ABSTRACT
Pancreatitis has the potential to occur with increasing cases of hyperlipidemia and alcohol consumption. Meanwhile, until now pharmacotherapy for pancreatitis is more emphasized for pain relief and lowering triglyceride levels (for HFD-induced pancreatitis). The polyphenol (quercetin) has several biological activities, including anti-cancer, antiallergic, anti-inflammatory and ant-diabetic. This study aimed to evaluate the potential effect of quercetin to treat pancreatitis. After verification, 19 relevant articles were included in the analysis, where Linear mix model analysis was applied to parameters including blood glucose, pancreas islet number, insulin level, TBARS level, nuclear factor kappa B (NF-κB), endogenous antioxidant (GSH, SOD, CAT, GPx), TNFα, IL−6, Caspase 3 (Casp3) and IL−1b gene expression. The level quercetin was used as fixed effect and study setting as random effect. The administration of quercetin was able to significantly increase the production of tissue insulin, restore the antioxidant defense system (increase SOD, GSH, decrease TBARS), and inhibit the progression of inflammation based on the expression of NF-kB, TNFα and IL−6 genes. Therefore, the results of meta-analysis support the hypothesis that quercetin is able to restore pancreatic function, and has the potential to be developed to treat pancreatitis.
Introduction
Hyperlipidemia is defined as an increase in plasma cholesterol, triglycerides, or both [Citation1,Citation2]. Hypertriglyceridemia has been reported to be positively correlated with cases of CVD and pancreatitis [Citation3]. Oxidative stress conditions on beta cells and changes in plasma viscosity by triglycerides are mentioned as triggers for pancreatic cell damage, ranging from inflammation to necrosis [Citation4,Citation5]. In the case of pancreatic cells, insulin secretion is impaired by the accumulation of free fatty acid (FFA), which can also lead to apoptosis [Citation6]. Therefore, treatment of damaged pancreatic cells is needed, especially to reduce the dependence of diabetic patients on insulin therapy.
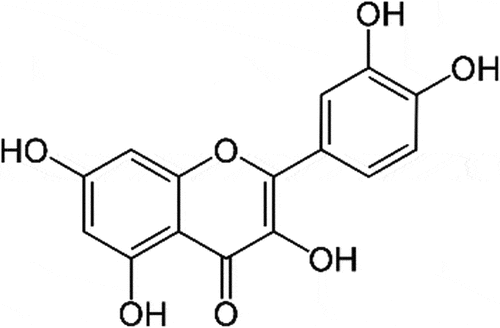
A number of natural compounds that have lipid lowering activity include flavonoids, coumarins, saponins, oligosaccharides, alkaloids, and organo sulfur [Citation7,Citation8]. One of the major members of flavonoids is quercetin (). Quercetin is often used as an equivalence standard in measuring total flavonoid levels although its oral absorption is classified as poor [Citation9,Citation10]. Quercetin is said to be able to overcome dyslipidemia by increasing the expression of the scavenger receptor, class B type 1 (SR-B1), as a multifunctional receptor for cholesterol influx and efflux. It also activates the Peroxisome proliferator-activated receptor pathway (PPARγ-LXRα), and upregulating the expression of p38-dependent Sp1 [Citation11].
In addition, quercetin has been explored for its activities including antioxidant, hepatoprotective, anti-inflammatory, antifungal, cytotoxic and anti-carcinogenic [Citation12]. The antioxidant activity of quercetin is closely related to the presence of hydroxyl groups at carbon numbers 3,5,7,3’, and 4’. The anti-inflammatory effects of quercetin have been reported through the down regulation of tumor necrosis factor alfa (TNFα), inflammatory enzymes (cyclo-oxygenase COX and lipo-oxygenase LOX), and inflammatory cytokines (interleukin IL−6, IL−1b, IL−1a). Quercetin up-regulates interferon gamma (IFN-γ) expression. Meanwhile, the anti-cancer activity of quercetin is said to be correlated with its antioxidant activity. Cancerous cells treated with quercetin were shown to decrease malondialdehyde (MDA), lipid peroxidation (LPO), increase in superoxide dismutase (SOD), glutathione (GSH) and catalase (CAT) as cancer cell apoptosis increased.
Since hypertriglyceridemia could cause pancreatitis and quercetin have triglyceride lowering and anti-inflammatory activity, this meta-analysis study aimed to provide evaluation of protective action of quercetin toward acute and chronic pancreatic damage based on scientific evidences of previously published researches.
Materials and methods
Search strategy
A meta-analysis of published studies reporting about the protective effect of quercetin on pancreatic damage was conducted in accordance with the Preferred Reporting Items for systematic reviews and Meta-Analyses (PRISMA) [Citation13] as shown in . The search for articles reporting experimental studies of the effects of quercetin on the pancreas was facilitated by the Publish Or Perish software [Citation14], a freeware to conduct article search from various scientific databases.
Figure 2. Selection and evaluation of articles related to quercetin effect on pancreatic damage according to PRISMA. **Indicate how many records were excluded by automation tools.
