis at Bambino Gesù Children’s Hospital (OPBG), Rome, Italy.
Three patients were males, with a median (interquartile range, IQR) age of 8 (7–9) years (). Three of them were affected by acute lymphocytic leukemia (ALL) (patients 1–3), while one was affected by metastatic neuroblastoma (patient 4). All were immunocompromised ().
Table 1. Pediatric patients’ characteristics.
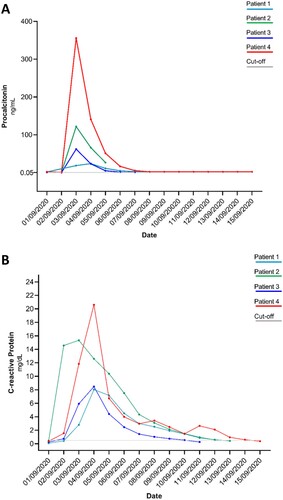
Approximately 24 h after transfusion, body temperature, C-reactive protein, and procalcitonin levels have been altered in all four patients, reaching elevated values (>38°C, >5 mg/dL, and >60 ng/mL, respectively) in three out of four patients (patients 2–4). Accordingly, a clinically defined sepsis was diagnosed in patients 2, 3, and 4 ( and ), while patient 1 experienced an acute self-limited illness. An empiric antimicrobial therapy mainly composed of tigecycline and aminoglycosides () was administered to each patient as soon as clinical manifestations were evident. In all patients, the vital signs returned to physiological ranges in a median (IQR) time of 4.5 (3.5–5.75) days.
Figure 1. Procalcitonin (Panel A) and C-reactive protein (Panel B) kinetics in the four paediatric patients receiving the platelet concentrates. All patients received platelet concentrates on 2 September 2020 except patient 1 who received the concentrates on 1 September 2020. Isolation of L. garvieae occurred for all patients (patients 1, 2, and 3) on 3 September 2020. Cut-off: normal value. Procalcitonin normal value <0.05 ng/mL; C-reactive protein normal value <0.50 mg/dL.
Phylogenetic relatedness of L. garvieae isolates
L. garvieae was isolated from the blood culture of all three patients developing sepsis (patients 2–4) 24 h post PCs transfusion, confirming the bloodstream infection. The blood culture of patient 1 resulted negative, confirming the self-limited illness.
The antimicrobial susceptibility testing defined resistance to the lincosamide clindamycin and penicillin G for all isolates (minimal inhibitor concentration [MIC] values: >256 and ≥0.5, respectively).
WGS experiment returned a total of 4.15 GB of Illumina 2 × 150 bp data by the sequencing of three L. garvieae isolates. De novo genome reconstruction retrieved an average genome size of 2.06 million base pairs with a GC content of 37.9%. The three L. garvieae sequences are available in the European Nucleotide Archive (ENA) under the following BioSample accession numbers: SAMEA12289652, SAMEA12289653, and SAMEA12289654.
Looking at the genome content, the three strains were characterized by 440 common SNPs, 44 of them were never described before (Table S2). Of these 43 SNPs, 19 were non-synonymous, and thus, involved in amino acid modifications in different L. garvieae enzymes.
The ML tree (based on the core SNP genomes of the three pediatric L. garvieae isolates plus 24 publicly available L. garvieae and L. lactis sequences, Table S1) showed that the three isolates shared a 99.9% identity, and clustered together within a bootstrap of 100% in the subtree of human and cow L. garvieae strains ((A)). The common origin of the three L. garvieae strains and their strong relatedness with human and cow strains were further confirmed by the Bayesian inference as suggested by the topology of the tree and the clusters’ posterior probabilities equal to 1.00 ((B)).
Figure 2. Estimated maximum likelihood (A) and Bayesian (B) phylogenies based on coreSNP genome of the three L. garvieae strains isolated from pediatric patients at Bambino Gesù Pediatric Hospital, IRCCS. Representative 20 L. garvieae and 4 L. lactis strains retrieved by public databases were also included to obtain an alignment of 27 coreSNP genomes 62,990 nucleotides long. The L. lactis and L. garvieae strains were annotated according to collection source (Orange: Dairy products, Blue: Fishes, Brown: Cow, Red: Humans; Other: Purple). BioSample accession numbers defined the strains. Panel (A) reported the ML tree, inferred using iqTree2 under the nucleotide substitution GTR+I+G4 model and 1000 bootstrap replicates. Panel (B) reported the Bayesian tree, performed by BEAST v.1.10.4 setting a chain length of 100 million states under a strict molecular clock model and the GTR+I+G4 substitution model. Bootstrap values and posterior probabilities are reported along the branches of the trees.
