ABSTRACT
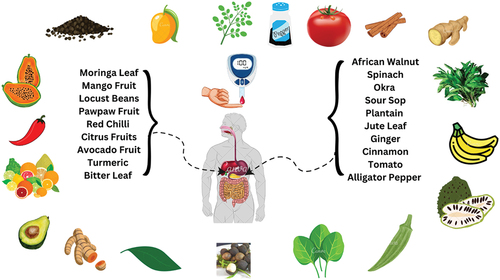
The prevalence of diabetes mellitus has increased over the years due to a high number of elderly people and sedentary lifestyles. Most synthetic drugs available lead to serious side effects compared to horticultural crops. A lot of plants have been reported as natural therapeutic plants for the therapy of diabetes worldwide. This review attempts to compile different horticultural crops in Nigeria and their antidiabetic properties for the therapy of diabetes mellitus. The horticultural crops evaluated in this review include; Citrus fruits, Aframomum melegueta (Alligator pepper), Persea americana (Avocado pear), Carica papaya (Pawpaw), Zingiber officinale (Ginger), Veronia amygdalina (Bitter leaf), Musa paradisiaca (plantain), Mangifera indica (Mango), Cinnamon cassia (Cinnamon), Parkia biglobosa (African locust bean), Moringa oleifera, Corchorus olitorius (Jute leaf), Annona muricata (soursop), Abelmoschus esculentus (Okra), Capsicum frutescens (Chilli pepper), Juglans regia (Walnut), Spinacia oleraceae (Spinach), Solanum lycopersicum (Tomato) and Curcuma longa (Turmeric). Various parts of these plants and their extracts were evaluated for hypoglycemic activity. These plants are easily available and have little or no negative side effects than conventional synthetic drugs. This review will be of help to researchers and diabetic patients for guidance.
Introduction
Recently, there has been a high rate of non-communicable diseases (NCD) affecting the general health of humans. To mitigate this, researchers have delved into seeking natural remedies with the use of horticultural crops. Horticultural crops have gained considerable attention from researchers across the globe as a possible option for the prevention and treatment of diseases. The use of horticultural crops is more or less going into extinction due to the advent of synthetic drugs. The alarming rate of NCD especially diabetes mellitus and the side effects of the synthetic drugs used have led to the search for alternatives that are cheap with little or no negative side effects [Citation1].
Diabetes mellitus is a long-term metabolic disorder that occurs as a result of the body’s inability to produce or use insulin and this affects human physical, social and psychological health. The pancreas produces insulin in the body which helps to convey blood sugar from the bloodstream to the cells which further breaks down and is used as fuel for normal body metabolism. The disease is typically attributed to lack of glucose homeostasis brought on by errors in insulin production or activity which in turn result in an improper metabolism of glucose and other sources of energy like protein and lipid. This is frequently accompanied by a loss of energy and body weight as well as significant alterations in lipid metabolism. Diabetes can cause serious side effects such as blindness, stroke, heart attack, liver malfunction, nerve damage and kidney failure if it is not treated properly [Citation2]. They occur in two types, namely, Type 1 (also known as Juvenile type) which is insulin-dependent and hereditary. It is an autoimmune disease in which the immune system accidentally attacks the insulin-producing cells of the pancreas. This type is common in children and adolescents.
The other is Type 2 (Adult type) which is prevalent among elderly individuals, it is non-insulin dependent and treated by controlling the diet. It is a metabolic disorder associated with insulin resistance and excess body weight. For people diagnosed with this type of diabetes, their pancreas produces insulin but their bodies find it difficult to make use of it. Hence, the pancreas cannot supply sufficient insulin to respond to the body’s needs and this type is the most prevalent in the world [Citation3]. The World Health Organization estimated that the number of diabetes mellitus will increase to over 366 million by 2030, and the large increase will be in developing countries amongst age groups between 45 and 64 years [Citation4]. Hence, the need to reduce these rates.
There are many ways of controlling diabetes mellitus (DM) and one of which is inhibiting the carbohydrate hydrolyzing enzymes present in the gastrointestinal tract as this will slow down digestion thereby prolonging the digestion time and decreasing blood glucose rise. Many horticultural crops have been investigated based on this effect. This review aims to identify different horticultural crops and their antidiabetic potential as a remedy for diabetes mellitus.
Methodology
A literature review was conducted using a compound of pertinent key terms such as ‘diabetes mellitus’, ‘horticultural crops’, ‘antidiabetic’, ‘hypoglycemic potential’, ‘extract’, ‘medicinal’ and ‘therapy’. As regards the search engine, PubMed, Science direct and Google Scholar, were reviewed. Only works published in English language published articles were examined. The years were not constrained. The EndNote software (version X7) was used to manage the references.
Horticultural crops
Citrus fruits
Rutaceae is the family of flowering plant that includes citrus. Citrus is grown globally for its juice and fruits (especially oranges, mandarins, tangors, grapefruits, lemon and lime) and is a common foodstuff for the masses. With a global population of 50, 186, 28.5, 934 and 7209 million metric tons for orange, tangerine, mandarin, lemons and limes, respectively, as of 2016/2017, it is extensively cultivated in the subtropical parts of the world [Citation5]. They contain a lot of nutrients including bioactive flavonoids, citric acid, minerals and vitamin C. Consuming citrus fruits has been attributed to a lower chance of developing chronic conditions including diabetes and cancer according to research [Citation2]. Citrus fruits have an effect on insulin tolerance and glucose homeostasis, as shown by laboratory, animal and epidemiological research.
Citrus paradisi (grapefruit): Lycopene and limonoids are two phytochemicals found in citrus paradisi. Moreover, it contains vitamins A, C, K, B5 and B9, iron, dietary fiber, calcium and other minerals [Citation6]. The two types (pink and red) are high in ß-carotene, high in fiber and low in calories according to research. They have protective phytochemicals including bioflavonoids, terpenes and phenolic acid, among others. The major bioflavonoid in grapefruit is Naringin which has been reported for its anti-diabetic potential and is also responsible for the characteristic bitter taste in grapefruit.
Citrus limonum (lemon) has shown to contain phytochemicals like cardiac glycosides, terpenoids, saponins, alkaloids, flavonoids and tannins.
Citrus naringin helps provide therapy against diabetes by reducing hyperglycemia-induced oxidative stress and pro-inflammatory cytokine production as demonstrated by Mahmoud et al. [Citation7]. Furthermore, naringin regulates the AMPK pathway, increasing insulin sensitivity and glucose tolerance. The study conducted by Mallick and Khan [Citation6] shows that as compared to diabetic control rats, diabetic rats given C. sinensis and C. paradisi had lower blood glucose concentrations. C. sinensis induced a hypoglycemic effect at doses of 2, 5 and 8 ml/kg, which might be as a result of its high contents of flavonoids and monoterpenes. This was also demonstrated by Tavafi et al. [Citation8]. The combination of the two demonstrated a synergistic effect in the reduction of blood glucose due to naringin in C. sinensis and polyphenols in C. paradisi. Another study by Kim et al. [Citation9] found that citrus fruit extract could be utilized to help diabetic individuals by controlling their sugar level through suppressing the intestinal tract enzymes. Additionally, the treatment with 100–600 mg/kg/day C. paradisi methanolic seed extract orally led to a considerable drop in blood glucose in the alloxan-induced diabetic rats. Therefore, citrus fruit consumption helps to prevent the risk of diabetes by improving insulin secretion and sensitivity.