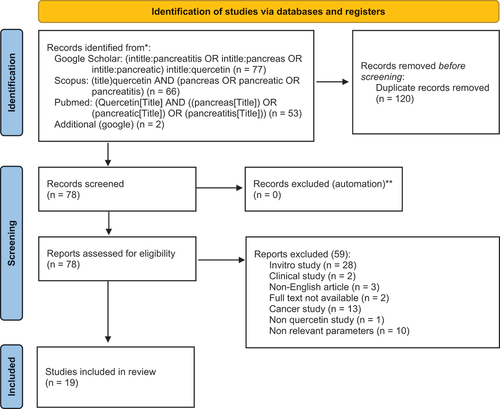
Literature searching was conducted from 10 June 2022 to 1 July 2022 on: PubMed, Google Scholar and Scopus databases. The following terms were used in the search for the article’s title: (quercetin AND (pancreatitis OR pancreatic OR pancreas)). The search was narrowed to only the titles of articles containing these terms and did not include citations or patents. There were no restrictions on the type of publication, sample size, study design, and methods of measuring exposure or outcomes.
Eligibility criteria
The articles obtained were then screened with validity benchmarks including: (1) In vivo test using rats or mice; (2) The samples tested are pure quercetin or extracts containing quercetin; (3) Measuring at least 1 physical or chemical parameter of pancreas; (4) Induction of animal model showed pancreatic damage in the control group; and (5) Not an anti-cancer study. Furthermore, the exclusion factor was also applied to articles that were: not in English language, the full text was not found, was a clinical trial, only an in vitro/ex vivo study, or was a review article. Articles that did not include a control group were also excluded in the study.
Data collection and exclusion
Data extraction includes first author’s last name, year of publication, kind and dose of inducer, duration of induction, study animal species, subject number, animal sex, route of administration, and dose of quercetin as well as physical and chemical parameters related to pancreas. After a more in-depth study, 19 articles were included in this meta-analysis study (). The parameters related to the pancreas that were reported included: (1) serum parameters (glucose, insulin, glucagon); (2) pancreatic tissue parameters (pancreas weight, pancreas islet number, insulin, thiobarbituric acid reactive species (TBARS), advanced glycation end products (AGE), nuclear factor kappa B (NF-κB), GSH, SOD, CAT, glutathione peroxidase (GPx), glucose transporter 2 and 4 (GLUT2/4), glucokinase, hexokinase, TNFα, IL−6, Caspase 3 (Casp3) and IL−1b gene expression).
Table 1. Collected studies included into the meta-analysis.
Quality assesment
The quality of study and risk of bias was assessed using the SYRCLE tool for animal studies [Citation34]. The risks of bias were declared as low, unclear, and high risk [Citation35]. The instrument addresses six biases: attrition bias, election bias, reporting bias, performance bias, detection bias, and other sources of bias.
Statistical analysis
The meta-analysis was conducted using a linear mixed model approach [Citation36]. The analysis was carried out using R software (lme4, lmerTest package) version 4.2.0 [Citation37]. The level of quercetin was considered as a fixed effect, while variation in the research setting was considered as a random effect. Mathematically, the regression equation was analyzed according to order 1 and order 2. Mixed model formula for each pancreas parameter (y):
st order = lmer(y ~ level_of_quercetin + (1|author), dataset)
nd order = lmer(y ~ (level_of_quercetin + I(level_of_quercetin^2)) + (1|author), dataset)
Model statistics used were p-value and root mean square error (RMSE). The significance of an effect was considered when p < 0.05.
Results
Quality and risk of bias
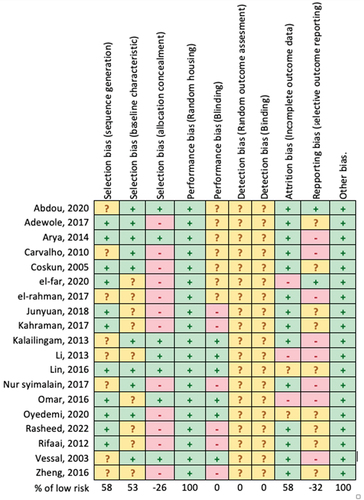
The analysis of the risk of bias presented in shows that all articles have a low risk of bias in terms of selection bias (sequence generation, baseline characteristic), performance bias (random housing), and other bias. However, several articles do show the potential for high risk of bias, especially in terms of allocation concealment, blinding, and reporting [Citation16,Citation18–23,Citation27,Citation29–31,Citation33]. In these studies, induction was carried out after grouping the animals. In addition, several studies have reported only a few parameters related to the physiological function of the pancreas.
Quercetin effect on glucose metabolism
The administration of quercetin to animal models with pancreatic tissue damage showed improvement in glucose metabolism (). Quercetin was able to significantly reduce serum glucose levels (p = 0.0057). The pattern of decline appears to follow a quadratic curve with the optimum effect achieved at a dose of 55.4 mg/kgBW/day. Meanwhile, the production of insulin in pancreatic beta cells showed a significant increase (p = 0.0292). However, the release of insulin from beta cells into the systemic circulation did not appear to increase significantly (p = 0.2177).
Table 2. Effect of quercetin administration on status of pancreatic damage in animal model.
Quercetin effect on antioxidant status of pancreatic tissue
As shown in , the administration of quercetin improved the antioxidant status of pancreatic tissue. The levels of the enzymes superoxide dismutase (SOD) (p = 0.0176) and glutathione (GSH) (p = 0.0085) increased significantly. However, the levels of catalase (CAT) (p = 0.1011) and glutathione peroxidase (GPx) (p = 0.3521) were not significantly increased. Quercetin was also shown to significantly inhibit the production of thiobarbituric acid reactive species (TBARS) (p = 0.0116), a product of the lipid peroxidation process.