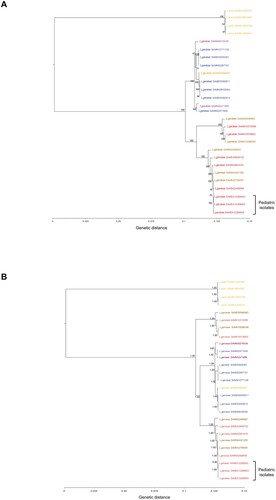
Of note, the isolates described in this work exhibit a genetic relatedness with L. garvieae isolates from meat and dairy products made with raw milk, previously pointed as the potential sources of human L. garvieae infection [Citation5].
Virulence factors and resistance genes characterizing the L. garvieae isolates
The isolates hold several virulence factors, such as hemolysin III family proteins, adhesin gene clusters, siderophores, and sortases (), suggesting a role of these strains in contributing to the pathogenic manifestations and the reported illnesses of the three pediatric patients.
Table 2. Putative virulence factors and resistance genes identified in the three L. garvieae strains isolated from pediatric patients at Bambino Gesù Pediatric Hospital, IRCCS.
Antimicrobial resistance profile is promoted in the three isolates by chromosomal lsa(D) gene, recently detected in L. garvieae fish pathogenic strain and described as a novel factor for resistance to lincosamide, pleuromutilins, and streptogramins [Citation30]. It acts as an ATP-dependent active transport and does not require additional cofactors to confer full resistance to upper mentioned antibiotics. The multidrug transporter Mdt(A) gene, originally described as a plasmid-dependent antimicrobial resistance factor in E. coli and L. lactis [Citation31], and recently found in the genomic content of L. garvieae [Citation32], was also shared in all the three isolates. Different from E. coli and L. lactis, this enzyme does not confer decreased susceptibility to erythromycin or tetracycline in L. garvieae species, probably due to two amino acid mutations in the C-motifs of Mdt(A), known to suppress the resistance phenotype of Tet(K) and Tet (L) [Citation33,Citation34].
The intermediate penicillin resistance found by the antimicrobial susceptibility test was probably promoted to a group of chromosomal penicillin binding proteins (PBP1a, PBP1b, PBPx, PBP2a, PBP2b, ) normally involved in wall cell biosynthesis [Citation35], and thus, capable of reacting with β-lactams. The intermediate, instead of being fully resistant to penicillin, can be explained by the absence of an auxiliary set of protein and regulatory elements encoded by a cassette chromosome called mec found in most of the penicillin-resistant Staphylococci, but absent in other non-pathogenic species [Citation36].
Of note, the genome content analysis also revealed a vanZ-like domain, known to be present in the genomes of clinically relevant bacteria, such as Bacillus, Streptococcus, Enterococcus, and Clostridium, and decreases their sensitivity to some lipoglycopeptide antibiotics, but not vancomycin [Citation37,Citation38].
Non-chromosomal genomic content in our isolates is represented by the plasmid pUC11C () [Citation39], known to encode two class C sortases, which are commonly involved in pilus biosynthesis [Citation40,Citation41].
The three L. garvieae genomes also contained the PLg-TB25 temperate prophage (coverage: 71.19%), recently described as a non-virulent prophage from a dairy strain of L. garvieae [Citation42], thus confirming the genetic relatedness of the three L. garvieae strains highlighted by the phylogenetic tree. This prophage carries with it several enzymes, such as resolvase and helicase, promoting exogenous DNA integration into the bacterial chromosome, and thus, paying an active role in acquiring and fixing genetic elements going under positive selection.
Donor’s platelet concentrates characterization
To confirm the source of L. garvieae infection, the microbial reads obtained by the donor’s PCs were profiled – thanks to MetaPhlAn2 (v.2.7.5). After removing low-quality and host genome reads, we obtained 5,459,177 reads with a quality score >30. The 4.98% (number of reads: 271,810) were properly classified as bacteria (n = 64,145), viruses (n = 200,356), or eukarya (n = 7,309), while 95.2% remained unclassified. Among the bacterial reads, 29.9% (number of reads: 19,154) belonged to Firmicutes, and 5.16% (number of reads: 3310) defined Streptococcaceae. The remaining bacterial reads mainly belonged to Proteobacteria (number of reads: 38,127).