Aframomum melegueta (Alligator pepper)
This is a spice plant in the family Zingiberaceae. Traditionally in Nigeria, it is referred to as ‘Atare’ in Yoruba, ‘Ose-oji’ in Igbo and ‘Citta’ in Hausa. It is a perennial herb that is grown in Africa’s tropical region for its seeds as stated by Osuntokun et al. [Citation10]. In height, it grows up to 1.5 m and possess a little, reddish-brown, aromatic and spicy seed in a long pod that grows from a purple bloom. Studies have shown that the seeds and leaves are rich in shogaols, paradol, gingerol and related substance. Shogaol boosts glucose utilization, which provides alligator pepper its hypoglycemic impact as indicated by Al Malki et al. [Citation11]. Therefore, the leaf sheath and seed extracts have the most observable secondary metabolite than the stem and fruit pulp [Citation12]. In West Africa, it is used as an aphrodisiac [Citation13], a remedy for snakebite and scorpion string, and also used to mitigate diarrhea, stomachache, diabetes, inflammation and cardiovascular diseases as elucidated by Ilic et al. [Citation14].
Nosiri et al. [Citation15] reported that aqueous extracts from Alligator pepper leaf decreased the blood glucose level of alloxan-induced diabetic rats in a dose-dependent manner. Also, the administration of 200/400 mg/kg A. melegueta ethanolic seed extract to diabetic rats induced with alloxan reduced blood glucose level and regenerate pancreatic ß-cells, though a higher concentration of the extract showed greater restorative outcome than a lower one. The seed extract of 200/400 mg/kg A. melegueta in water significantly reduced glycemia in alloxan-induced diabetic rats as opposed to the control group. The use of alligator pepper ensures the regulation of blood sugar in the body.
Persea americana (Avocado pear)
It is a flowering plant of the Lauraceae family. Botanically, the fruit contains a large seed referred to as a ‘pit’ or ‘stone’. It is fatty and of smooth, almost creamy texture. The antioxidant activity it possesses is due to the phenolic content of the seeds, which is greater than 70%. Because it contains lipids including phytosterols, campesterol, ß-sitosterol, stigmasterol and oleic acid, it provides healthy fats [Citation16]. The same authors demonstrated that the blood glucose levels of the alloxan-induced diabetic rats were markedly reduced by daily oral administration of dosages of the ethanol fruit pulp extract of P. americana, approaching the normal value. This is reportedly because it contains insulin-stimulating compounds such as steroids, alkaloids, tannins and saponins.
Ebifa et al. [Citation17] showed that the aqueous extract of avocado pear significantly reduced glycemia in diabetic-treated rats compared with non-treated rats. This is in agreement with the findings of Alhassan et al. [Citation18]. The anti-diabetic effects of the extract may be due to the availability of minerals such as calcium, zinc, magnesium, etc., as these play a vital role in blood glucose homeostasis. The existence of healthy fats in avocado pear helps to slow down the rate of carbohydrate breakdown, thereby stabilizing the blood sugar level in the body.
Carica papaya (Pawpaw)
Pawpaw belongs to the family Caricaceae, the genus Carica. Locally, it is called ‘ibepe’ (Yoruba), ‘Okworo’ (Igbo) and Gwanda (Hausa) in Nigeria. It is an abundant source of vitamins C, A and E (antioxidant), B vitamins (B5 and B9) and minerals (magnesium and potassium). The leaves, seeds, latex and fruits are of great medicinal value. Pawpaw contains papain and chymopapain, present in the fruit, stem and leaves. These are proteolytic enzymes that aid digestion [Citation4]. Studies have demonstrated that the unripe pulp of C. papaya has anti-hyperglycemic activities by reducing blood glucose levels [Citation19] and this is due to the bioactive constituents of the plant. Leaf’s extract physicochemical analysis showed the presence of glycosides, flavonoids, alkaloids, saponins, phenols, tannins and steroids [Citation20]. Phenolic compounds inhibit a rise in blood glucose concentration by blocking alpha glucosidase and pancreatic lipase activities [Citation21]. A study conducted by Ebifa et al. [Citation17] on the effect of C. papaya aqueous leaf extract on rats with diabetes revealed that the blood sugar level was reduced significantly compared to the control rats. This is in correspondence with the givens of Apea and Faruq [Citation22]. Another investigation was done by Ogundele et al. [Citation23] on extracting C. papaya leaves using methanol, and it was found out that it contains a number of bioactive constituents like polyphenol, tannins, saponins, terpenoids, flavonoids, glycosides and alkaloids. Also, it is capable of enhancing the usage of glucose in the body thereby controlling the blood glucose level. Other studies showed that Carica papaya seed extract administered at two different doses of 100 mg/kg and 200 mg/kg to Streptozotocin-Nicotinamide diabetic rats caused a significant reduction of blood glucose levels and maximum reduction was observed after 14 days. Due to its excellent source of vitamins, especially vitamin C, which is known to boost immunity, it can help to mitigate the risk of diabetes.
Zingiber officinale (Ginger)
Zingiber officinale known as ginger belongs to the family Zingiberaceae. It is usually referred to as ‘atale’ in Yoruba. It contains antioxidants including terpenes, polysaccharides, phenolic compounds, lipids, raw fibers and organic acids. Its active chemical compound is gingerol and shogaol, which is responsible for their antidiabetic potential [Citation24]. Gingerol inhibits pancreatic ß-cells from oxidative stress, hence delaying the formation of diabetes as indicated by Son et al. [Citation25].
A study was conducted on diabetic rats in which 3.0 g/100 ml aqueous extract of ginger was added to their normal feed and a remarkable lowering of the glucose level was observed as opposed to non-treated ones. These findings were in agreement with the work of Abdulrazaq et al. [Citation26] who reported the anti-hyperglycemic effect of ginger by reducing glucose levels. This was reported to be due to the ability of the aqueous extract to increase the synthesis of glycogen, increase glucose uptake and raise insulin receptor phosphorylation according to Khandouzi et al. [Citation27]. The result from animal studies reported that administration of ginger ethanolic extract orally at 100–800 mg/kg markedly decreased fasting blood sugar levels upon 1 hr treatment in diabetic rats induced with streptozotocin. After 4 hr, the effect maximized, with ginger creating a 24–53% decrease in blood glucose [Citation28]. Hence, consumption of ginger in diabetic patients has been demonstrated to reduce sugar levels.
Veronia amygdalina (Bitter leaf)
Veronia amygdalina, commonly called bitter leaf, is a plant that grows all over tropical Africa, and it is most common in the southeastern part of Nigeria. It belongs to the family Compositae. In Nigeria, Hausas refer to it as ‘Chuserdek’ Yorubas ‘Ewuro’ Igbos ‘Onugwu’. Research has indicated that it is an abundant source of minerals (P, Ca, Mg, Zn, Fe, K), vitamins (A, C, E). Findings were conducted by Nwaoguikpe [Citation29] on the effects of 2–10% aqueous bitter leaf extract on diabetic rats for 15 days. It was revealed that the highest reduction was observed in the rats that received 8% of the extract. It was reported that the phytochemicals present in the extract include flavonoids, saponins, polyphenols, tannins and glycosides. Saponins have been found in studies to lower blood sugar levels through regenerating insulin activity, stimulating glycogen synthesis and reducing gluconeogenesis. All these influenced its anti-diabetic potential since they are antioxidants. Also, the presence of the vitamins protects against degenerative changes. These findings are similar to the work of Mazumder et al. [Citation30]. Other findings revealed that the phenolic bitter leaf extract inhibits the activity of α-amylase and α-glucosidase in vitro in a dose-dependent approach (4–16 µg/ml) which helps to slow down the breakdown of carbohydrate to glucose thereby reducing the amount of glucose absorbed into the body [Citation31], although α-glucosidase has a stronger inhibitory effect. This agrees with the findings of Adefegha et al. [Citation32] who reported that the enzyme inhibitory activity is associated with the phenolic content of the extract. Hence, making it a strong antioxidant. These aforementioned showed that regular consumption of bitter leaf decreases the risk of type 2 diabetes.
