ABSTRACT
Cisplatin (Cp) is an anti-neoplastic drug. Nephrotoxicity is the major side effect of Cp. Spirulina (Sp) is a blue-green alga with the ability to alleviating side effects of chemotherapeutic drugs in clinic. The aim of the study was to evaluate the ability of Sp to protect from cisplatin-induced nephrotoxicity. Adult male rat was orally administered Sp two days before single dose of Cp over 21 days (Sp + Cp group), 2nd group that intake Sp two days after with the same dose of Cp (Cp + Sp group) and two further groups were served as controls treated with either Sp or Cp only, respectively, as well as normal healthy control was included. At the end of experiment, Cp drug was induced acute kidney injury elevated levels of BUN, creatinine, KIM-1, NGAL and microalbumin in significant compared to that of normal control group, while the opposite effects were found in the rats who received Sp only. Furthermore, the prophylactic group showed improvements in biochemical parameters compared to those received Cp only. The effects of Cp were found to be diminished in the treated group. In addition, evaluation of Bax and SIRT genes expression in combination with histomorphological alterations of the kidney indicated the protective effect of Sp against Cp. In conclusion, pretreatment with Sp protected Cp-induced nephrotoxicity, probably via its antiapoptotic properties.
Introduction
Cisplatin (cis-diamminedichloridoplatinum(II)) is one of the most potent chemotherapeutic antitumor drugs used in clinical practice for treatment of solid tumors in lung, head and neck, ovarian, and urinary bladder cancer. Cisplatin activates apoptotic pathway and inflicts cellular damage via oxidative stress and inflammation. However, the use of cisplatin is frequently limited by various significant side effects such as bone marrow suppression, neurotoxicity and nephrotoxicity [Citation1]. More than 25% of treated patients develop acute nephrotoxicity after receiving cisplatin. Actually, kidneys are the major targets for the toxic effects of various chemical agents and thus drug-induced acute kidney injury (AKI) is a frequent entity in clinical medicine that is defined as a clinical syndrome characterized by a rapid decrease in renal function together with the accumulation of waste products such as urea. It is known also as an independent risk factor for mortality that increases the risk of death by 10–15 fold and results in a mortality rate up to 50% [Citation1–Citation3]. In addition, the increases in circulating kidney neutrophils with AKI are in concert with a decrease in kidney function are found in cisplatin-treated patients [Citation4–Citation7].
The mechanism of cisplatin nephrotoxicity is complicated; it involves oxidative stress as well as apoptosis, inflammatory processes [Citation8], proximal tubule injury and vascular injury, but they are not completely understood [Citation9]. In proximal tubule injury, the organic cation transporters 2 (OCT2) which are primarily in the basolateral membrane of the renal proximal tubules are considered important mediators of cisplatin uptake and a cause of renal tubular cell injury [Citation10]. So far there is no treatment to prevent this side effect of cisplatin. Numerous agents have shown a nephroprotective effect in models of renal damage induced by cisplatin and some of them are antioxidants [Citation11].
Blue-green algae is claimed to be a potential antioxidant [Citation12].
The aim of this work is to clarify the effect of spirulina extract on cisplatin-induced nephrotoxicity either before cisplatin treatment as a prophylactic measure or post-cisplatin treatment as a therapeutic measure with the determination of nephrotoxicity predictors among studies markers.
Spirulina platensis is a unicellular cyanoba-cterium that is full of nutritional wonders and has been used extensively in many countries as a dietary supplement due to its nutritional value [Citation13]. It is widely known by its contents of powerful antioxidants and free radical scavenging agents, including a potent two phycobiliproteins; phycocyanin and allophycocyanin [Citation14]. It has been implicated in several pharmacological properties demonstrated in preclinical studies, such as prevention of anemia, antimicrobial, antiviral, reduction of HIV replication velocity, anti-carcinogenic, immunostimulant, antioxidant and also participates in the prevention of heavy metal poisoning stimulation of antibody production as well as hepatoprotective, neuroprotective and antigenotoxic activities [Citation14–Citation21] . Spirulina also has a multiple advantages related to its high nutritional value that are considered as a food additive, its simple production method due to its moderate requirements for growth, its excellent conservation after recollection, and its security in relation to consumption (no toxicities) [Citation22]. In addition, Spirulina platensis is a potent antioxidant that could ameliorate the effect of chromium-induced nephrotoxicity and provided a near complete protection in terms of tissue and biochemical changes [Citation23].
Materials and methods
Spirulina, a fine dark blue-green spray-dried powder, was extracted from Spirulina platensis that was prepared kindly in the Botany Department (Faculty of Science, Zagazig University, Egypt). In total, 50 gm of Spirulina powder was dissolved in one liter of sterile distilled water; used as a stock solution to treat the animals in a dose of 1000 mg/kg/day. Cisplatin was purchased from Sigma Alderich Chemical Co. (St. Louis, USA) and it was prepared to inject in animal models for a dose of 7 mg/kg intraperitoneal at experimental days 1 and 11.