Quercetin effect on cellular pancreatic cells damage
Cellularly, the administration of quercetin was shown to be able to significantly reduce the production of TNFα (p = 0.0050), NF-κB (p = 0.0068) as well as the level of necrosis (p = 0.0301) (). Casp3 expression (p = 0.3921) appeared to decrease, while BCl2 (p = 0.4039) and pancreatic islet number (p = 0.1037) appeared to increase although not significant. The expression of the IL−6 cytokine gene appeared to be significantly decreased (p = 0.0016) while the IL−1b gene was not significant (p = 0.3898). Meanwhile, quercetin did not appear to significantly improve the status of pancreatic edema based on the parameters of pancreatic relative weight (p = 0.2689) and pancreatic vacuolation (p = 0.1007).
Discussion
Pancreatitis is an inflammatory condition of the pancreas characterized by fever, increased heart rate, nausea and abdominal pain. Other pathological signs are elevated pancreatic enzymes in blood plasma, pancreatic tissue edema, necrosis of acinar cells and hemorrhage [Citation5]. There are two types of pancreatitis: acute and chronic [Citation33]. Generally, acute pancreatitis is caused by infection, trauma, gallstones, alcohol consumption and metabolic disorders. While the chronic type is mostly caused by hypertriglyceridemia, alcohol, cystic fibrosis, and hereditary history [Citation38].
The mechanism of pancreatitis caused by hypertriglyceridemia is still not clearly understood. Until now, it is estimated that the increase of triglyceride (VLDL and chylomicron) levels will increase plasma viscosity so that it triggers ischemia in pancreatic tissue. Ischemic conditions accompanied by high free fatty acids (FFA) will activate trypsinogen to stimulate pancreatic inflammation [Citation39].
Pharmacotherapy to treat hypertriglyceridemia-induced pancreatitis (dietary changes, insulin therapy, apheresis, heparin therapy, fibrates, antisense Apo-B) is almost entirely aimed at lowering plasma triglyceride (TG) levels by increasing lipoprotein lipase (LPL) activity [Citation4]. To the best of the authors’ knowledge, there are no drugs used to modulate tissue to repair itself or promote cell survival.
According to previous review, quercetin has various pharmacological activities. Most of its activity is related to its antioxidant property [Citation12]. Abdou et.al [Citation15] reported that quercetin from Trifolium alexandrinum was able to modulate pancreatic function in diabetic animal models. Another study reported that quercetin was able to overcome ferroptosis in pancreatic beta cells due to iron accumulation in animal models of diabetes [Citation40]. Quercetin which was supplemented into the diet was able to restore shrunken mitochondrial beta cells back to normal in T2DM animals. Meanwhile, quercetin has been widely reported to affect triglyceride level [Citation15,Citation32,Citation41–44].
In this meta-analysis, a total of 19 articles were included in this study. Publications that tested quercetin in the form of glycosides were excluded from this study because the authors limited the study to quercetin alone without a glycone group due to the wide variety of quercetin glycosylation and so as not to lead to a QSAR study (Quantitative structure-activity relationship).
From the results of statistical analysis, it is known that the administration of quercetin, up to a dose of 55 mg/kgBW/day, could reduce serum glucose linearly. At doses above 55 mg/kgBW glucose lowering activity did not increase with increasing dose (quadratic relation; y = 295.129–6.098× + 0.055x2; p = 0.0057). In line with the meta-analysis of a previous review regarding the anti-diabetic effect of quercetin [Citation45], it was stated that up to a dose of 50 mg/kgBW the glucose lowering activity of quercetin was strongly correlated with the dose administered. The activity of quercetin as anti-diabetic seems to be more effective at low doses than high doses as reported by Henagan, et al. [Citation46].
Insulin production in pancreatic beta cells was seen to increase significantly with increasing quercetin dose (up to 100 mg/kgBW/day) (p = 0.0292). This implies that the dysfunction of beta cells as insulin producers was normalized by the administration of quercetin. Interestingly, the level of insulin release into the systemic circulation did not change significantly (p = 0.2177) with increasing doses. This strengthens the evidence that quercetin can increase insulin receptor sensitivity [Citation47]. Insulin receptors in peripheral tissues are said to be more sensitive and mitochondrial biogenesis activity is also significantly increased.
Moreover, quercetin is a chemical compound with high antioxidant activity. The antioxidant activity of quercetin is known to be highly dependent on the presence of a hydroxyl group at carbon number 3,3’,4’ and the C2-C3 double-bond [Citation10,Citation48]. Rusmana et al. [Citation49] compared the antioxidant activity of quercetin against rutin (a 3-O-glycosilated quercetin) against the radicals DPPH, ABTS, and FRAP. DPPH and ABTS bleaching decreased dramatically in the presence of glycosyl. IC50 DPPH rutin vs quercetin (5.56 vs 0.55 µg/mL), IC50 ABTS (17.16 vs 1.17 µg/mL), ferrous formation FRAP (23.65 vs 211.5 µM Fe2+/µg at 1.56 µg/mL specimen).