Musa paradisiaca (Plantain)
Plantain belongs to the family Musaceae. It has a tall herb with an aerial pseudo stem that dies after flowering. On ripening, the fruits become golden yellow and are in several clusters. It is a better source of vitamin A, B (B1, B3, B2, B6) and vitamin C. Studies have indicated that flavonoids, sterols, alkaloids and polyphenols are the bioactive anti-diabetic components in Musa paradisiaca.
From the findings of Reddy and Hemachandran [Citation33], it was shown that the extract from the fruit and stem has a significant inhibition of α-amylase and α-glucosidase activity than its leaf and flower. The oral administration of 200 mg/kg of extracts of M. paradisiaca in diabetic rats induced with streptozotocin lowers the blood sugar concentration significantly as opposed to the control. Additionally, excess weight was observed which could be due to the improvement in metabolic activity of the system to maintain glucose homeostasis. Diabetes causes excess glucose that is present in the blood to react with amino groups of lysine residues to form HbA1c, which is an important biochemical marker for assessing diabetes and the rate of protein glycation. Therefore, taking the flower extract orally decreases glycosylated Hb level due to its hypoglycemic activity [Citation34].
Another author reported that the flower’s methanolic and aqueous extract resulted in an anti-diabetic impact in diabetic rats induced with streptozotocin, although the methanolic extract yielded the best glucose resistance effect after 15 days of treatment. This might result from the existence of flavonol glycoside and anthocyanin in the extract, which has been shown to exhibit anti-hyperglycemic activity [Citation35]. Shodehinde et al. [Citation36] indicated that the unripe pulp of Musa paradisiaca reduced sugar levels in diabetic rats upon 14 days of oral administration by inhibiting pancreatic α-amylase and intestinal α-glucosidase. Other authors reported that the physicochemical analysis of the fraction of M. paradisiaca leaves indicated the existence of rutin, which is a flavonol glycoside reported to modulate glucose homeostasis, increases secretion of insulin and inhibits α-glucosidase, which helps to delay the absorption of ingested carbohydrate and decrease insulin peaks [Citation37]. Hence, plantain is effective at inhibiting sugar spikes which supports in the management of type 2 diabetes.
Mangifera indica (Mango)
This belongs to the family Anacardiaceae, genus Mangifera. The active compound present in all parts of mango is mangiferin, which has the potential of inhibiting the key enzymes associated in the metabolism of glucose as declared by Ganogpichayagrai et al. [Citation38]. In addition, it is a good source of phenolic acids (caffeic acid, tannic acid, gallic acid), carbohydrates, amino acids, vitamins and organic acid [Citation39]. The stem bark’s ethanolic extract of M. indica demonstrated a gradual decrease in sugar absorption in the gut of rats with type II diabetes. Therefore, it exhibits an anti-hyperglycemic effect [Citation40]. According to reports, the mango peel is an abundant source of phytochemicals like carotenoids, vitamin C, vitamin E, polyphenols and dietary fiber. Addition of mango peel powder at 5% and 10% in the diet of diabetic rats induced with streptozotocin enhanced the activities of antioxidant enzyme and prevented hyperglycemia. This may be due to the action of the bioactive compounds and dietary fiber [Citation41]. Saleem et al. [Citation21] noticed that the hydro-alcoholic extract of mango leaves of 550–950 mg/kg was administered to diabetic-induced rats for 7 consecutive days and it prevented rise in the fasting blood glucose. It also revealed that in diabetic rats, the extract had a protective impact on the pancreas by reducing the damage to ß-cells and restoring the pancreatic structure. Also, an increase in body weight was reported. The findings of Azhar et al. [Citation42] on the ethanol extract of mango seed revealed a substantial reduction in fasting blood sugar levels at 200 mg/kg administered for 21 days in diabetic induced rats as opposed to the control. This could be due to the existence of flavonoids, phenolic acid in the mango seed. Hence, the presence of mangiferin in mango supports to alleviate the risk of diabetes.
Cinnamon cassia
Cinnamon cassia from the family Lauraceae is an aromatic plant. The Cinnamon cassia’s parts include camphor, eugenol, cinnamaldehyde, caryophyllene, hydrocarbons, trans-cinnamyl acetate terpenes, caryophyllene, alphacopane, terpenoids and alpha-bergamotene [Citation43]. Eugenol inhibits glucose metabolism by blocking α- glucosidase, resulting in a drop in blood sugar as noticed by Singh et al. [Citation44]. The oral dosage of cinnamon extract of 50–200 mg/kg was given to diabetic mice for 6 weeks and it lowers the blood sugar levels significantly [Citation45], although alterations in body weight were not significant. This is in agreement with the findings of Khan et al. [Citation46]. The Phyto-constituents present in the C. cassia’s bark extract are terpenoids, phenols, tannins and alkaloids. The bark’s ethanolic extract had high inhibitory activity against carbohydrate hydrolyzing enzyme in an in vitro model. Additionally, it increased the level of insulin secretion. Hence, this explains the effect of hypoglycemia of the extract. Although methanol, ethanol and aqueous extracts possess high phytoconstituents compared to hexane, chloroform and ether, ethanol has more inhibitory activity. Association [Citation47] reported that a protein of interest that is connected to glucose homeostasis and used for the therapy of diabetes and dyslipidemia known as PPARα/γ has more affinity and interaction with 9-Octadecenoic acid present in C. cassia’s bark extract, by that way enhancing insulin secretion. The presence of this acid may account for its putative anti-diabetic properties [Citation43]. Huttada et al. [Citation48] support these findings. Based on these, cinnamon is a powerful antioxidant that improves the release of insulin, thereby helping in the management of diabetes.
Parkia biglobosa (African locust bean)
African locust bean belongs to the Leguminosae family, subfamily Mimosoideae. It is commonly found in Nigeria and other countries in West Africa [Citation49]. The tree has pods which when matured range from pink-brown to dark brown and is reported to consist of 30 seeds enclosed in a yellow pericarp [Citation50]. Oyedemi et al. [Citation51] evinced that the oral daily administration of P. biglobosa stem bark hydro-methanolic extracts for 28 days exhibited a substantial decrease in the fasting blood glucose level in type 2 diabetic rats induced with streptozotocin and it also enhanced the test for oral glucose tolerance compared with a standard metformin. This was reported to be due to the insulin-mimetic activity of the plant extract by enhancing insulin secretion from the existing pancreatic ß-cells. Similar findings by Ibrahim et al. [Citation52] reported a reduction in diabetic rats administered with fermented seeds and leaf extracts from P. biglobosa.