Animals and experimental design
Fifty adult male inbred Sprague-Dawley rats (weighing; 180–200 g) were divided into five equal groups. Animals were kept in stainless steel cages and maintained at controlled room temperature (24 ± 2ᵒC) on 12-hour light/dark under standard hygienic conditions. Free food access and distilled water were allowed in ad libitum. These experiments were carried out in Urology & Nephrology Center, Mansoura University according to the protocol approved by the Institutional Animal Care and Ethical Committee at Mansoura University. The groups of animal models were achieved as follows: The prophylactic group (Sp + Cp group) was received Sp for 21 successive days starting at two days before Cp induction, the treated rat group (Cp + Sp group) which was received Sp for 21 successive days after two days of administration Cp, Spirulina-treated control group (Sp group) received Sp daily by oral intubation for 21 days and Cisplatin-positive control group (Cp group) received two doses of Cisplatin (7 mg/kg; IP) in day 1 and day 11. The normal, healthy control group was included which received 1 ml of 0.9% NaCl solution (normal saline), daily.
Careful observation of animals in each group was carried out along the experimental period and clinical observations were recorded. The body weight and the weight of both kidneys for each animal were recorded at the end of the study. The animal models were anesthetized using a mixture of equal volumes of (diethyl ether, chloroform and acetone) then they were sacrificed for sample collection. Blood samples were collected by heart puncture and divided in two samples, one without anticoagulant that was centrifuged at 3000 rpm for 5 min for determination of serum urea and creatinine levels and the other withdrawal on EDTA for investigating of complete blood counts.
Collection of urine samples
Rats were placed in metabolic cages for 24 h in order to collect 24-hour urine specimens. The urine volume was measured and then a sample from each rat was taken for estimating urine creatinine value.
Harvesting of kidney specimens
At the end of experiments, rats were euthanized and then, abdomen was opened whereas one of the kidneys was perfused briefly with phosphate-buffered saline (PBS) through a cannula inserted into the abdominal aorta to rinse out the blood. Perfusion with formalin was done through the same channel and the kidney was removed and preserved in a container containing 10% neutral buffered formalin for histopathological examination while the other kidney was inserted into RNA Stabilization Reagent (RNA later, Qiagen, USA) solution to keep for gene expression evaluation and were frozen in liquid nitrogen [Citation24].
Biochemical analysis
Freshly voided urine samples were collected and centrifuged at 1000 g for 5 min. The supernatant was divided into aliquots of 1.5 ml Eppendorf tube and processed in varying manners for biochemical characterization. The tubes were frozen at −80°C within the first 20 min following collection. Both serum and urine creatinine concentrations were measured by the Jaffe assay using Roche/Hitachi 917 automated systems. According to manufacturer’s instruction, urinary soluble KIM-1 protein was quantified by a modified ELISA system using polyclonal KIM-1 antibodies, urinary NGAL concentration was measured using a commercially available ELISA kit as well as microalbumin (Antibody Shop, Gentofte, Denmark). Urinary biomarker values were normalized using urinary creatinine content in each sample by dividing the concentration of its biomarker by the urinary concentration of creatinine.
Histopathological investigations
A portion of kidney in each rat was fixed in 10% formasal (formalin diluted to 10% with saline) for histopathology. Serial sections of 5 µm thickness were prepared from the fixed tissues and then stained with hematoxylin and eosin to evaluate the details of renal architecture in each group using light microscopy.
Real-time PCR
Total RNA was extracted from preserved tissue samples manually using a mortar and pestle. Briefly, the tissue was frozen immediately and grind to a fine powder under liquid nitrogen. According to the manual instructs for RNA extraction kit (RNeasy Plus, Qiagen Sciences, Maryland, USA), RNA was isolated and purified. RNA concentration was quantified by using the Nanodrop (Thermo scientific, USA) and its integrity was determined using 1.5% agarose gel electrophoresis stained by ethidium bromide. The only samples that showed two RNA characteristic bands and without degradation was used for real-time PCR test. 3 µg of total RNA was converted into cDNA by random primers using the RT2 First strand kit (Qiagen Sciences, Maryland, USA). The PCR reaction was prepared by 10 µl of the SYBR green master mix. (Maxima, thermo fisher, USA), 2 µl (150 ng) of template, 2 µl (10 pmol) of primers and 6 µl of sterile distilled water. The samples were introduced to thermal cycle (CFX96, BioRad, USA) with amplification program as follows: initial denaturation at 95oC for 5 min. following by 40 cycles of denaturation at 94oC for 15 s, annealing at 55oC for 30 s and finally extension at 60oC for 30 s. Gene expression was performed for Bax, and Sirt1 genes which were designed online at the NCBI site (). Each sample was achieved in triplicate and statistical analysis was done according to the equation of M. Pfaffl [Citation25].