Pancreatic damage, both acute and chronic, is mediated by reactive oxygen species (ROS) toxicity [Citation30,Citation50–52]. Streptozotocin (STZ) triggers ROS production by activating xanthine oxidase and DNA alkylation [Citation52]. High fat diet induces pancreatic damage through lipotoxicity mechanisms, de novo ceramide synthesis which triggers endoplasmic reticulum stress and mitochondrial dysfunction [Citation30,Citation51]. Fructose and glucose promote ROS production by increasing NADPH, an electron donor, on the outer mitochondrial wall. Meanwhile, cerulein triggers ROS production by activating NADPH oxidase [Citation50].
In the endogenous antioxidant defense system, SOD, CAT, GSH, GPx, GR and Fe2+ play a very important role as shown in [Citation53]. SOD is in charge of converting superoxide O2* (the result of over activation of NADPH oxidase/xanthine oxidase) into hydrogen peroxide H2O2. Furthermore, H2O2 is converted to H2O by CAT and GPx. In diabetes conditions, it is reported that Fe2+ accumulation (ferroptosis) will convert H2O2 into hydroxyl radicals (OH*) [Citation40].
Figure 4. Quercetin effect on tissue antioxidant status. (Abbr.: SOD = superoxide dismutase, O2 = oxygen, H2O2 = hydrogen peroxide, Fe = iron, CAT = catalase, GPx = glutathione peroxidase, GR = glutathione reductase, GSH = glutathione, GSSG = glutathione disulfide).
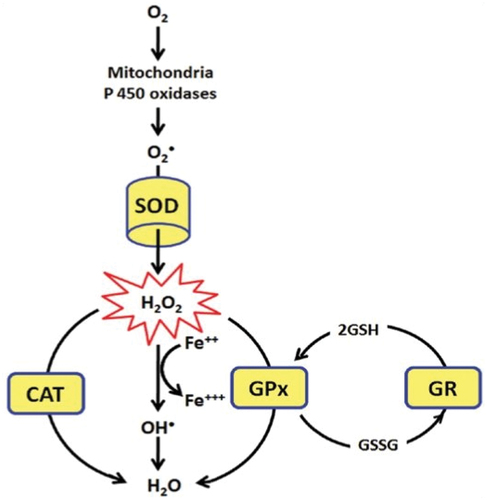
The results of this meta-analysis showed that the administration of quercetin was able to improve the antioxidant status of pancreatic tissue. The high activity of NADPH oxidase and xanthine oxidase stimulates the production of superoxide. However, with the administration of quercetin, cells were able to significantly increase the expression of SOD (p = 0.0176). This increase in SOD activity was also accompanied by an increase in SOD expression at the protein level [Citation54]. In addition, GSH as a hydrogen donor was also significantly increased (p = 0.0085). However, the levels of catalase (p = 0.1011) and glutathione peroxidase (GPx) (p = 0.3521) were not significantly increased.
These results are similar to the Granado-Serrano study which stated that the stimulation of cellular antioxidants by quercetin was mediated by the activation of the transcription factor NrF2 which regulates the expression of GSH, GPx, glutathione reductase (GR) and glutamylcysteine-synthetase. In addition, it is estimated that quercetin also acts to scavenge the OH* radicals of Fe2+ oxidation products as shown by the ABTS assay test [Citation55].
In terms of lipid metabolism, quercetin was also seen to significantly inhibit the production of TBARS, a product of the lipid peroxidation process (p = 0.0116). Malondialdehyde (MDA), a type of TBARS, is produced through various mechanisms including enzymatic and non-enzymatic. Enzymatic production of MDA comes from the decomposition of arachidonic acid by cyclooxygenase (COX) and thromboxane synthase (TXAS) as a cascade of inflammation and cell injury [Citation56]. Meanwhile, non-enzymatically begins with the reaction of polyunsaturated fatty acids (PUFA) with reactive oxygen species. These PUFA radicals will form bicyclic endoperoxides by releasing MDA molecules [Citation56]. Based on the results of the meta-analysis, quercetin is thought to inhibit MDA production in both mechanisms. The enzymatic mechanism was proven by a significant decrease in inflammatory mediators (TNFα) (p = 0.0050), which in turn would decrease arachidonic acid production. While the non-enzymatic mechanism is estimated that quercetin stops the propagation of lipid peroxide radicals by donating a hydrogen atom.
Pancreatic tissue damage varied from inflammation to necrosis and apoptosis as seen in the control group. Inflammation is characterized by increased transcription factor nuclear factor kappa B (NF-kB); the inflammatory mediators TNF-a, IL−1b, and IL−6; and platelet-activating factor (PAF) [Citation18].
After administering quercetin, signs of cell survival were obtained. Cell survival was shown by reduction of inflammation severity through the mechanism of decreasing production of TNFa significantly (p = 0.0050), IL−6 (p = 0.0016), and increasing BCl2 expression although not significant (p = 0.4039). The increase in BCl 2 level will prevent BAX/BAK oligomerization which will eventually prevent apoptosis [Citation57]. Moreover, quercetin has been reported that it is non-cytotoxic for normal cells [Citation58–63].
Meanwhile, from the statistics on the number of islet cells per area, the quercetin treatment group did not significantly increase the islet number (p = 0.1037) as well as its relative weight of the pancreas (p = 0.2689). From these results, it is estimated that the effect of quercetin on pancreatic tissue is to reduce the severity of inflammation or inhibit the progression of inflammation in the early stages, but does not promote regeneration of damaged tissue. The initial stage of inflammation that becomes the point of action for quercetin is ROS radical scavenging which in turn inhibits the activation of NF-kB, a transcription factor of various inflammatory mediators ().