All Streptococcaceae reads (ENA accession number: ERS9886497) shared a >97% homology with L. garvieae isolates, thus confirming the presence of L. garvieae in the PCs transfusion.
Discussion
Here, we describe three cases of sepsis-related to the drug-resistant L. garvieae transmission in three onco-hematologic pediatric patients caused by a platelet transfusion obtained by the same healthy adult donor. The fourth patient, who had received a transfusion first, developed a self-limited illness, accompanied by a blood culture that remained negative. To our knowledge, this is the first report that described a clinically defined sepsis in a pediatric setting caused by this emerging human pathogen during a blood transfusion procedure.
The cases of L. garvieae infection in humans described so far are characterized by a favourable clinical course and regard manifestations such as endocarditis, septicaemia, urinary tract infection, peritonitis, and liver abscess [Citation3,Citation9,Citation11]. L. garvieae was also the cause of bacterial contamination of PCs [Citation43,Citation44], and thus, represents a serious problem in transfusions, as demonstrated by the first case of sepsis caused by this transfusion-transmitted pathogen [Citation43].
In this regard, the safety of the blood supply, including bacterial contamination of platelet products and transfusion-transmission risks associated with emerging pathogens [Citation45,Citation46] continues to represent a challenge for clinical blood centers. In the pediatric setting, sepsis without source account for 3.4%–13.6% of cases seen in emergency departments [Citation47], and all are characterized by a challenging diagnosis. Today, there are certain technologies that mitigate this risk either as commercially available products or as investigational protocols. These include pathogen-reduction technology (PRT) for apheresis platelets and plasma, rapid tests for bacterial detection in PCs, and investigational screening assays for emerging pathogens. The implementation of these technologies has enhanced the safety of the blood supply in the last years [Citation48], even if full prevention of transfusion-related bacterial infection cannot be completely achieved. In 2019, according to the latest FDA report, approximately 1.9 million apheresis platelets were transfused, and one death due to bacterial contamination occurred [Citation49].
Here, the three cases of sepsis in pediatric recipients developed 24 h after PCs transfusion. All three patients (patients 2–4) were characterized by a peak fever and a significant C-reactive protein and procalcitonin increase (). Consistent with clinical manifestations, the blood culture revealed L. garvieae infection in all three patients. The genome content analysis of L. garvieae isolates from the three blood cultures suggested their clonal origin and a well-defined homology with L. garvieae derived from meat and dairy products [Citation7]. The source and the transmission chain were revealed by the metagenomics analysis that confirmed the presence of L. garvieae traces in PCs.
Even if all patients had a favourable clinical course after a fully active antimicrobial therapy composed mostly of tigecycline and aminoglycosides (Amikacin), the illnesses reported in three out four patients after transfusion were typical of a pathogenic microorganism invasion.
In view of this and other reports that described sepsis associated with L. garvieae infection [Citation11,Citation13,Citation50,Citation51], the knowledge of virulence factors and resistance mechanisms associated with the Lactococcus genera and L. garvieae species is detrimental.
Here, we implemented the knowledge of the virulent L. garvieae circulating strains by providing a capillary description of chromosomal and extrachromosomal content of these bacteria, focusing the attention to all factors that might confer a selective advantage in host invasion [Citation7]. Indeed, the three strains hold the virulence factors necessary to survive and feed in iron rich-environment, like human blood [Citation52]. Collected strains also shared several groups of adhesins, haemolysin, fibronectin-binding proteins, and penicillin acylase that actively promote bacterial colonization of mucosal tissues [Citation53,Citation54], and thus, increase the chance of bacteria being present in blood transfusable components [Citation52,Citation54]. Some adhesins such as MucBP domain-containing protein LCGL 1005 also confer to the bacteria the ability to form biofilm and to escape immune surveillance systems [Citation55]. Furthermore, the presence of WxL domain-containing proteins (already characterized in Enterococcus faecium) increases the L. garvieae ability to overcome the osmotic stress and to aggregate in a complex population [Citation56].