Table 1. Primers sequences for Real-Time PCR.
Statistical analysis
The evaluation and differences within groups were analyzed by one-way analysis of variance with SPSS software (version 10; SPSS Inc., Chicago, IL, USA). P < 0.05 was considered to indicate a statistically significant difference. Mean ± standard deviation values were calculated for each group to determine the significance of inter-group differences.
Results
Effect of spirulina and cisplatin on body weight measuring
The changes in body weight between different groups showed the group that received only spirulina increased in its weight compared to the normal, healthy group of animals (p < 0.01) while the development of body weight was depressed in all animals treated with cisplatin drug (p < 0.01). Decreasing in body weight in cisplatin pre- and post-treated rats were changed by increasing their weight with the administration of spirulina ().
Table 2. Measurements of body and kidney weight for the experimental rats.
Effect of spirulina on cisplatin-induced renal dysfunction
To evaluate the effect of spirulina on preventing kidney dysfunction induced by cisplatin, we analyzed plasma creatinine and plasma BUN presented in . Cisplatin-treated group demonstrated a marked elevation of plasma creatinine and BUN compared to that of the normal group. On the other hand, pretreatment with Spirulina at a dose of 1000 mg/kg significantly improved the concentration of both creatinine and BUN as shown by a significant reduction in plasma creatinine and BUN concentration when compared with cisplatin-treated group.
Table 3. Comparison between studied groups according to kidney function.
For evaluation of cisplatin-induced renal damage, we performed urinary NGAL, KIM-1 and microalbumin () they illustrate that Cp injection induced increase in NGAL, KIM-1 and microalbumin, respectively, early markers for assessment of acute kidney injury, significantly when compared to both normal and spirulina groups. Interestingly, the concentration of NGAL, KIM-1 and microalbumin was reduced in spirulina pre- and post-treated group as compared to that of cisplatin-treated group.
Table 4. Biochemical investigations among different groups.
Effect of spirulina on blood count
In this study, the mean values of blood parameters are all highly significantly reduced (P < 0.001) in cisplatin (Cp)-treated rat group in comparison with their corresponding levels in healthy control one (). On the other hand, oral administration of spirulina (Sp) dosing 1000 mg/kg in rat previously injected with Cp significantly improved the lowered levels of hematological parameters. Furthermore, the improvement in the lowered hematological parameters was more significant; their levels nearly approached the control levels if Sp (1000 mg/kg/day) was ingested before Cp (). Whilst, the administration of Sp ingested alone in control rat showed nearly the same levels as their corresponding levels of the control models.
Table 5. Blood counts parameters among different rat groups.
Kidney histopathological alterations
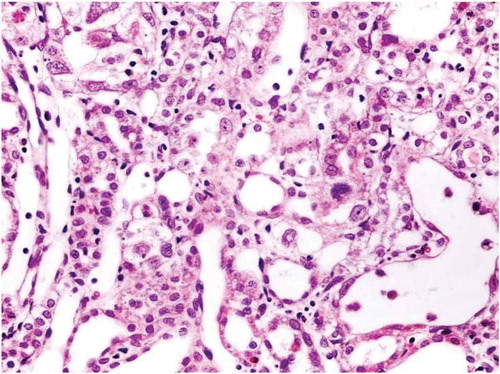
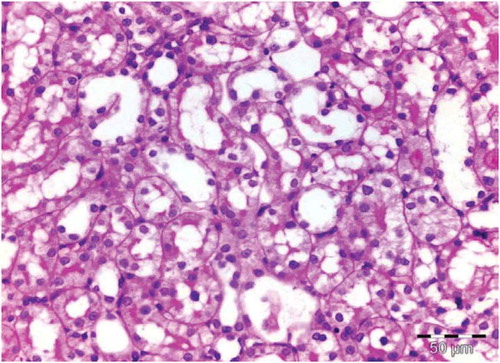
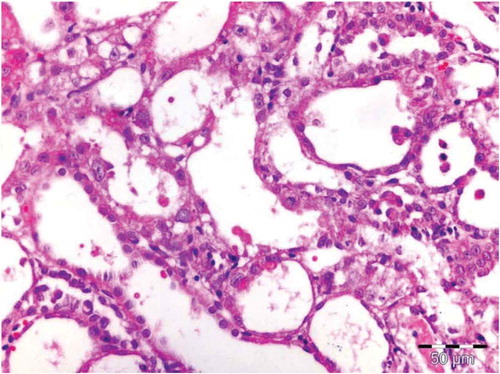
There was no histological alteration in the kidney morphology of normal rats ( and ) as well as Spirulina (1000 m/kg) induction (). The spirulina pretreated rat group was also showing normal kidney morphology except minimal tubular atrophy with dilatation, distortion and flattened lining, affecting the tubules in a specific field (). In line with the progression of renal damage, Cisplatin control at group showed prominent multiple tubular necrosis, degeneration, inflammatory cell infiltration, vocalization and loss of architecture of tubules ( and ). Interestingly, post-treatment with spirulina group ameliorated the pathological changes induced by cisplatin drug injection ( and ).