Figure 5. Quercetin inhibit further inflammation by neutralizing ROS, and in consequence inactivate NF-Kb and Casp3 signaling. (Abbr.: ROS = reactive oxygen species, Casp3 = caspase − 3, PARP = Poly [ADP-ribose] polymerase, IKK = IκB kinase, IκB = Inhibitor of NF-κB, NF-κB = Nuclear factor-kappa B, TNF-α = Tumor necrosis factor alpha, IL = interleukin).
![Figure 5. Quercetin inhibit further inflammation by neutralizing ROS, and in consequence inactivate NF-Kb and Casp3 signaling. (Abbr.: ROS = reactive oxygen species, Casp3 = caspase − 3, PARP = Poly [ADP-ribose] polymerase, IKK = IκB kinase, IκB = Inhibitor of NF-κB, NF-κB = Nuclear factor-kappa B, TNF-α = Tumor necrosis factor alpha, IL = interleukin).](/cms/asset/68a2a33a-5e31-40ea-b246-9b8fa0e98dda/teba_a_2222467_f0005_oc.jpg)
Finally, from various in vitro and in vivo tests and supported by the results of this meta-analysis, quercetin appears promising for use at the clinical level, especially as a complementary agent. However, to step into this realm requires a standardization and also the development of an optimal delivery system (e.g. encapsulation, emulsification, hydrogel, co-crystallization). Quercetin is a flavonoid with a large chemical structure with low bioavailability (<10%) [Citation64]. The closest step in the use of quercetin is as a nutraceutical. In terms of regulation, nutraceuticals are easier than drugs [Citation65].
Conclusions
This meta-analysis showed that the administration of quercetin was able to restore pancreatic function. Cellular damage in animal models of acute and chronic pancreatitis was inhibited by quercetin so that it did not progress to necrosis or apoptosis. At doses of quercetin above 55 mg/kgBW/day, there appears to be no linear correlation between the dose and blood glucose levels. However, this study did not consider delivery system factors or the type of adjuvant used, which greatly affect the bioavailability of quercetin. Further research needs to be done, in particular to overcome the problem of quercetin bioavailability in the body, as well as an appropriate delivery system for its instability issue of rapid oxidation by dissolved oxygen.
Abbreviations
| 6PGD | = | 6-Phosphogluconate dehydrogenase |
| ABTS | = | 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| AGE | = | advanced glycation end product |
| Casp3 | = | caspase-3 |
| CAT | = | catalase |
| COX | = | cyclo-oxygenase |
| CVD | = | cardiovascular disease |
| DPPH | = | 2, 2′-diphenyl-1-picrylhydrazyl |
| F16bp | = | Fructose 1,6-bisphosphate |
| FFA | = | free fatty acid |
| FRAP | = | Feric Reducing Activity |
| G6p | = | glucose 6 phosphate |
| G6PDH | = | Glucose-6-phosphate dehydrogenase |
| GLUT2/4 | = | glucose transporter-2/4 |
| GPx | = | glutathione peroxidase |
| GSH | = | glutathione |
| H2O2 | = | hydrogen peroxide |
| HFD | = | high fat diet |
| IL-6 | = | interleukin 6 |
| LOX | = | lipo-oxygenase |
| LPL | = | lipoprotein lipase |
| MDA | = | malondialdehyde |
| NADPH | = | Nicotinamide adenine dinucleotide phosphate |
| NF-κB | = | nuclear factor kappa B |
| ROS | = | reactive oxygen species |
| SD | = | Sprague Dawley |
| SOD | = | superoxide dismutase |
| STZ | = | streptozotocin |
| T2DM | = | type 2 diabetes melitus |
| TBARS | = | thiobarbituric acid reactive substances |
| TNFα | = | tumor necrosis factor alfa |
| VLDL | = | very low density lipoprotein |
Authors Contribution
All authors have substantial contribution including: design of the work (T Wiyono, K Nisa, S Handayani, A Windarsih, SN Hayati, MP Wulanjati, EN Sholikhah, WR Pratiwi); the acquisition (T Wiyono), analysis (T Wiyono), have drafted the work (T Wiyono), revised and approved (K Nisa, S Handayani, A Windarsih, SN Hayati, MP Wulanjati, EN Sholikhah, WR Pratiwi).
Acknowledgments
Researchers would like to thank the Indonesian Institute of Sciences and the Ministry of Research and Higher Education of the Republic of Indonesia for their funding support.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. Interested parties can contact the corresponding author via the email provided: https://docs.google.com/spreadsheets/d/11E_B7JgjLzAWmPmWktWiUqgNq1wKrdSb/edit?usp=sharing&ouid=105511364159406934633&rtpof=true&sd=true.