We also identified chromosomal contents conferring drug resistance to lincosamides (IsaD gene) and penicillins (penicillin binding proteins) (), and to some lipoglycopeptide antibiotics [Citation31,Citation35,Citation37,Citation38,Citation57], thus providing the genetic basis of the antimicrobial susceptibility testing results, that defined resistance to clindamycin and intermediate resistance to penicillin G. These results also confirmed the drug-resistant genetic backbone of the L. garvieae isolated in the three pediatric patients.
Even if the three cases described here support the circulation of drug-resistant L. garvieae strains in humans and its potential role as a human pathogen, more insights and evidence are needed to better define L. garvieae pathogenicity and related outcomes, and thus, to guide its significance in clinical practice. Most efforts in WGS are also needed to better characterize L. garvieae circulation in pediatric and adult settings.
Regarding transfusion-transmitted infection risk, although four patients had received the same platelet concentrate bag, only three developed sepsis. A discriminating factor that can justify the different grades of illness is the time of transfusion administration.
According to our hospital procedure [Citation58], PCs are generally stored on a platelet shaker at room temperature (22 ± 2°C) before being transfused. Due to the ability of L. garvieae to grow at temperatures as low as 10°C in red blood cells [Citation59], the platelets storage at room temperature could have provided suitable conditions for bacterial proliferation [Citation48].
In line with this evidence, we observed that the first patient who received the transfusion was the only one who did not develop sepsis. On the contrary, the patient who last received the platelet concentrate bag was the first to have a positive blood culture. Interestingly, this patient was also characterized by a higher level of procalcitonin than the others (), providing further evidence that in the case of bacterial contamination, the longer is the time of platelets storage, the higher is the risk to transmit high bacterial load [Citation60]. It is worth knowing that the irradiation performed according to internal hospital procedures is used to prevent the Graft versus Host Disease (GvHD), but is not able to prevent bacterial growth as stated in the National Guidelines [Citation58].
In conclusion, despite advances in donor screening and infectious disease testing, the risk of transfusion-transmitted infections continues to be of particular concern, as defined by the three episodes of sepsis due to a transfusion-associated transmission of drug-resistant L. garvieae in pediatric hospitalized onco-hematologic patients. This highlights the importance to implement the screening of platelet components with new human-defined pathogens to ensure the safety of blood supply, and more broadly, for surveillance of emerging pathogens.
Supplemental Material
Download Zip (31.5 KB)Acknowledgements
We thank Biodiversa s.r.l. for providing technical support. We also thank the whole staff of the Microbiology Laboratory of Bambino Gesù Paediatric Hospital IRCCS for outstanding technical support in processing samples and performing laboratory analyses.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Collins MD, Farrow JAE, Phillips BA, et al. Streptococcus garvieae sp. nov. and Streptococcus plantarum sp. nov. J Gen Microbiol. 1983;129:3427–3431. DOI:10.1099/00221287-129-11-3427.
- Vendrell D, Balcázar JL, Ruiz-Zarzuela I, et al. Lactococcus garvieae in fish: a review. Comp Immunol Microbiol Infect Dis 2006;29:177–198. DOI:10.1016/j.cimid.2006.06.003.
- Tariq EF, Irshad Y, Khalil HB, et al. Urinary tract infection caused by the novel pathogen, Lactococcus garvieae: a case report. Cureus. 2020;12:e9462. DOI:10.7759/cureus.9462.
- Teixeira LM, Merquior VLC, Vianni MDCE, et al. Phenotypic and genotypic characterization of atypical Lactococcus garvieae strains isolated from water buffalos with subclinical mastitis and confirmation of L. garvieae as a senior subjective synonym of Enterococcus seriolicida. Int J Syst Bacteriol. 1996;46:664–668. DOI:10.1099/00207713-46-3-664.
- Tejedor JL, Vela AI, Gibello A, et al. A genetic comparison of pig, cow and trout isolates of Lactococcus garvieae by PFGE analysis. Lett Appl Microbiol. 2011;53:614–619. DOI:10.1111/j.1472-765X.2011.03153.x.
- Wang CYC, Shie HS, Chen SC, et al. Lactococcus garvieae infections in humans: possible association with aquaculture outbreaks. Int J Clin Pract. 2007;61:68–73. DOI:10.1111/j.1742-1241.2006.00855.x.
- Gibello A, Galán-Sánchez F, Blanco MM, et al. The zoonotic potential of Lactococcus garvieae: an overview on microbiology, epidemiology, virulence factors and relationship with its presence in foods. Res Vet Sci. 2016;109:59–70. DOI:10.1016/j.rvsc.2016.09.010.