Reports from Odetola et al. [Citation53] have shown that the seeds of P. biglobosa contain glycosides and alkaloids and high protein and amino acid content, which might be responsible for the anti-diabetic potential. Alkaloids prevent diabetes through enhancing insulin sensitivity and regulating oxidative stress [Citation54]. Also, from the investigation conducted, it revealed that both aqueous and methanolic seed extracts of Parkia biglobosa markedly decreased the sugar level of diabetic rats induced with alloxan to a level that was comparable to that of diabetic and non-diabetic control rats treated with glibenclamide. Furthermore, the high protein and amino acid content of the seed may have contributed to the aqueous extract’s ability to prevent weight loss, as opposed to the methanolic extract’s inability to do so due to the lack of these nutrients. Hence, the consumption of African locust bean can prevent weight loss and help to ameliorate blood sugar level.
Moringa oleifera
This belongs to the Moringaceae family, which is a drought resistant plant that is grown in tropical and subtropical regions. The soil and amount of rainfall in the area where the tree grows are very variable [Citation55]. It is popularly referred to as a ‘wonder tree’ due to the multipurpose uses of its plant parts [Citation56], particularly those leaves utilized in traditional treatment as reported by Popoola and Obembe [Citation57]. Anwar et al. [Citation58] stated that the antioxidants present in moringa leaves are phenolics, ascorbic acid and flavonoids, while the roots have shown to possess saponins, flavonoids, alkaloids, terpenoids, tannins and steroids, as appeared by Raj et al. [Citation59]. Givens from Tshabalala et al. [Citation55] have shown that the roots of the moringa tree contain less total phenol than the leaves. Also, there are more flavonoid content (myricetin, quercetin, kaempferol, isorhamnetin) and condensed tannin as reported by Leone et al. [Citation60] in the lateral roots than in the leaves. Quercetin provides antidiabetic action by preventing damage to the pancreas and ameliorating endogenous antioxidant enzymes, while isorhamnetin acts by stimulating insulin as indicated by Al-Ishaq et al. [Citation61].
The extract fraction of ethyl acetate at 200 mg/kg of M. oleifera significantly reversed all the symptoms associated with diabetes in rats such as loss of body weight, inflammation, etc., as reported by Bamagous et al. [Citation62]. Omodanisi et al. [Citation63] reported a similar findings. Another author assessed the impact of M. oleifera leaf aqueous extract on diabetic rats [Citation64]. It revealed that the blood sugar level was decreased by the aqueous extract, enhanced glucose tolerance, and it was more effective than the reference drug (Glipizide) used. Additionally, treating diabetic rats induced with streptozotocin with low doses of mango seed powder (50–100 mg/kg) was able to control diabetic nephropathy as reported by Al-Malki and El Rabey [Citation65]. Therefore, moringa might help the body to process sugar better and this may positively affect how the body releases insulin.
Corchorus olitorius (Jute leaf)
Jute leaf is from the family Tiliaceae, genus Corchorus, a green leafy vegetable, native to the people in Tropical Africa and Asia popularly called ‘Ewedu’ among the Yorubas in Nigeria. It was reported to contain lipids, protein, crude fiber, carbohydrates, vitamins (A, C, E) and minerals (Ca, Na, K, P, Fe) [Citation66]. It has a lot of beneficial phytochemicals but the most important ones are phytol and monogalactosyldiacylglycerol, and they are antioxidants that react with free radicals in the body. Hence, indicating their antidiabetic potential. The study conducted on C. olitorius ethanolic extract reduced fasting serum glucose levels significantly by 27% indicating that it possesses a hypoglycemic effect. A similar report was done by Omeje et al. [Citation67]. It is rich in polyphenolic compounds which interact with protein and inhibit the activity of the enzymes. The enzyme inhibitory activity was as a result of the existence of caffeic acid, chlorogenic acid and isorhamnetin [Citation1]. Previous reports have attributed the inhibition of postprandial glucose and utilization of fat in the body to the presence of chlorogenic acid. Chigurupati et al. [Citation68] reported C. olitorius to be rich in soluble dietary fiber and phenols, which has a lowering effect on blood glucose by inhibiting α-amylase and α-glucosidase enzymes, thereby preventing the absorption of glucose from the intestine. Mohammed et al. [Citation69] also revealed that the aqueous extract of C. olitorius leaf reduced the amount of blood sugar in diabetic alloxan-induced male rodents administered for 28 consecutive days. Hence, consumption of jute leaf assists in the prevention of diabetes as a result of its bioactive components.
Annona muricata (Soursop)
It is commonly known as soursop and belongs to the family Annonaceae, a tropical plant species with natural antioxidants. Research has shown that historically, the leaves have been used to cure, cystitis, diabetes, insomnia, headaches, etc. Agu et al. [Citation70] conducted an investigation using different extracts on the parts of A. muricata such as the root bark, stem-bark, fruit-pulp and leaf for their potential to impede the activity of pancreatic tissue and plasma amylase in albino Wistar male rats. For the methanolic extract at various dosages of 0–800 mg/kg body weight, it was revealed that the fruit pulp gave a better inhibitory effect in vivo compared to the other parts, while the stem-bark demonstrated the greatest inhibitory effect in vitro. It could be associated with the high phenolic content or other constituents such as terpenes, flavonoids and alkaloids. Also, the methanolic extract of the leaf showed the highest α-glucosidase inhibitory effect compared to others. Fruit pulp provided the strongest inhibition against α-glucosidase for the ethyl acetate extract. Then, for the dichloromethane extract, the root bark provided better inhibition effect against α-glucosidase. Hence, it can be implied that the plant exhibits strong inhibitory activity and can be used for the management of diabetes mellitus. The active compound in soursop leaf possessing antidiabetic potential is tannin which has an insulin-like compound and flavonoid (particularly quercetin) which stimulates the pancreatic secretion of insulin and repairs the damage of ß-cells as observed by Ratya [Citation71]. Due to its enzyme inhibitory effect, it can slow down how quickly glucose is absorbed into the blood.
Abelmoschus esculentus fruit (Okra)
Okra, also known as ‘ladyfinger’ or ‘gumbo’, is a tropical vegetable, which belongs to the family Malvaceae. The numerous uses of its seeds, flowers, pods, stems and leaves has made it a multipurpose crop. Reports have shown that the pods consist of polyphenolic compounds, folic acid, carotene, thiamine, riboflavin, niacin, vitamin C, oxalic acid and amino acids, the seeds are mainly oligomeric catechins and flavonol derivatives, protein and oil fraction (oleic, palmitic, linoleic acids), for roots are carbohydrates and flavonol glycosides and minerals, tannins and flavonol glycosides for leaves [Citation72]. The major flavonoid in okra which was shown to be responsible for their antidiabetic activity is isoquercetin and quercetin [Citation73]. However, several reports have revealed that okra polysaccharides (especially rhamnogalacturonan) are linked to the management of diabetes, consequently possessing hypoglycemic activity [Citation74]. By boosting the absorption of glucose and effectively enhancing glucose tolerance, okra polysaccharide may be able to restore insulin signaling hence controlling diabetes [Citation75]. Yaradua et al. [Citation76] reported that okra binds to glucose and delays its absorption from the intestinal lumen due to the existence of dietary fiber in it. Also, the administration of the whole peel and seed to alloxan-induced diabetic rats markedly decreased fasting blood sugar, and this was linked to some factors associated with okra such as high viscosity, high fiber content and inhibition of intestinal enzymes. These findings were supported by Subrahmanyam et al. [Citation77] who reported a similar hypoglycemic effect of okra fruit. Hence, okra prevents the body from absorbing too much sugar during digestion due to its fiber content, which assists to stabilize the blood sugar.