Figure 1. Control (saline injected) normal rat. Tubules show intact architecture, no intervening fibrosis.
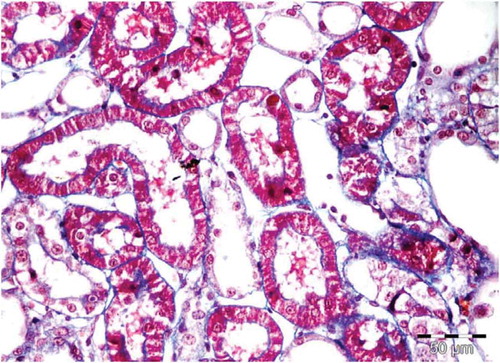
Figure 2. Control (saline injected) normal rat. Tubules of the OSOM show intact architecture, no degenerative or regenerative changes.
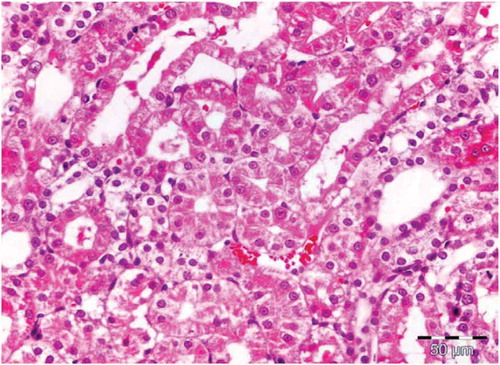
Figure 3. Kidney for rats receiving Spirulina. High magnification showing tubules of the OSOM with intact architecture, no degenerative or regenerative changes.
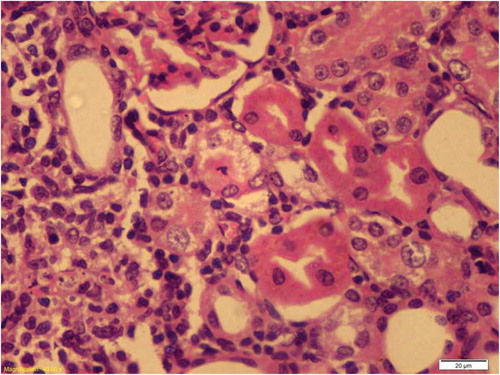
Figure 4. OSOM of rat receiving spirulina and cisplatin showing minimal tubular atrophy with dilatation, distortion and flattened lining affecting the tubules in this field (HX&E X 400).
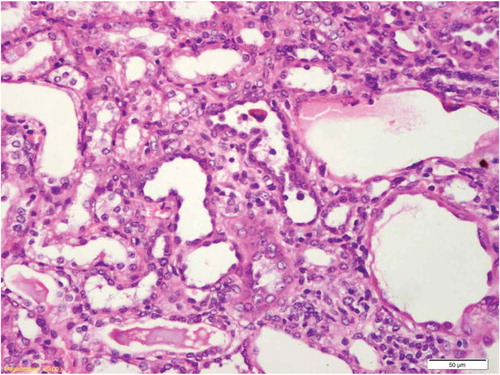
Figure 5. Kidney of Cisplatin group the proximal tubule showed apoptotic body near the luminal border of the epithelium with few prominent nucleoli.
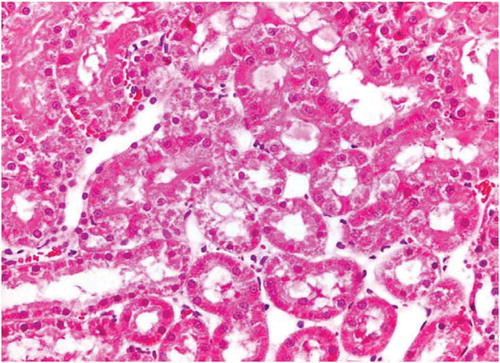
Figure 6. Cisplatin group tubular injury evidenced with apoptotic epithelial cells with shedding into lumen.