Additional information
Funding
References
- Agrawal S, Zaritsky JJ, Fornoni A, et al. Dyslipidaemia in nephrotic syndrome: mechanisms and treatment. Nat Rev Nephrol. 2018;14:57–70. doi: 10.1038/nrneph.2017.155
- Hamad Zubi ZB, Hamad Alfarisi HA. Hyperlipidemia and male infertility. Egypt J Basic Appl Sci. 2021;8:385–396. doi: 10.1080/2314808X.2021.1977080
- Oh RC, Lanier JB. Management of hypertriglyceridemia. Am Fam Physician. 2007;75:1365–1371. https://www.aafp.org/pubs/afp/issues/2007/0501/p1365.html
- Pretis N, Amodio A, Frulloni L. Hypertriglyceridemic pancreatitis: epidemiology, pathophysiology and clinical management. United Eur Gastroenterol J. 2018;6:649–655. doi: 10.1177/2050640618755002
- Kichler A, Jang S. Chronic pancreatitis: epidemiology, diagnosis, and management updates. Drugs. 2020;80:1155–1168. doi: 10.1007/s40265-020-01360-6
- Thupakula S, Nimmala SSR, Ravula H, et al. Emerging biomarkers for the detection of cardiovascular diseases. Egypt Heart J. 2022;74:1–17. doi: 10.1186/S43044-022-00317-2
- Tejada S, Martorell M, Capo X, et al. Coumarin and derivates as lipid lowering agents. Curr Top Med Chem. 2016;17:391–398. doi: 10.2174/1568026616666160824102322
- Chavan S, Dias R, Magdum C. Garuga pinnata attenuates oxidative stress and liver damage in chemically induced hepatotoxicity in rats. Egypt J Basic Appl Sci. 2021;8:235–251. doi: 10.1080/2314808X.2021.1961207
- Fakhrudin N, Wiyono T, Putra AR, et al. The evaluation on anti-platelet and antithrombosis activities of cinnamomum sintoc bark extract. Thai J Pharm Sci. 2019;43:219–226. http://www.tjps.pharm.chula.ac.th/ojs/index.php/tjps/article/view/43
- Wiyono T, Frediansyah A, Sholikhah EN, et al. UHPLC-ESI-MS analysis of Javanese Tamarindus indica leaves from various tropical zones and their beneficial properties in relation to antiobesity. J Appl Pharm Sci. 2022;12:137–147. doi: 10.7324/JAPS.2022.120814
- Ji X, Shi S, Liu B, et al. Bioactive compounds from herbal medicines to manage dyslipidemia. Biomed Pharmacother. 2019;118:109338. doi: 10.1016/j.biopha.2019.109338
- Batiha G-S, Beshbishy AM, Ikram M, et al. The pharmacological activity, biochemical properties,and pharmacokinetics of the major natural polyphenolic flavonoid: quercetin. Foods. 2020;9:374. doi: 10.3390/foods9030374
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021:n71. doi: 10.1136/bmj.n71
- Harzing A. Publish or Perish (Software) 2007. https://harzing.com/resources/publish-or-perish (accessed May 1, 2022).
- Abdou HM, Hamaad FA, Ali EY, et al. Antidiabetic efficacy of Trifolium alexandrinum extracts hesperetin and quercetin in ameliorating carbohydrate metabolism and activating IR and AMPK signaling in the pancreatic tissues of diabetic rats. Biomed Pharmacother. 2022;149:112838. doi: 10.1016/j.biopha.2022.112838
- Adewole S, Caxton-Martins E, Ojewole J. Protective effect of quercetin on the morphology of pancreatic β-cells of streptozotocin-treated diabetic rats. Afr J Tradit Complement Altern Med. 2007;4:64–74. doi: 10.4314/ajtcam.v4i1.31196
- Arya A, Jamil Al-Obaidi MM, Shahid N, et al. Synergistic effect of quercetin and quinic acid by alleviating structural degeneration in the liver, kidney and pancreas tissues of STZ-induced diabetic rats: a mechanistic study. Food Chem Toxicol. 2014;71:183–196. doi: 10.1016/j.fct.2014.06.010
- Carvalho KMMB, Morais TC, de Melo TS, et al. The natural flavonoid quercetin ameliorates cerulein-induced acute pancreatitis in mice. Biol Pharm Bull. 2010;33:1534–1539. doi: 10.1248/bpb.33.1534
- Coskun O, Kanter M, Korkmaz A, et al. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and β-cell damage in rat pancreas. Pharmacol Res. 2005;51:117–123. doi: 10.1016/j.phrs.2004.06.002
- El-Far AH, Lebda MA, Noreldin AE, et al. Quercetin attenuates pancreatic and renal D-Galactose-induced aging-related oxidative alterations in rats. Int J Mol Sci. 2020;21:4348. doi: 10.3390/ijms21124348
- El-Rahman SNA, Al-Jameel SS. Synergistic effect of quercetin nanoparticles on liver and pancreas tissues and oxidative stress of streptozotocin-induced diabetic rats. Asian J Chem. 2017;29:1003–1010. doi: 10.14233/ajchem.2017.20389