Capsicum frutescens (Red pepper)
This is commonly referred to as ‘red pepper’ or ‘African chili’ from the family Solanaceae. It is extensively grown in Africa and globally as reported by Anthony et al. [Citation78]. The fruit is small, spicy and pungent and has yellow seeds. Its phytochemical screening revealed that it contains alkaloids, flavonoids, terpenoids, tannins and saponins, while the fruits contain compounds such as δ-elemene, ß-bisabolene, α-humelene, capsaicin, vanillyl-nonanamide and γ-himachalene. Of all the phytochemicals, capsaicin is the most active ingredient that has been widely researched. Capsaicin works by decreasing inflammation and activating TRPV1 (Transient receptor potential vanilloid subtype 1), which reduces insulin resistance and maintains glucose homeostasis [Citation79]. The findings of Anthony et al. [Citation78] demonstrated the addition of red pepper in the diet of diabetic alloxan-induced rats normalizes fasting sugar concentration and body weight. The extract of C. frutescens using ethanol at 5 mg/ml inhibited the essential enzymes that hydrolyze polysaccharide to glucose, i.e. α-amylase&α-glucosidase, thereby preventing too much glucose absorption in the blood as reported by Watcharachaisoponsiri et al. [Citation80]. Another author revealed the hypoglycemic impact of C. frutescens powder in rabbits [Citation81]. Also, Kim et al. [Citation82] investigated the oral administration of extract from red pepper seeds at 200 mg/kg body weight for 8 weeks and it resulted in the improvement of fasting glucose, hemoglobin (HbA1c) and insulin levels in diabetic rats. Therefore, supplementation of African chili in diabetic patient’s diets can contribute greatly to insulin regulation.
Juglans regia (Walnut)
Walnut, which belongs to the family Juglandaceae, genus Juglans, is an abundant source of bioactive components and nutrients. Moreover, there is evidence that the leaves can mitigate hyperglycemia [Citation83]. Reports have shown that walnut fruit tends to prevent non-communicable diseases (such as diabetes, cancer and cardiovascular diseases) which have been related to the existence of minerals, ω-3 fatty acids, dietary fiber, polyphenols and vitamins in walnut [Citation84]. Hosseini et al. [Citation85] conducted a human trial on patients with type 2 diabetes to ascertain the hypoglycemic effect of aqueous extract of J. regia leaf, and it was revealed that the patients showed significantly reduced levels of serum fasting HbA1c, blood glucose and insulin. Some secondary metabolites present in the methanolic extract of walnut leaf such as chlorogenic acid, 3-p-coumaroylquinic acid, trihydroxynaphthalene-hexoside, quercetin glycosides and kaempferol have been notified to be responsible for the leaf activity as revealed by Nour et al. [Citation86]. Kaempferol stimulates insulin synthesis and release from ß-cells, offering anti-diabetic benefits [Citation87]. However, Forino et al. [Citation83] reported megastigmane derivatives as the principal molecules responsible for walnut leaf’s antidiabetic effects. Furthermore, the ethanolic extract of the walnut fruit’s interior septum significantly decreased sugar levels in diabetic rats as opposed to the control, which might be due to the availability of high amount of polyphenolic compounds in it as reported by Ghiravani et al. [Citation88]. Hence, eating walnut can help to control excess weight due to its fiber content and in turn control type 2 diabetes.
Spinacia oleraceae (Spinach)
This is a leafy green vegetable belonging to the family Amaranthaceous, which originated from Asia and is currently grown in different parts of the world. It is rich in fiber, vitamins B9, K, C, A and E and minerals such as magnesium, iron and manganese. It contains pigments such as carotenoids, beta carotene and lutein [Citation89]. The methanolic extract’s phytochemical screening indicated the existence of saponins, tannins and alkaloids in it and had no antioxidant activity using DPPH free radical scavenging activity method. Saponins have anti-diabetic properties due to its ability to decrease gastric emptying, block carbohydrate digesting enzymes and stimulate insulin secretion as described by El Barky et al. [Citation90].
Upon administration of the extract at 250 mg/kg and 500 mg/kg to alloxan-induced diabetic mice, the blood glucose concentration decreased significantly [Citation91]. The abundant source of fiber in spinach prevents immediate spike in sugar level, consequently preventing the risk of diabetes.
Solanum lycopersicum (Tomato)
One of the vegetables that is most commonly consumed worldwide is the tomato. It contains some nutrients such as minerals, fiber, essential amino acids, monounsaturated fatty acids, vitamins, phytosterols, carotenoids (lycopene) [Citation92] and bioactive phenolic compounds including; kaempferol, naringenin, lutein, quercetin, caffeic, ferulic and chlorogenic acids [Citation93]. It was reported by Ali and Agha [Citation94] that lycopene present in tomatoes plays a great role in protecting the DNA against oxidative damage, thus hindering the occurrence of diseases.
The hydro-methanolic extract activity of tomato leaves studied by Figueiredo-Gonzalez et al. [Citation95] on diabetic rats induced with streptozotocin revealed that it hindered the key carbohydrate hydrolyzing enzyme (α-glucosidase and α-amylase) in a concentration-dependent approach due to the existence of rutin and chlorogenic acid which are the predominant compounds in the extract. Reports have shown that rutin decreases the absorption of carbohydrate from the small intestine and activates secretion of insulin from the ß-cells, while chlorogenic acid has the ability to reduce insulin resistance and increase insulin sensitivity, thus providing anti-diabetic impact. The same inhibitory effect was exhibited in snake tomato as demonstrated by Ademosun et al. [Citation96]. Therefore, it can be inferred that the consumption of tomato assists in banning the risk of diabetes due to the presence of lycopene.
Curcuma longa (Turmeric)
Curcuma longa belonging to the family Zingiberaceae, genus Curcuma, is grown in various regions of the world and is recognized for its medicinal value. In India, it is referred to as ‘golden spice’ [Citation97]. The major bioactive components of turmeric rhizome were reported to be curcuminoids and turmerin. Both have been shown to exhibit a high tendency to hinder α-amylase and α-glucosidase, thus retarding the absorption of glucose and suppressing postprandial hyperglycemia [Citation98]. The presence of curcumin in turmeric helps to keep the blood sugar levels steady.
Conclusion
At the long run, diabetes leads to multiple complications and most of the available synthetic drugs are effective for controlling it, but they are associated with serious side effects such as liver disorders, diarrhea, etc. However, there is an increasing attention to the use of horticultural crops with little or no side effects. These crops have the tendency to prevent hyperglycemic effects due to their bioactive compounds, they restore the pancreatic tissue by increasing its insulin output or reducing glucose absorption. Furthermore, they elicit their anti-hyperglycemic activity through the inhibition of carbohydrate hydrolyzing enzymes (α-amylase & α-glucosidase). More awareness should be done to educate people on the health benefits of these horticultural crops in order to mitigate diseases. Besides, dietician have to exert great efforts to prepare functional food products that contain extracts of these natural horticultural crops and make it readily available in the market.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Oboh G, Ademiluyi AO, Akinyemi AJ, et al. Inhibitory effect of polyphenol-rich extracts of jute leaf (Corchorus olitorius) on key enzyme linked to type 2 diabetes (α-amylase and α-glucosidase) and hypertension (angiotensin I converting) in vitro. J Funct Foods. 2012;4(2):450–458.