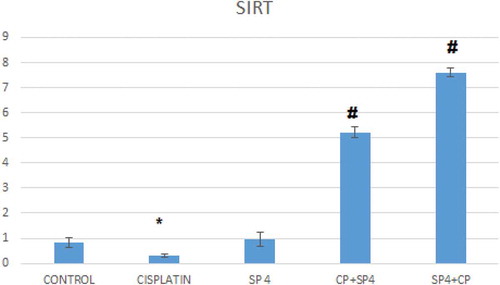
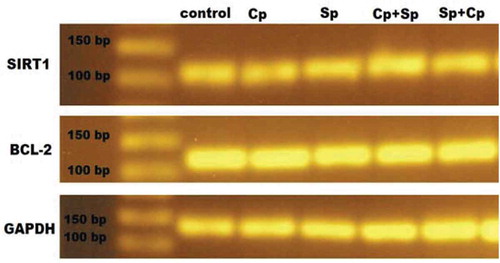
Effect of cisplatin and spirulina on Sirt-1 and Bax gene expressions
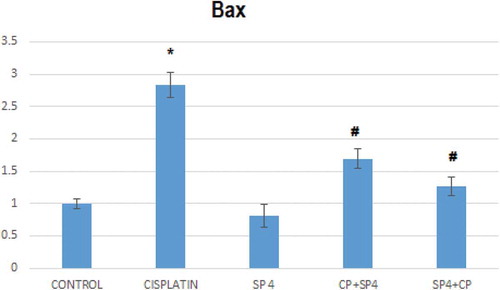
The injection of cisplatin increased the mRNA expression of Bax significantly in rat models about three-folds compared to that of the normal, healthy rat group; this increase was attenuated by pretreatment with Sp though no significant difference was seen when comparing to control group (). Similarly, Cp injection decreased the mRNA expression of Sirt-1 significantly as compared to that of normal group, and again this level was increased by pretreatment with spirulina ( and ).
Figure 9. Effects of pre- and post-treatment with spirulina 1400 mg/kg orally once daily for 21 days on the mRNA levels Bax in kidney of cisplatin-injected rats.
*compared to normal group
# compared to Cp group.
Discussion
Cisplatin-induced AKI is associated with inflammation, renal tubular cell death and tissue injury, which eventually leads to renal dysfunction. The molecular mechanism and pathogenesis of cisplatin nephrotoxicity have been studied for many decades and the recent evidence has shown the acute necrosis in kidney tubules is the predominant lesion associated with cisplatin-induced AKI [Citation26–Citation28]. Previous studies of rat models have demonstrated that cisplatin given intraperitoneally in a single dose of 7 mg/kg induced significant increase in nephrotoxicity markers, such as plasma creatinine and BUN and decreased creatinine clearance [Citation29–Citation31]. In accordance to the previous studies, we determined cisplatin injection in rats at a dose of 7 mg/kg induced significant increase in the markers of renal function, including plasma creatinine and plasma BUN and other urinary biomarkers ( & ). In accordance, other studies showed an increase in sCr, and blood urea nitrogen after cisplatin treatment [Citation32,Citation33]. Spirulina pretreatment inhibited the increasing of sCr and BUN induced by cisplatin and this is more affected than giving Spirulina after cisplatin injected. Actually, animal models receiving these agents undergo severe side effects as hepatotoxicity, nephrotoxicity and bone marrow toxicity that limited the dose which can be administered. The ability to minimize side effects is essential for the success of cancer chemotherapy. Different natural compounds have been recently investigated to potentially protect against cisplatin-induced toxicity. One of these compounds is Spirulina platensis (Sp) which has been studied to ameliorate the toxic side effects of chemotherapeutic drugs through its renoprotective efficiency. In this study, we assessed the protective effects of spirulina on cisplatin-induced nephropathy, we found that cisplatin administration affected by a significant decrease in kidney weight and kidney weight/body weight ratio compared to healthy group and these changes were alleviated with spirulina pre- and post-treatments (). These results were in agreement with Matsushima [Citation34] who observed that cisplatin injection caused an increase in urine volume and induced a significant decrease in body weight compared with control rats. Significant nephrotoxicity was observed after cisplatin administration as detected by elevations in serum creatinine (sCr) and BUN and these were taken as indications of an abnormal glomerular function [Citation35]. Spirulina, as an antioxidant agent, may have inhibited the chain reactions of the cisplatin-generated free radicals, or scavenged the free radicals before they reached the cell targets and damaging the glomerular kidney function. Under the experimental condition of the present study, spirulina showed protection on cisplatin-induced damage in adult rat kidneys.