- Junyuan Z, Hui X, Chunlan H, et al. Quercetin protects against intestinal barrier disruption and inflammation in acute necrotizing pancreatitis through TLR4/MyD88/p38 MAPK and ERS inhibition. Pancreatology. 2018;18:742–752. doi: 10.1016/j.pan.2018.08.001
- Kahraman A, Vurmaz A, Koca HB, et al. The effect of quercetin on cerulein-induced acute pancreatitis. Med Express. 2017;4:1–7. doi: 10.5935/medicalexpress.2017.05.02
- Kalailingam P, Balasubramanian K, Kannaian B, et al. Isolation and quantification of flavonoids from ethanol extract of Costus igneus rhizome (CiREE) and impact of CiREE on hypoglycaemic, electron microscopic studies of pancreas in streptozotocin (STZ)-induced diabetic rats. Biomed Prev Nutr. 2013;3:285–297. doi: 10.1016/j.bionut.2013.01.001
- Li J-M, Wang W, Fan C-Y, et al. Quercetin preserves β -Cell Mass and function in fructose-induced hyperinsulinemia through modulating pancreatic Akt/FoxO1 Activation. Evid Based Complement Alternat Med. 2013;2013:1–12. doi: 10.1155/2013/303902
- Lin C-F, Kuo Y-T, Chen T-Y, et al. Quercetin-Rich Guava (Psidium guajava) Juice in combination with trehalose reduces autophagy, apoptosis and pyroptosis formation in the kidney and pancreas of type ii diabetic rats. Molecules. 2016;21:334. doi: 10.3390/molecules21030334
- Nur Syimal’ain A, Nooraain H, N S, et al. Effect of azadirachta excelsa and quercetin on the endocrine pancreas of the experimentally induced diabetic rats: histological and insulin secretion. Adv Nat Appl Sci. 2017;11:35+. https://go.gale.com/ps/anonymous?id=GALE%7CA601551043&v=2.1&it=r&linkaccess=abs&issn=19950772
- Omar H-D, Ragaa SM, Elghaffar SKA, et al. Berberine, Quercetin and O-Coumaric acid phytochemicals ameliorate the impact of experimentally fed high-fat/high-sucrose diet on pancreas β-cells and glycemic control indices. Austin J Endocrinol Diabetes. 2016;3:1–6. https://austinpublishinggroup.com/endocrinology-diabetes/fulltext/ajed-v3-id1042.php
- Oyedemi SO, Nwaogu G, Chukwuma CI, et al. Quercetin modulates hyperglycemia by improving the pancreatic antioxidant status and enzymes activities linked with glucose metabolism in type 2 diabetes model of rats: in silico studies of molecular interaction of quercetin with hexokinase and catalase. J Food Biochem. 2020;44:1–16. doi: 10.1111/jfbc.13127
- Rasheed RA, Elshikh MS, Mohamed MO, et al. Quercetin mitigates the adverse effects of high fat diet on pancreatic and renal tissues in adult male albino rats. J King Saud Univ - Sci. 2022;34:101946. doi: 10.1016/j.jksus.2022.101946
- Rifaai RA, El-Tahawy NF, Ali Saber E. Effect of quercetin on the endocrine pancreas of the experimentally induced diabetes in male albino rats: a histological and immunohistochemical study. J Diabetes Metab. 2012;3:1000182. doi: 10.4172/2155-6156.1000182
- Vessal M, Hemmati M, Vasei M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp Biochem Physiol - C Toxicol Pharmacol. 2003;135:357–364. doi: 10.1016/S1532-0456(03)00140-6
- Zheng J, Wu J, Chen J, et al. Therapeutic effects of quercetin on early inflammation in hypertriglyceridemia-related acute pancreatitis and its mechanism. Pancreatology. 2016;16:200–210. doi: 10.1016/j.pan.2016.01.005
- Hooijmans CR, Rovers MM, De Vries RB, et al. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. doi: 10.1186/1471-2288-14-43
- De Brito WA, Ferreira MRA, De Sousa Dantas D, et al. Biological activities of Eugenia uniflora L. (pitangueira) extracts in oxidative stress-induced pathologies: a systematic review and meta‐analysis of animal studies. Pharmanutrition. 2022;20:100290. doi: 10.1016/j.phanu.2022.100290
- Hayanti SY, Hidayat C, Jayanegara A, et al. Effect of vitamin E supplementation on c hicken sperm quality: a meta-analysis. Vet World. 2022;15:419–426. doi: 10.14202/vetworld.2022.419-426
- Team RC. R: a language and environment for statistical computing (Software) 2020. https://www.r-project.org/ (accessed May 1, 2022).