- Srinivasan S, Vinothkumar V, Murali R. Antidiabetic efficacy of citrus fruits with special allusion to flavone glycosides bioactive food as dietary interventions for diabetes. 2nd ed. Cambridge, Massachusetts: Elsevier Academic Press; 2019pp. 335–346.
- Preethi PJ. Herbal medicine for diabetes mellitus: a Review. Asian J Pharmaceutical Res. 2013;3(2):57–70.
- Alhassan AJ, Lawal TA, Dangambo M. Antidiabetic properties of thirteen local medicinal plants in Nigeria, a review. World J Pharma Res. 2017;6(8):2170–2189.
- USDA. (2020). Citrus: World Markets and Trade.
- Mallick N, Khan RA. Effect of Citrus paradisi and Citrus sinensis on glycemic control in rats. Afr J Pharm Pharmacol. 2015;9(3):60–64.
- Mahmoud AM, Ashour MB, Abdel-Moneim A, et al. Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats. J Diabetes Complications. 2012;26(6):483–490.
- Tavafi M, Ahmadvand H, Tamjidipoor A, et al. Satureja khozestanica essential oil ameliorates progression of diabetic nephropathy in uninephrectomized diabetic rats. Tissue Cell. 2011;43(1):45–51.
- Kim G-N, Shin J-G, Jang H-D. Antioxidant and antidiabetic activity of Dangyuja (Citrus grandis Osbeck) extract treated with Aspergillus saitoi. Food Chem. 2009;117(1):35–41.
- Osuntokun OT, Olumekun V, Ajayi A, et al. Assessment of in-vitro antioxidant/enzymes inhibitory potentials of aframomum melegueta [Roscoe] K. Schum (grains of paradise) leaf, stem bark, seed bark and seed extracts. Archiv Curr Res Int. 2020;20(2):40–57.
- Al Malki WH, Abdel-Raheem IT, Dawoud MZ, et al. 6-shogaol protects against diabetic nephropathy and cardiomyopathy via modulation of oxidative stress/NF-κB pathway. Pak J Pharm Sci. 2018;31(5):2109–2117.
- Ajayi A, Osuntokun O, Olumekun V, et al. Systemic evaluation of anti-diabetics, anti-Inflammatory and secondary metabolite potentials of Aframomum melegueta [Roscoe] K. South Asian J Parasitol. 2022;6(4):66–82.
- Kamtchouing P, Mbongue G, Dimo T, et al. Effects of Aframomum melegueta and Piper guineense on sexual behaviour of male rats. Behav Pharmacol. 2002;13(3):243–247.
- Ilic N, Schmidt BM, Poulev A, et al. Toxicological evaluation of grains of paradise (Aframomum melegueta)[Roscoe] K. J Ethnopharmacol. 2010;127(2):352–356.
- Nosiri C, Okereke S, Anyanwu C, et al. Responses of liver and pancreatic cells to ethanolic seed extract of Aframomum melegueta in alloxan-induced diabetic rats. J Med Plant Res. 2016;4(5):112–116.
- Umoh I, Samuel O, Kureh T, et al. Antidiabetic and hypolipidaemic potentials of ethanol fruit pulp extract of Persea americana (avocado pear) in rats. J Afr Assos Physiol Sci. 2019;7(1):59–63.
- Ebifa JO, Elechi-Amadi H, Abiakam H, et al. Comparative effects of Carica papaya, avocado pear and ginger extracts on the histological structure of the pancreas of streptozotocin-induced diabetic rats. Asian J Med Principles Clin Pract. 2021;4(4):1–11.
- Alhassan A, Sule M, Atiku M, et al. Effects of aqueous avocado pear (Persea americana) seed extract on alloxan induced diabetes rats. Greener J Med Sci. 2012;2(1):5–11.
- Sunday A, Uzoma K. Hypoglycemic, hypolipidemic and body weight effects of unripe pulp of Carica papaya using diabetic Albino rat model. J Pharmacogn Phytochem. 2014;2(6):109–114.
- Ikeyi A, Ogbonna A, Eze F. Phytochemical analysis of paw-paw (Carica papaya) leaves. Int J Life Sci Biotechnol Pharma Res. 2013;2(3):347–351.
- Saleem M, Tanvir M, Akhtar MF, et al. Antidiabetic potential of Mangifera indica L. cv. Anwar Ratol leaves: medicinal application of food wastes. Medicina. 2019;55(7):353–361.
- Apea OB, Faruq UZ. Antidiabetic activity of pawpaw leaf extract: chemical composition and biokinetic modeling. J Sci Innovat Dev. 2013;1(2):13–21.
- Ogundele AV, Otun KO, Ajiboye A, et al. Anti-diabetic efficacy and phytochemical screening of methanolic leaf extract of pawpaw (Carica papaya) grown in North Central Nigeria. J Turk Chem Soc A Chem. 2017;4(1):99–114.
- Mao Q-Q, Xu X-Y, Cao S-Y, et al. Bioactive compounds and bioactivities of ginger (Zingiber officinale Roscoe). Foods. 2019;8(6):185.
- Son MJ, Miura Y, Yagasaki K. Mechanisms for antidiabetic effect of gingerol in cultured cells and obese diabetic model mice. Cytotechnology. 2015;67(4):641–652.
- Abdulrazaq NB, Cho MM, Win NN, et al. Beneficial effects of ginger (Zingiber officinale) on carbohydrate metabolism in streptozotocin-induced diabetic rats. British Journal Of Nutrition. 2012;108(7):1194–1201.
- Khandouzi N, Shidfar F, Rajab A, et al. The effects of ginger on fasting blood sugar, hemoglobin A1c, apolipoprotein B, apolipoprotein A-I and malondialdehyde in type 2 diabetic patients. Iranian J Pharm Res. 2015;14(1):131–140.
- Ojewole JA. Analgesic, antiinflammatory and hypoglycaemic effects of ethanol extract of Zingiber officinale (Roscoe) rhizomes (Zingiberaceae) in mice and rats. Phytother Res. 2006;20(9):764–772.
- Nwaoguikpe RN. The effect of extract of bitter leaf (Vernonia amygdalina) on blood glucose levels of diabetic rats. Int J Biol Chem Sci. 2010;4(3):721–729.
- Mazumder U, Gupta M, Manikandan L, et al. Evaluation of anti-inflammatory activity of Vernonia cinerea Less. extract in rats. Phytomedicine. 2003;10(2–3):185–188.
- Saliu J, Ademiluyi A, Akinyemi A, et al. In vitro antidiabetes and antihypertension properties of phenolic extracts from bitter leaf (Vernonia amygdalina Del.). J Food Biochem. 2012;36(5):569–576.
- Adefegha A, Oboh G, Akinyemi A, et al. Inhibitory effects of aqueous extract of two varieties of ginger on some key enzymes linked to type-2 diabetes in vitro. J Food Nutr Res. 2010;49(1):14–20.
- Reddy J, Hemachandran J. Comparative evaluation of the antidiabetic and hypoglycaemic potentials of the parts Musa paradisiaca plant extracts. Int J Sci Res. 2014;4:1–5.