Kidney injury molecule (KIM-1), neutrophil gelatinase-associated lipocalin (NGAL) and microalbumin are some of protein expressions that might function as novel biomarkers for the initiation phase of acute kidney injury [Citation32]. In our study, NGAL, Kim-1 and microalbumin were highly increased in animal urine following nephrotoxic AKI by cisplatin. This increase in urine parameters levels were decreased in spirulina-treated animals and the effect of spirulina were more efficient when administered before cisplatin injection as a prophylactic regimen (). After injury, Kim-1 is expressed on the apical membrane of the epithelial cells of the proximal tubules, increasing of the Kim-1 level in the urine means that cisplatin caused epithelial cell and proximal tubule injury and was decreased in spirulina-treated groups clarifying that spirulina could be an effective therapeutic agent for kidney injury and can eliminate nephrotoxic compounds early in the drug development process. Sasaki has demonstrated that the expression of KIM-1 increased after administration of cisplatin to rat models [Citation36]. Elevated Microalbumin (MAU) concentrations were indicative of high permeability for albumin in the renal glomerulus and it is more sensitive than either sCr or BUN [Citation37,Citation38]. Its highly increasing level was a prediction of progressive cisplatin nephrotoxicity which is characterized as primary glomerular injury and secondary tubular injury. The result of Kuhad et al. was in accordance with our finding who tested the renoprotective effects of Spirulina fusiformis on cisplatin-induced renal injury due to oxidative stress in rats [Citation39]. We found that Cp caused marked renal oxidative and significantly deranged renal function and these changes in renal function observed in the rat system correlate well with the nephrotoxic effects of cisplatin in men [Citation40].
In the present study, it was found that the mean values of red blood cells, hemoglobin, white blood cells, platelets, MCV, MCH and MCHC, were significantly decreased in rats receiving Cp. Also, no significant differences in all hematological parameters in healthy rats receiving Sp were found when compared to a healthy control group. The anemia found in Cp-treated rats might be of the type caused by chronic disease; however, other causes include autoimmune hemolytic anemia, iron deficiency anemia, anemia of chronic renal failure and Cp myelotoxicity. Thrombocytopenia occurred in Cp-treated rats and it is a part of a broader hematological disturbance. All these disturbances in hematological parameters were improved in Sp-treated rats in comparison to their corresponding values in Cp-treated rat. These findings are in agreement with the result of another researcher who reported that disturbance in hematological parameter were more minimized if Sp was given before Cp and this indicates that Sp may have a great role deal with the process of erythropoiesis [Citation41]. The present study found that, Cp administration induced elevation of Bax gene expression in kidney tissues, and this result was similar to those demonstrated by other researchers who indicated that the ratio of Bax/Bcl-2 protein expression in kidney tissues was increased following cisplatin injection. Indeed, the increase ratio of pro-apoptotic protein, Bax, and antiapoptotic protein, Bcl-2, determines the potential of cells to apoptosis process [Citation42,Citation43]. It is clear that Sp may be attenuated Cp effect and down regulated the pro-apoptotic protein (Bax) and this effect may be by up regulated the antiapoptotic protein (Bcl-2) corresponded with reduced apoptosis and improved renal function and this suggest a correlation between the changes in the mitochondrial pathway of apoptosis and the administration of Spirulina. Importantly, when the Bax gene was deleted, the animals became resistant to cisplatin and this illustrated that spirulina administration caused a protective effect on kidney or increased the antiapoptotic gene and decreasing the Bax apoptotic gene. In our experimental study, it is found that overexpression of Sirtuin 1 (SIRT1) in spirulina pre- and post-treatment has a significant protective effect against cisplatin-induced AKI. Actually, SIRT1 gene activation exerted cytoprotective effects through several mechanisms, including antiapoptosis, antioxidative and anti-inflammatory responses as well as the regulation of mitochondrial biogenesis, autophagy and metabolism in response to cellular energy and redox status [Citation44]. SIRT1 deacetylates p53 and in turn decreases apoptosis through deacetylation of p53 inhibition. P53 may also increase the survival of the cancer cells and thus reduce the therapeutic efficiency of cisplatin [Citation45,Citation46]. Thus, spirulina exerts cytoprotective effects through SIRT1 activation.
The kidneys of Cp-treated rats showed marked histological alterations, especially in the outer cortex and medullary region of the kidneys, compared with kidneys of normal control group. Spirulina significantly regressed these structural changes in the kidney, suggesting possible involvement of ROS in mediating these histological alterations. A beneficial effect on kidney function was found when adult rats were treated with spirulina in higher amounts before cisplatin injected. Under the experimental condition of the present study, spirulina showed protection on cisplatin-induced oxidative damage in adult rat kidneys. The renoprotective effect of spirulina may be due to the presence of phycocyanin pigment present in spirulina. Thus, the plausible mechanism of the renoprotective effect of spirulina may be due to its antioxidant effect, from these results we can say that Cp as platinum-based agents can affect the different parts of the nephron, producing a variety of renal complications such as acute renal failure, nephrotic syndrome and hydroelectrolytic disorders, with clinical manifestations that range from an asymptomatic elevation of serum creatinine to renal impairment.
In conclusion, we have demonstrated the protective effects of spirulina on kidney against cisplatin-induced kidney damage. Our data highlight that the protective effects of spirulina might be attributable to their antiapoptotic and anti-inflammatory effects along with their already-proven antioxidant activity. Further study is needed to identify and isolate the active principle of spirulina, which can have offer antioxidant and renoprotective properties.
