ABSTRACT
Diabetic nephropathy (DN) is one of the most significant complications of diabetes mellitus. Annona squamosa has been demonstrated to have anti-inflammatory, anti-diabetic, and antioxidant properties. This study aimed to assess the modulatory role of A. squamosa fruit aqueous extract against DN. Forty male rats were divided into four groups: control group, A. squamosa extract (AE)-supplemented, diabetic (injected with a single dose of STZ), and diabetic + AE group. The diabetic rats treated with AE for four weeks revealed remarkable restoration in the levels of blood glucose and kidney functions disrupted by diabetes. These were manifested by significant improvement in the levels of urea, creatinine, and uric acid, as well as DN markers (β2 microglobulin, ceruloplasmin, NGAL, and plasma KIM-1). Additionally, the activity of lipid profiles (total cholesterol, triglycerides, LDH, and HDL), oxidative stress (MDA), and kidney antioxidants (CAT, SOD, GPx, and GSH) returned to normalcy. Also, the altered histological architecture of the kidney was markedly recovered after treatment with AE. Further, AE showed a proliferative and anti-inflammatory role, as indicated by modulation of PCNA and TNF-α expression in the kidney tissues. Annona squamosa extract significantly improved diabetic nephropathy in male rats via its antidiabetic, antioxidant and anti-inflammatory potential.
Introduction
Diabetes mellitus (DM) is a metabolic condition characterized by high blood sugar levels resulting from the body’s decreased ability to produce or respond to the hormone insulin [Citation1]. Concurrently, high blood glucose and/or low insulin levels may have detrimental effects on the body’s physiology over time [Citation2]. Prolonged hyperglycemia increases the synthesis of advanced glycosylated end products and reactive free radicals [Citation3], vasoactive amines (angiotensin-II), and some cytokines, which result in functional damage followed by structural damage to kidney tubules [Citation4]. Renal functional disorder is represented by tubular hypertrophy as a result of enhanced glucose absorption and malfunction of transporter proteins [Citation5,Citation6] that causes a rise in protein excretion. This can be used as a biomarker for diagnosing renal functional disorder while glomerular function remains normal.
DM presents a major concern to public health since it can lead to long-lasting impairments in physiological functioning and malfunctions in organs with widespread impediments. The disease’s incidence is also on the rise [Citation7]. In people with diabetes, for example, diabetic nephropathy accounts for 40% of instances of end-stage renal disease (ESRD) [Citation8]. Among the conditions which promote the development of DN are hyperglycemia, glycosuria, hypertension, ketoacidosis, coronary heart disease, obesity, ketoacidosis, and aging; however, the precise mechanism is yet unknown [Citation9]. Notably, inflammation and oxidative stress are associated with the progression of DN [Citation10].
Long-term or end-stage renal disease is brought on by DN, a microvascular consequence of DM [Citation11]. The pathophysiology of diabetic nephropathy involves a multifactorial interaction including lipid problems, oxidative stress, alterations in renal hemodynamics, polyol activation, inflammatory pathways, and mitogen-activated protein kinase signaling pathways [Citation12,Citation13]. The levels of some biomarkers like serum ceruloplasmin, kidney injury molecule-1 (Kim-1) and neutrophil gelatinase-associated lipocalin (NGAL) are up-regulated in chronic kidney injury. Up-regulation of these biomarkers was reported to be identical for developing DN [Citation14,Citation15].
Therefore, the fundamental objective of diabetic nephropathy therapy continues to be the control of hyperglycemia. The oral hypoglycemic medications used in DM treatment therapy need to both suppress hepatic glucose production by down-regulating gluconeogenesis and enhance insulin secretion and/or sensitivity in order to ameliorate the pathophysiology [Citation16]. It had been reported that the DN-relieving oral hypoglycemia medications are adipogenic [Citation17], and numerous hazards are closely linked to synthetic oral hypoglycemic medications [Citation18].