- Elliott JA, Collins MD, Pigott NE, et al. Differentiation of Lactococcus lactis and Lactococcus garvieae from humans by comparison of whole-cell protein patterns. J Clin Microbiol 1991;29:2731–2734. DOI:10.1128/jcm.29.12.2731-2734.1991.
- Malek A, De La Hoz A, Gomez-Villegas SI, et al. Lactococcus garvieae, an unusual pathogen in infective endocarditis: case report and review of the literature. BMC Infect Dis. 2019;19:301. DOI:10.1186/s12879-019-3912-8.
- Aubin GG, Bémer P, Guillouzouic A, et al. First report of a hip prosthetic and joint infection caused by Lactococcus garvieae in a woman fishmonger. J Clin Microbiol. 2011;49:2074–2076. DOI:10.1128/JCM.00065-11.
- Mofredj A, Baraka D, Cadranel JF, et al. Lactococcus garvieae septicemia with liver abscess in an immunosuppressed patient [5]. Am J Med. 2000;109:513–514. DOI:10.1016/S0002-9343(00)00534-9.
- Vinh DC, Nichol KA, Rand F, et al. Native-valve bacterial endocarditis caused by Lactococcus garvieae. Diagn Microbiol Infect Dis. 2006;56:91–94. DOI:10.1016/j.diagmicrobio.2006.02.010.
- Nakamura S, Nakai K, Sakata M, et al. Recipient sepsis caused by Lactococcus garvieae contamination of platelets from a donor with colon cancer. Vox Sang. 2019;114:182–184. DOI:10.1111/vox.12740.
- Lafolie J, Labbé A, L’Honneur AS, et al. Assessment of blood enterovirus PCR testing in paediatric populations with fever without source, sepsis-like disease, or suspected meningitis: a prospective, multicentre, observational cohort study. Lancet Infect Dis. 2018;18:1385–1396. DOI:10.1016/S1473-3099(18)30479-1.
- Casado-Flores J, Blanco-Quirós A, Asensio J, et al. Serum procalcitonin in children with suspected sepsis: a comparison with C-reactive protein and neutrophil count. Pediatr Crit Care Med. 2003;4:190–195. DOI:10.1097/01.PCC.0000059420.15811.2D.
- Jackman SD, Vandervalk BP, Mohamadi H, et al. ABySS 2.0: resource-efficient assembly of large genomes using a Bloom filter. Genome Res. 2017;27:768–777. DOI:10.1101/gr.214346.116.
- Gurevich A, Saveliev V, Vyahhi N, et al. QUAST: quality assessment tool for genome assemblies. Bioinformatics. 2013;29:1072–1075. DOI:10.1093/bioinformatics/btt086.
- Shakya M, Ahmed SA, Davenport KW, et al. Standardized phylogenetic and molecular evolutionary analysis applied to species across the microbial tree of life. Sci Rep.2020;10:1–15. DOI:10.1038/s41598-020-58356-1.
- Minh BQ, Schmidt HA, Chernomor O, et al. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic Era. Mol Biol Evol. 2020;37:1530–1534. DOI:10.1093/MOLBEV/MSAA015.
- Kozlov AM, Darriba D, Flouri T, et al. RAxML-NG: A fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics. 2019;35:4453–4455. DOI:10.1093/bioinformatics/btz305.
- Suchard MA, Lemey P, Baele G, et al. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 2018;4:vey016. DOI:10.1093/VE/VEY016.
- Eraclio G, Ricci G, Fortina MG. Insertion sequence elements in Lactococcus garvieae. Gene. 2015;555:291–296. DOI:10.1016/j.gene.2014.11.019.
- Aguado-Urda M, López-Campos GH, Blanco MM, et al. Genome sequence of Lactococcus garvieae 21881, isolated in a case of human septicemia. J Bacteriol. 2011;193:4033–4034. DOI:10.1128/JB.05090-11.
- Maki T, Santos MD, Kondo H, et al. A transferable 20-kilobase multiple drug resistance-conferring R plasmid (pKL0018) from a fish pathogen (Lactococcus garvieae)ls highly homologous to a conjugative multiple drug resistance-conferring enterococcal plasmid. Appl Environ Microbiol. 2009;75:3370–3372. DOI:10.1128/AEM.00039-09.