Ethical Approval
The approvals of this study were kindly obtained from the ethical committee of the Mansoura University. Also, statement of animal rights were done as approved and supervised by an institution veterinarian, the animals were kept in individual well-ventilated cages at a room temperature of 24ºC and humidity of 50% and were provided with a standardized diet of bread, milk, cocked chicken and water ad libitum.
Acknowledgments
The authors would like to thank the organizers of the 14th International Conference on Chemistry and its Role In Development (14th ICCRD 2019), held at Mansoura-Hurghada 25-28 March 2019 for the kind acceptance for the oral presentation of this study.
Disclosure statement
No potential conflicts of interest were disclosed.
References
- Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380(9843):756–766.
- Chertow GM, Levy EM, Hammermeister KE, et al. Independent association between acute renal failure and mortality following cardiac surgery 12. Am J Med. 1998;104(4):343–348.
- Ramesh G, Reeves WB. Inflammatory cytokines in acute renal failure. Kidney Int. 2004;66:S56–S61.
- Tadagavadi RK, Gao G, Wang WW, et al. Dendritic cell protection from cisplatin nephrotoxicity is independent of neutrophils. Toxins (Basel). 2015;7(8):3245–3256.
- Bhat ZY, Cadnapaphornchai P, Ginsburg K, et al. Understanding the risk factors and long-term consequences of cisplatin-associated acute kidney injury: an observational cohort study. PloS One. 2015;10(11):e0142225.
- Jakob S, Arnold W, Marti H-P. Progressive renal failure after cisplatin therapy. Nephrol Dialysis Transplantation. 1996;11(2):370–373.
- Latcha S, Jaimes EA, Patil S, et al. Long–term renal outcomes after cisplatin treatment. Clin J Am Soc Nephrol. 2016;11(7):1173–9. CJN. 08070715.
- Troxell ML, Higgins JP, Kambham N. Antineoplastic Treatment and renal injury: an update on renal pathology due to cytotoxic and targeted therapies. Adv Anat Pathol. 2016;23(5):310–329.
- Ozkok A, Edelstein CL. Pathophysiology of cisplatin-induced acute kidney injury. BioMed Res Int. 2014.
- Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73(9):994–1007.
- Ali BH, Al Moundhri MS. Agents ameliorating or augmenting the nephrotoxicity of cisplatin and other platinum compounds: a review of some recent research. Food Chem Toxicol. 2006;44(8):1173–1183.
- Kuriakose GC, Kurup MG. Evaluation of renoprotective effect of Aphanizomenon flos-aquae on cisplatin-induced renal dysfunction in rats. Ren Fail. 2008;30(7):717–725.
- Chu W-L, Lim Y-W, Radhakrishnan AK, et al. Protective effect of aqueous extract from Spirulina platensis against cell death induced by free radicals. BMC Complement Altern Med. 2010;10(1):53.
- Rodríguez-Salgueiro S, Ramírez-Carmenate Z, González-Núñez L. An update on potential applications of Spirulina sp. and C-phycocyanin to treat kidney diseases. Ann Res Antioxid. 2017;2:1.
- Ferreira-Hermosillo A, Torres-Durán P, Shamosh-Halabe S, et al. Biological effects of Spirulina and current research on its antioxidant activity. Toctli RICTB. 2011;2(1):1–13.
- Hoseini S, Khosravi-Darani K, Mozafari M. Nutritional and medical applications of spirulina microalgae. Mini Rev Med Chem. 2013;13(8):1231–1237.
- Abdel-Daim MM, Abuzead SM, Halawa SM. Protective role of Spirulina platensis against acute deltamethrin-induced toxicity in rats. PloS One. 2013;8(9):e72991.
- Gargouri M, Ghorbel-Koubaa F, Bonenfant-Magné M, et al. Spirulina or dandelion-enriched diet of mothers alleviates lead-induced damages in brain and cerebellum of newborn rats. Food Chem Toxicol. 2012;50(7):2303–2310.
- Wu Q, Liu L, Miron A, et al. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: an overview. Arch Toxicol. 2016;90(8):1817–1840.
- Hemalatha K, Pugazhendy K, Jayachandran K, et al. Studies on the protective efficacy of Spirulina against lead acetate induced hepatotoxicity in Rattus norvegicus. group. 2012;2(3.17):0.15.
- Ayehunie S, Belay A, Baba TW, et al. Inhibition of HIV-1 replication by an aqueous extract of Spirulina platensis (Arthrospira platensis). J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18(1):7–12.
- Gutiérrez-Salmeán G, Fabila-Castillo L, Chamorro-Cevallos G. Aspectos nutricionales y toxicológicos de Spirulina (arthrospira). Nutricion Hospitalaria. 2015;32(1):34–40.