- Shanmuga SC, Subramanian S. Biochemical evaluation of hypoglycemic activity of Musa paradisiaca (plantain) flowers in STZ-induced experimental diabetes in rats. Asian J Res Chem. 2011;4(5):827–833.
- Vilhena RO, Figueiredo ID, Baviera AM et al. Antidiabetic activity of Musa x paradisiaca extracts in streptozotocin-induced diabetic rats and chemical characterization by HPLC-DAD-MS. J Ethnopharmacol. 2020;254:112666.
- Shodehinde SA, Ademiluyi AO, Oboh G, et al. Contribution of Musa paradisiaca in the inhibition of α-amylase, α-glucosidase and Angiotensin-I converting enzyme in streptozotocin induced rats. Life Sci. 2015;133:8–14.
- Kappel VD, Cazarolli LH, Pereira DF, et al. Beneficial effects of banana leaves (Musa x paradisiaca) on glucose homeostasis: multiple sites of action. Revista Brasileira de Farmacognosia. 2013;23(4):706–715.
- Ganogpichayagrai A, Palanuvej C, Ruangrungsi N. Antidiabetic and anticancer activities of Mangifera indica cv. Okrong leaves. J Adv Pharm Technol Res. 2017;8(1):19–24.
- Pino JA, Mesa J, Muñoz Y, et al. Volatile components from mango (Mangifera indica L.) cultivars. J Agric Food Chemistry. 2005;53(6):2213–2223.
- Bhowmik A, Khan LA, Akhter M, et al. Studies on the antidiabetic effects of Mangifera indica stem-barks and leaves on nondiabetic, type 1 and 2 diabetic model rats. Bangladesh J Pharmacol. 2009;4(2):110–114.
- Gondi M, Basha SA, Bhaskar JJ, et al. Anti‐diabetic effect of dietary mango (Mangifera indica L.) peel in streptozotocin‐induced diabetic rats. J Sci Food Agric. 2015;95(5):991–999.
- Azhar A, Aamir K, Asad F, et al. Therapeutic effect of mango seed extract in diabetes mellitus. Professional Med J. 2019;26(9):1551–1556.
- Vijayakumar K, Prasanna B, Rengarajan R, et al. Anti-diabetic and hypolipidemic effects of Cinnamon cassia bark extracts: an in vitro, in vivo, and in silico approach. Arch Physiol Biochem. 2020;129(2):338–348.
- Singh P, Jayaramaiah RH, Agawane SB, et al. Potential dual role of eugenol in inhibiting advanced glycation end products in diabetes: proteomic and mechanistic insights. Sci Rep. 2016;6(1):18798–18810.
- Kim SH, Hyun SH, Choung SY. Anti-diabetic effect of cinnamon extract on blood glucose in db/db mice. J Ethnopharmacol. 2006;104(1–2):119–123.
- Khan A, Safdar M, Ali Khan MM, et al. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care. 2003;26(12):3215–3218.
- Association AD. Economic costs of diabetes in the US in 2002. Diabetes Care. 2003;26(3):917–932.
- Huttada L, Hiremath M, D’Souza N. Enhancing the activity of peroxisome proliferator-activated receptor’s (PPAR) activity through natural ligand binding in diabetes: substantial computational approach. Nat Prod Chem Res. 2016;4(3):2–7.
- Ogunyinka BI, Oyinloye BE, Osunsanmi FO, et al. Comparative study on proximate, functional, mineral, and antinutrient composition of fermented, defatted, and protein isolate of Parkia biglobosa seed. Food Sci Nutri. 2017;5(1):139–147.
- Gloria NE, Babajide OE, Olufunmilola OO, et al. Comprehensive investigation into the nutritional composition of dehulled and defatted African locust bean seed (Parkia biglobosa). Afr J Plant Sci. 2011;5(5):291–295.
- Oyedemi SO, Eze K, Aiyegoro OA, et al. Computational, chemical profiling and biochemical evaluation of antidiabetic potential of Parkia biglobosa stem bark extract in type 2 model of rats. J Biomol Struct Dynamics. 2022;40(20):9948–9961.
- Ibrahim MA, Habila JD, Koorbanally NA, et al. Butanol fraction of Parkia biglobosa (Jacq.) G. Don leaves enhance pancreatic β-cell functions, stimulates insulin secretion and ameliorates other type 2 diabetes-associated complications in rats. J Ethnopharmacol. 2016;183:103–111.
- Odetola A, Akinloye O, Egunjobi C, et al. Possible antidiabetic and antihyperlipidaemic effect of fermented parkia biglobosa (Jacq) extract in alloxan‐induced diabetic rats. Clin Exp Pharmacol Physiol. 2006;33(9):808–812.
- Ajebli M, Khan H, Eddouks M. Natural alkaloids and diabetes mellitus: a review. Endocr Metab Immune Disord Drug. 2021;21(1):111–130.
- Tshabalala T, Ndhlala A, Ncube B, et al. Potential substitution of the root with the leaf in the use of Moringa oleifera for antimicrobial, antidiabetic and antioxidant properties. South Afri J Bot. 2020;129:106–112.
- Ashfaq M, Basra SM, Ashfaq U. Moringa: a miracle plant for agro-forestry. J Agric Soc Sci. 2012;8(3):115–122.
- Popoola JO, Obembe OO. Local knowledge, use pattern and geographical distribution of Moringa oleifera Lam.(Moringaceae) in Nigeria. J Ethnopharmacol. 2013;150(2):682–691.
- Anwar F, Latif S, Ashraf M, et al. Moringa oleifera: a food plant with multiple medicinal uses. Phytother Res. 2007;21(1):17–25.
- Raj AJ, Gopalakrishnan VK, Yadav SA, et al. Antimicrobial activity of Moringa oleifera (Lam.) root extract. J Pharm Res. 2011;4(5):1426–1427.
- Leone A, Spada A, Battezzati A, et al. Cultivation, genetic, ethnopharmacology, phytochemistry and pharmacology of Moringa oleifera leaves: an overview. Int J Mol Sci. 2015;16(6):12791–12835.
- Al-Ishaq RK, Abotaleb M, Kubatka P, et al. Flavonoids and their anti-diabetic effects: cellular mechanisms and effects to improve blood sugar levels. Biomolecules. 2019;9(9):430.
- Bamagous GA, Al Ghamdi SS, Ibrahim IAA, et al. Antidiabetic and antioxidant activity of ethyl acetate extract fraction of Moringa oleifera leaves in streptozotocin-induced diabetes rats via inhibition of inflammatory mediators. Asian Pac J Tropical Biomedicine. 2018;8(6):320.
- Omodanisi EI, Aboua YG, Oguntibeju OO. Assessment of the anti-hyperglycaemic, anti-inflammatory and antioxidant activities of the methanol extract of Moringa oleifera in diabetes-induced nephrotoxic male Wistar rats. Molecules. 2017;22(4):439.
- Jaiswal D, Rai PK, Kumar A, et al. Effect of Moringa oleifera Lam. leaves aqueous extract therapy on hyperglycemic rats. J Ethnopharmacol. 2009;123(3):392–396.
- Al-Malki AL, El Rabey HA. The antidiabetic effect of low doses of Moringa oleifera Lam. seeds on streptozotocin induced diabetes and diabetic nephropathy in male rats. Bio Med Res Int. 2015;1–13. DOI:10.1155/2015/381040
- Ali MM, Asrafuzzaman M, Tusher M, et al. Comparative study on antidiabetic effect of ethanolic extract of jute leaf on neonatal streptozotocin-induced type-2 diabetic model rat. J Pharm Res Int. 2020;32(31):60–71.