- Zhao J-B. Animal models of pancreatitis: can it be translated to human pain study? World J Gastroenterol. 2013;19:7222. doi: 10.3748/wjg.v19.i42.7222
- Kiss L, Fűr G, Pisipati S, et al. Mechanisms linking hypertriglyceridemia to acute pancreatitis. Acta Physiol. 2023;237:1–21. DOI:10.1111/apha.13916
- Li D, Jiang C, Mei G, et al. Quercetin alleviates ferroptosis of pancreatic β cells in type 2 diabetes. Nutrients. 2020;12:1–15. doi: 10.3390/nu12102954
- El-Tantawy WH, Temraz A, Hozaien HE, et al. Anti-hyperlipidemic activity of an extract from roots and rhizomes of {Panicum} repens {L}. on high cholesterol diet-induced hyperlipidemia in rats. Z Naturforschung C J Biosci. 2015;70:139–144. doi: 10.1515/znc-2014-4147
- Shao Y, Yu Y, Li C, et al. Synergistic effect of quercetin and 6-gingerol treatment in streptozotocin induced type 2 diabetic rats and poloxamer P-407 induced hyperlipidemia. RSC Adv. 2016;6:12235–12242. doi: 10.1039/C5RA16493A
- Vijayakumar K, Rengarajan RL, Radhakrishnan R, et al. Hypolipidemic effect of psidium guajava leaf extract against hepatotoxicity in rats. Pharmacogn Mag. 2018;14:4–8. doi: 10.4103/pm.pm_167_17
- Zeni ALB, Moreira TD, Dalmagro AP, et al. Evaluation of phenolic compounds and lipid-lowering effect of {Morus} nigra leaves extract. An Acad Bras Cienc. 2017;89:2805–2815. doi: 10.1590/0001-3765201720160660
- Bule M, Abdurahman A, Nikfar S, et al. Antidiabetic effect of quercetin: a systematic review and meta-analysis of animal studies. Food Chem Toxicol. 2019;125:494–502. doi: 10.1016/j.fct.2019.01.037
- Henagan TM, Lenard NR, Gettys TW, et al. Dietary quercetin supplementation in mice increases skeletal muscle pgc1α expression, improves mitochondrial function and attenuates insulin resistance in a time-specific manner. PLoS ONE. 2014;9:e89365. doi: 10.1371/journal.pone.0089365
- Shi G-J, Li Y, Cao Q-H, et al. In vitro and in vivo evidence that quercetin protects against diabetes and its complications: a systematic review of the literature. Biomed Pharmacother. 2019;109:1085–1099. doi: 10.1016/j.biopha.2018.10.130
- Ozgen S, Kilinc OK, Selamoğlu Z. Antioxidant activity of quercetin: a mechanistic review. Turk J Agric - Food Sci Technol. 2016;4:1134. doi: 10.24925/turjaf.v4i12.1134-1138.1069
- Rusmana D, Wahyudianingsih R, Elisabeth M, et al. Antioxidant activity of phyllanthus niruri extract, rutin and quercetin. Indones Biomed J. 2017;9:84. doi: 10.18585/inabj.v9i2.281
- Kim H. Cerulein pancreatitis: oxidative stress, inflammation, and apoptosis. Gut Liver. 2008;2:74–80. doi: 10.5009/gnl.2008.2.2.74
- Oh YS, Bae GD, Baek DJ, et al. Fatty acid-induced lipotoxicity in pancreatic beta-cells during development of type 2 diabetes. Front Endocrinol. 2018;9:384. doi: 10.3389/fendo.2018.00384
- Wang N, Zhang J, Qin M, et al. Amelioration of streptozotocin‑induced pancreatic β cell damage by morin: involvement of the AMPK‑FOXO3‑catalase signaling pathway. Int J Mol Med. 2017:1409–1418. doi: 10.3892/ijmm.2017.3357
- Pandey KB, Rizvi SI. Markers of oxidative stress in erythrocytes and plasma during aging in humans. Oxid Med Cell Longev. 2010;3:2–12. doi: 10.4161/oxim.3.1.10476
- Alía M, Mateos R, Ramos S, et al. Influence of quercetin and rutin on growth and antioxidant defense system of a human hepatoma cell line (HepG2). Eur J Nutr. 2006;45:19–28. doi: 10.1007/s00394-005-0558-7
- Granado-Serrano AB, Martín MA, Bravo L, et al. Quercetin modulates Nrf2 and glutathione-related defenses in HepG2 cells: involvement of p38. Chem Biol Interact. 2012;195:154–164. doi: 10.1016/j.cbi.2011.12.005
- Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014. 1–31. doi:10.1155/2014/360438
- Tzifi F, Economopoulou C, Gourgiotis D, et al. The Role of BCL2 family of apoptosis regulator proteins in acute and chronic leukemias. Adv Hematol. 2012;2012:1–15. doi: 10.1155/2012/524308
- Guo Y, Tong Y, Zhu H, et al. Quercetin suppresses pancreatic ductal adenocarcinoma progression via inhibition of SHH and TGF-β/Smad signaling pathways. Cell Biol Toxicol. 2021;37:479–496. doi: 10.1007/s10565-020-09562-0
- Herr. Dietary polyphenol quercetin targets pancreatic cancer stem cells. Int J Oncol. 2010;37:551–561. 10.3892/ijo_00000704
- Nwaeburu CC, Bauer N, Zhao Z, et al. Up-regulation of microRNA let-7c by quercetin inhibits pancreatic cancer progression by activation of Numbl. Oncotarget. 2016;7:58367–58380. doi: 10.18632/oncotarget.11122
- Sak K. Site-specific anticancer effects of dietary flavonoid quercetin. Nutr Cancer. 2014;66:177–193. doi: 10.1080/01635581.2014.864418
- Valentich MA, Eynard AR, Barotto NN, et al. Effect of the co-administration of phenobarbital, quercetin and mancozeb on nitrosomethylurea-induced pancreatic tumors in rats. Food Chem Toxicol. 2006;44:2101–2105. doi: 10.1016/j.fct.2006.07.013
- Elreedy HA, Elfiky AM, Mahmoud AA, et al. Neuroprotective effect of quercetin through targeting key genes involved in aluminum chloride induced Alzheimer’s disease in rats. Egypt J Basic Appl Sci. 2023;10:174–184. doi: 10.1080/2314808X.2022.2164136
- Kandemir K, Tomas M, McClements DJ, et al. Recent advances on the improvement of quercetin bioavailability. Trends Food Sci Technol. 2022;119:192–200. doi: 10.1016/j.tifs.2021.11.032
- Amer SS, Mamdouh W, Nasr M, et al. Quercetin loaded cosm-nutraceutical electrospun composite nanofibers for acne alleviation: preparation, characterization and experimental clinical appraisal. Int J Pharm. 2022;612:121309. doi: 10.1016/j.ijpharm.2021.121309