- Aguado-Urda M, Gibello A, Blanco MM, et al. Characterization of plasmids in a human clinical strain of Lactococcus garvieae. PLoS ONE 2012;7:e40119. DOI:10.1371/journal.pone.0040119.
- Galata V, Fehlmann T, Backes C, et al. PLSDB: a resource of complete bacterial plasmids. Nucleic Acids Res. 2019;47:D195–D202. DOI:10.1093/nar/gky1050.
- Li H, Handsaker B, Wysoker A, et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. DOI:10.1093/bioinformatics/btp352.
- Segata N, Waldron L, Ballarini A, et al. Metagenomic microbial community profiling using unique clade-specific marker genes. Nat Methods 2012;9:811–814. DOI:10.1038/nmeth.2066.
- Truong DT, Franzosa EA, Tickle TL, et al. Metaphlan2 for enhanced metagenomic taxonomic profiling. Nat Methods 2015;12:902–903. DOI:10.1038/nmeth.3589.
- Shi YZ, Yoshida T, Fujiwara A, et al. Characterization of lsa(D), a novel gene responsible for resistance to lincosamides, streptogramins A, and pleuromutilins in fish pathogenic Lactococcus garvieae Serotype II. Microb Drug Resist. 2021;27:301–310. DOI:10.1089/mdr.2020.0218.
- Perreten V, Schwarz FV, Teuber M, et al. Mdt(A), a new efflux protein conferring multiple antibiotic resistance in Lactococcus lactis and Escherichia coli. Antimicrob Agents Chemother. 2001;45:1109–1114. DOI:10.1128/AAC.45.4.1109-1114.2001.
- Walther C, Rossano A, Thomann A, et al. Antibiotic resistance in Lactococcus species from bovine milk: presence of a mutated multidrug transporter mdt(A) gene in susceptible Lactococcus garvieae strains. Vet Microbiol. 2008;131:348–357. DOI:10.1016/j.vetmic.2008.03.008.
- Ginn SL, Brown MH, Skurray RA. The TetA(K) tetracycline/H+ antiporter from Staphylococcus aureus: mutagenesis and functional analysis of motif C. J Bacteriol.2000;182:1492–1498. DOI:10.1128/JB.182.6.1492-1498.2000.
- De Jesus M, Jin J, Guffanti AA, et al. Importance of the GP dipeptide of the antiporter motif and other membrane-embedded proline and glycine residues in tetracycline efflux protein Tet(L). Biochemistry. 2005;44:12896–12904. DOI:10.1021/bi050762c.
- David B, Duchêne MC, Haustenne GL, et al. PBP2b plays a key role in both peripheral growth and septum positioning in Lactococcus lactis. PLoS ONE. 2018;13:e0198014. DOI:10.1371/journal.pone.0198014.
- Zapun A, Contreras-Martel C, Vernet T. Penicillin-binding proteins and β-lactam resistance. FEMS Microbiol Rev. 2008;32:361–385. DOI:10.1111/j.1574-6976.2007.00095.x.
- Arthur M, Depardieu F, Reynolds P, et al. Moderate-level resistance to glycopeptide LY333328 mediated by genes of the vanA and vanB clusters in enterococci. Antimicrob Agents Chemother. 1999;43:1875–1880. DOI:10.1128/aac.43.8.1875.
- Vimberg V, Zieglerová L, Buriánková K, et al. Vanz reduces the binding of lipoglycopeptide antibiotics to Staphylococcus aureus and Streptococcus pneumoniae cells. Front Microbiol. 2020;11. DOI:10.3389/fmicb.2020.00566.
- Kelleher P, Mahony J, Bottacini F, et al. The lactococcus lactis pan-plasmidome. Front Microbiol. 2019;10:707. DOI:10.3389/fmicb.2019.00707.
- Lebeer S, Claes I, Tytgat HLP, et al. Functional analysis of lactobacillus rhamnosus GG pili in relation to adhesion and immunomodulatory interactions with intestinal epithelial cells. Appl Environ Microbiol. 2012;78:185–193. DOI:10.1128/AEM.06192-11.
- Von Ossowski I, Reunanen J, Satokari R, et al. Mucosal adhesion properties of the probiotic lactobacillus rhamnosus GG SpaCBA and SpaFED pilin subunits. Appl Environ Microbiol. 2010;76:2049–2057. DOI:10.1128/AEM.01958-09.