- Omeje KO, Ezike TC, Omeje HC, et al. Effect of ethanol extract of Corchorus olitorus leaf on glucose level and antioxidant enzymes of Streptozotocin-induced hyperglycemic rat. Biokemistri. 2016;28(3):121–127.
- Chigurupati S, Aladhadh HS, Alhowail A, et al. Phytochemical composition, antioxidant and antidiabetic potential of methanolic extract from Corchorus olitorius Linn. grown in Saudi Arabia. Med Plants Int J Phytomed Relat Ind. 2020;12(1):71–76.
- Mohammed A, Luka C, Ngwen A, et al. Evaluation of the effect of aqueous leaf extract of jute mallow Corchorus olitorius on some biochemical parameters in alloxan-induced diabetic rats. Eur J Pharm Med Res. 2019;6(10):652–658.
- Agu KC, Eluehike N, Ofeimun RO, et al. Possible anti-diabetic potentials of Annona muricata (soursop): inhibition of α-amylase and α-glucosidase activities. Clin Phytoscience. 2019;5(1):1–13.
- Ratya A. Antidiabetic potential of soursop leaf extract (Annona muricata L.) as a treatment for type 2 diabetes mellitus. J Agromed. 2014;1(1):61–66.
- Yonas M, Garedew W, Debela A. Multivariate analysis among okra (Abelmoschus esculentus (L.) Moench) collection in South Western Ethiopia. J Plant Sci. 2014;9(2):43–50.
- Durazzo A, Lucarini M, Novellino E, et al. Abelmoschus esculentus (L.): bioactive components’ beneficial properties—focused on antidiabetic role—for sustainable health applications. Molecules. 2018;24(1):38–50.
- Liu J, Zhao Y, Wu Q, et al. Structure characterisation of polysaccharides in vegetable “okra” and evaluation of hypoglycemic activity. Food Chem. 2018;242:211–216.
- Liao Z, Zhang J, Liu B, et al. Polysaccharide from okra (Abelmoschus esculentus (L.) Moench) improves antioxidant capacity via PI3K/AKT pathways and Nrf2 translocation in a type 2 diabetes model. Molecules. 2019;24(10):1906.
- Yaradua I, Ibrahim M, Matazu K, et al. Antidiabetic activity of Abelmoschus esculentus (Ex-Maradi Okra) fruit in alloxan-induced diabetic rats. Niger J Biochem Mol Biol. 2017;32(1):44–52.
- Subrahmanyam G, Sushma M, Alekya A, et al. Antidiabetic activity of Abelmoschus esculentus fruit extract. Int J Res Pharm Chem. 2011;1:17–20.
- Anthony OE, Ese AC, Lawrence EO. Regulated effects of Capsicum frutescens supplemented diet (C.F.S.D) on fasting blood glucose level, biochemical parameters and body weight in alloxan induced diabetic Wistar rats. Br J Pharm Res. 2013;3(3):496–507.
- Jeszka-Skowron M, Zgoła-Grześkowiak A, Grześkowiak T, et al. Analytical methods in the determination of bioactive compounds and elements in food. New York City: Springer; 2021.
- Watcharachaisoponsiri T, Sornchan P, Charoenkiatkul S, et al. The α-glucosidase and α-amylase inhibitory activity from different chili pepper extracts. Int Food Res J. 2016;23(4):1439–1445.
- Dougnon TJ, Gbeassor M. Evaluation of the effects of the powder of Capsicum frutescens on glycemia in growing rabbits. Vet World. 2016;9(3):281–286.
- Kim HK, Jeong J, Kang EY, et al. Red pepper (Capsicum annuum L.) seed extract improves glycemic control by inhibiting hepatic gluconeogenesis via phosphorylation of FOXO1 and AMPK in obese diabetic db/db mice. Nutrients. 2020;12(9):2546.
- Forino M, Stiuso P, Lama S, et al. Bioassay-guided identification of the antihyperglycaemic constituents of walnut (Juglans regia) leaves. J Funct Foods. 2016;26:731–738.
- Hardman WE. Walnuts have potential for cancer prevention and treatment in mice. J Nutr. 2014;144(4):555S–560S.
- Hosseini S, Huseini HF, Larijani B, et al. The hypoglycemic effect of Juglans regia leaves aqueous extract in diabetic patients: a first human trial. DARU J Pharma Sci. 2014;22:1–5.
- Nour V, Trandafir I, Cosmulescu S. HPLC determination of phenolic acids, flavonoids and juglone in walnut leaves. J Chromatogr Sci. 2013;51(9):883–890.
- Zhang Z, Ding Y, Dai X, et al. Epigallocatechin-3-gallate protects pro-inflammatory cytokine induced injuries in insulin-producing cells through the mitochondrial pathway. Eur J Pharmacol. 2011;670(1):311–316.
- Ghiravani Z, Hosseini M, Taheri MMH, et al. Evaluation of hypoglycemic and hypolipidemic effects of internal septum of walnut fruit in alloxan-induced diabetic rats. Afr J Traditional Complementary Altern Med. 2016;13(2):94–100.
- Hussain F, Bashir S, Bashir S. Antioxidant, antidiabetic and structural analysis of Spinacia oleracea leaf. Pakistan J Biochem Biotechnol. 2022;3(1):1–11.
- El Barky AR, Hussein S, Alm-Eldeen A, et al. Saponins and their potential role in diabetes mellitus. Diabetes Manag. 2017;7(1):148–158.
- Shaheen SM, Ohidul I, Azad K, et al. Phytochemical profiling and evaluation of antioxidant and antidiabetic activity of methanol extract of spinach (Spinacia oleracea L.) leaves. Int J Pharm Sci Scient Res. 2018;4(1):24–27.
- Elbadrawy E, Sello A. Evaluation of nutritional value and antioxidant activity of tomato peel extracts. Arabian J Chem. 2016;9:Supp. 2 S1010–S1018.
- Navarro-González I, García-Alonso J, Periago MJ. Bioactive compounds of tomato: cancer chemopreventive effects and influence on the transcriptome in hepatocytes. J Funct Foods. 2018;42:271–280.
- Ali MM, Agha FG. Amelioration of streptozotocin‐induced diabetes mellitus, oxidative stress and dyslipidemia in rats by tomato extract lycopene. Scand J Clin Lab Invest. 2009;69(3):371–379.
- Figueiredo-Gonzalez M, Valentao P, Andrade PB. Tomato plant leaves: from by-products to the management of enzymes in chronic diseases. Ind Crops Prod. 2016;94:621–629.
- Ademosun O, Oboh G, Adewuni T, et al. Antioxidative properties and inhibition of key enzymes linked to type-2 diabetes by snake tomato (Tricosanthes cucumerina) and two tomato (Lycopersicon esculentum) varieties. Afr J Pharm Pharmacol. 2013;7(33):2358–2365.
- Razzaq PA, Iftikhar M, Faiz A, et al. A comprehensive review on antidiabetic properties of turmeric. Life Sci J. 2020;17(10):26–39.
- Lekshmi P, Arimboor R, Raghu K, et al. Turmerin, the antioxidant protein from turmeric (Curcuma longa) exhibits antihyperglycaemic effects. Nat Prod Res. 2012;26(17):1654–1658.