- Gargouri M, Saad HB, Amara IB, et al. Spirulina exhibits hepatoprotective effects against lead induced oxidative injury in newborn rats. Cell Mol Biol. 2016;62(10):85–93.
- Medeiros M, Sharma V, Ding R, et al. Optimization of RNA yield, purity and mRNA copy number by treatment of urine cell pellets with RNAlater. J Immunol Methods. 2003;279(1–2):135–142.
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001;29(9):e45–e45.
- Linkermann A, Bräsen JH, Darding M, et al. Two independent pathways of regulated necrosis mediate ischemia–reperfusion injury. Proc Nat Acad Sci. 2013;110(29):12024–12029.
- Linkermann A, De Zen F, Weinberg J, et al. Programmed necrosis in acute kidney injury. Nephrol Dialysis Transplantation. 2012;27(9):3412–3419.
- Kim J, Long KE, Tang K, et al. Poly (ADP-ribose) polymerase 1 activation is required for cisplatin nephrotoxicity. Kidney Int. 2012;82(2):193–203.
- Kalpana C, Sudheer A, Rajasekharan K, et al. Comparative effects of curcumin and its synthetic analogue on tissue lipid peroxidation and antioxidant status during nicotine-induced toxicity. Singapore Med J. 2007;48(2):124.
- Ugur S, Ulu R, Dogukan A, et al. The renoprotective effect of curcumin in cisplatin-induced nephrotoxicity. Ren Fail. 2015;37(2):332–336.
- Topcu-Tarladacalisir Y, Sapmaz-Metin M, Karaca T. Curcumin counteracts cisplatin-induced nephrotoxicity by preventing renal tubular cell apoptosis. Ren Fail. 2016;38(10):1741–1748.
- Palipoch S, Punsawad C, Koomhin P, et al. Hepatoprotective effect of curcumin and alpha-tocopherol against cisplatin-induced oxidative stress. BMC Complement Altern Med. 2014;14(1):111.
- Shimeda Y, Hirotani Y, Akimoto Y, et al. Protective effects of capsaicin against cisplatin-induced nephrotoxicity in rats. Biol Pharm Bull. 2005;28(9):1635–1638.
- Matsushima H, Yonemura K, Ohishi K, et al. The role of oxygen free radicals in cisplatin-induced acute renal failure in rats. J Lab Clin Med. 1998;131(6):518–526.
- Nagai J, Takano M. Molecular aspects of renal handling of aminoglycosides and strategies for preventing the nephrotoxicity. Drug Metab Pharmacokinet. 2004;19(3):159–170.
- Sasaki D, Yamada A, Umeno H, et al. Comparison of the course of biomarker changes and kidney injury in a rat model of drug-induced acute kidney injury. Biomarkers. 2011;16(7):553–566.
- Xie H-G, Wang S-K, Cao -C-C, et al. Qualified kidney biomarkers and their potential significance in drug safety evaluation and prediction. Pharmacol Ther. 2013;137(1):100–107.
- Dieterle F, Sistare F, Goodsaid F, et al. Renal biomarker qualification submission: a dialog between the FDA-EMEA and predictive safety testing consortium. Nat Biotechnol. 2010;28(5):455.
- Kuhad A, Tirkey N, Pilkhwal S, et al. Renoprotective effect of Spirulina fusiformis on cisplatin-induced oxidative stress and renal dysfunction in rats. Ren Fail. 2006;28(3):247–254.
- Segelov E, Mann G, Harnett PR. Mechanisms determining sensitivity to cisplatin in three mutant Chinese hamster ovary cell lines. Mutat Res/DNA Repair. 1998;407(3):243–252.
- Simsek N, Karadeniz A, Kalkan Y, et al. Spirulina platensis feeding inhibited the anemia-and leucopenia-induced lead and cadmium in rats. J Hazard Mater. 2009;164(2–3):1304–1309.
- Wada Y, Iyoda M, Matsumoto K, et al. Epidermal growth factor receptor inhibition with erlotinib partially prevents cisplatin-induced nephrotoxicity in rats. PloS One. 2014;9(11):e111728.
- Humanes B, Lazaro A, Camano S, et al. Cilastatin protects against cisplatin-induced nephrotoxicity without compromising its anticancer efficiency in rats. Kidney Int. 2012;82(6):652–663.
- Kitada M, Kume S, Takeda-Watanabe A, et al. Sirtuins and renal diseases: relationship with aging and diabetic nephropathy. Clin Sci. 2013;124(3):153–164.
- Vaziri H, Dessain SK, Eaton EN, et al. hSIR2SIRT1 functions as an NAD-dependent p53 deacetylase. cell. 2001;107(2):149–159.
- Hasegawa K, Wakino S, Yoshioka K, et al. Kidney-specific overexpression of Sirt1 protects against acute kidney injury by retaining peroxisome function. J Biol Chem. 2010;285(17):13045–13056.