- Eraclio G, Fortina MG, Labrie SJ, et al. Characterization of prophages of Lactococcus garvieae. Sci Rep. 2017;7:1856. DOI:10.1038/s41598-017-02038-y.
- Kozakai M, Matsumoto M, Matsumoto C, et al. First report of the isolation of Lactococcus garvieae from a platelet concentrate in Japan. Transfusion. 2016;56:2602–2606. DOI:10.1111/trf.13752.
- Pietersz RNI, Reesink HW, Panzer S, et al. Bacterial contamination in platelet concentrates. Vox Sang. 2014;106:256–283. DOI:10.1111/vox.12098.
- Dodd RY. Emerging pathogens and their implications for the blood supply and transfusion transmitted infections. Br J Haematol. 2012;159:135–142. DOI:10.1111/BJH.12031.
- Kreuger AL, Middelburg RA, Kerkhoffs JLH, et al. Storage medium of platelet transfusions and the risk of transfusion-transmitted bacterial infections. Transfusion. 2017;57:657–660. DOI:10.1111/trf.13969.
- Alpern ER, Stanley RM, Gorelick MH, et al. Epidemiology of a pediatric emergency medicine research network: The PECARN core data project. Pediatr Emerg Care. 2006;22:689–699. DOI:10.1097/01.pec.0000236830.39194.c0.
- Blajchman MA, Beckers EAM, Dickmeiss E, et al. Bacterial detection of platelets: current problems and possible resolutions. Transfus Med Rev. 2005;19:259–272. DOI:10.1016/j.tmrv.2005.05.002
- Fda, Cber. Fatalities reported to FDA following blood collection and transfusion annual summary for fiscal year 2019. 2019.
- Sahu KK, Sherif AA, Syed MP, et al. A rare cause of sepsis: Lactococcus garvieae. QJM. 2019;112:447–448. DOI:10.1093/qjmed/hcz078.
- Nadrah K, Cerar T, Papst L, et al. Lactococcus garvieae septicaemia in a patient with artificial heart valves. Wien Klin Wochenschr. 2011;123:677–679. DOI:10.1007/s00508-011-0059-z.
- Skaar EP. The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog 2010;6:1–2. DOI:10.1371/journal.ppat.1000949.
- Salighehzadeh R, Sharifiyazdi H, Akhlaghi M, et al. Serotypes, virulence genes and polymorphism of capsule gene cluster in Lactococcus garvieae isolated from diseased rainbow trout (Oncorhynchus mykiss) and mugger crocodile (Crocodylus palustris) in Iran. Iran J Vet Res. 2020;21:26–32.
- Eraclio G, Ricci G, Quattrini M, et al. Detection of virulence-related genes in Lactococcus garvieae and their expression in response to different conditions. Folia Microbiol. 2018;63:291–298. DOI:10.1007/s12223-017-0566-z.
- Devi SM, Halami PM. Diversity and evolutionary aspects of mucin binding (MucBP) domain repeats among Lactobacillus plantarum group strains through comparative genetic analysis. Syst Appl Microbiol. 2017;40:237–244. DOI:10.1016/j.syapm.2017.03.005.
- Galloway-Peña JR, Liang X, Singh KV, et al. The identification and functional characterization of WxL proteins from Enterococcus faecium reveal surface proteins involved in extracellular matrix interactions. J Bacteriol. 2015;197:882–892. DOI:10.1128/JB.02288-14.
- Walther-Wenke G. Incidence of bacterial transmission and transfusion reactions by blood components. Clin Chem Lab Med. 2008;46:919–925. DOI:10.1515/CCLM.2008.151.
- Liumbruno G, Bennardello F, Lattanzio A, et al. Raccomandazioni SIMTI sul corretto utilizzo degli emocomponenti e dei plasmaderivati. Società Italiana di Medicina Trasfusionale e Immunoematologia. 2008;1:37–39.
- Facklam R, Elliott JA. Identification, classification, and clinical relevance of catalase-negative, gram-positive cocci, excluding the streptococci and enterococci. Clin Microbiol Rev. 1995;8:479–495. DOI:10.1128/cmr.8.4.479.
- Haesebaert J, Bénet T, Michallet M, et al. Septic shock during platelet transfusion in a patient with acute myeloid leukaemia. BMJ Case Rep. 2013;2013:bcr2013010412. DOI:10.1136/bcr-2013-010412.