The World Health Organization (WHO) considers natural products from plants and animals to be safe and effective in managing DM, with minimal or no adverse effects. Indeed, such natural components are being considered as viable options for oral therapy [Citation19]. Consequently, some antioxidants may be effective in alleviating conditions linked to diabetes [Citation20] because ROS plays a crucial role in the development of diabetic nephropathy [Citation21]. Historically, local societies have utilized medicinal plants as a new approach for treating several diseases [Citation22]. There are more than 800 plant species known to have anti-diabetic qualities [Citation23]. Among the several phytoconstituents included in these plants that are extremely powerful and effective at lowering blood glucose are flavonoids, alkaloids, polyphenols, tannins, and other phytochemicals. As a result, they may aid in the discovery and development of novel medications to treat DM [Citation22,Citation24].
The custard apple, or Annona squamosa, is a tropical tree that belongs to the Annonaceae family and grows throughout many countries [Citation25,Citation26]. The leaves, fruits, roots, bark, and seeds of A. squamosa are used to treat or alleviate a variety of diseases, including parasite infections, diabetes, gastritis, diarrhea, and dysentery [Citation22].
Different parts of Annona squamosa were found to have antioxidant [Citation27], anti-inflammatory [Citation28] anti-diabetic [Citation29], renoprotective [Citation30], and hepatoprotective [Citation31] activities. In addition, a previous study demonstrated that Annona squamosa fruit extract has a potential hypoglycemic and hypolipidmic effects in alloxan-induced diabetic rabbits [Citation32]. Furthermore, earlier research has shown that A. squamosa contains phytomolecules like kaempferol, rutin, quercetin, and β-caryophyllene, which have antidiabetic and antioxidant properties [Citation25,Citation33]. Studies conducted in vitro using A. squamosa water extract showed a decrease in the absorption of glucose and protein glycation. It also enhanced the activities of β-cells, plasma insulin levels, and glucose tolerance in obese rats [Citation25].
Streptozotocin (STZ) is an alkylating antineoplastic drug that occurs naturally and is especially harmful to the β-cells in mammals’ pancreas that produce insulin. In medical research, it is used to create an animal model for hyperglycemia, Alzheimer’s disease, type 2 diabetes, or type 1 diabetes at several low dosages. It is also used to treat specific tumors of the islets of Langerhans. Additionally, STZ is utilized to trigger inflammation, hyperlipidemia, and hyperuricemia in diabetic kidney damage models [Citation11,Citation34].
With regard to that, the purpose of this study is to assess the possible ameliorative effect of A. squamosa extract against streptozotocin-induced diabetic nephropathy in male rats by comparing the histological and biochemical alterations across treatment groups.
Material and methods
Experimental materials
i. Streptozotocin
STZ was purchased from Sigma Chemical Inc. (St. Louis, MO, USA).
ii. Preparation of Annona squmaosa Extract (AE)
Five kilograms of A. squamosa fruit were purchased from the Damanhour City local market. Fruit was categorized and identified by staff members of the Botany Department, Faculty of Science, Damanhour University. The fruit has been peeled and the seeds removed off. The fruit pulp samples have been dried at 45°C. Before being used again, the dried samples were separated, ground into a fine powder, and stored at 4°C. Following an extraction using 1:20 w/v of distilled water, the powdered samples were heated for 30 minutes at 70°C while being stirred. After centrifuging the extract for 25 minutes at 10,000 rpm, it was filtered, lyophilized, and kept in a freezer at −20°C until used [Citation35]. The percentage yield was approximately 9% (450 g).
Animal handling and treatment
In the present study, 40 male Wistar albino rats weighing 180–190 g have been used. Throughout the experiment, the animals were kept in standard laboratory settings with a 12-hour light/dark cycle and a temperature at 25 ± 2°C. Prior to and during the trials, the animals were given tap water and regular rat chow without restriction. The rats were categorized into the following groups (n = 10)
Group 1 (Control): The rats feed on a standard diet in addition to a single intraperitoneal injection of 0.1 M citrate buffer pH 4.5.
Group 2 (AE-treated): the rats were given AE (350 mg/kg/day) orally via gavage [Citation29] in addition to a single dose of citrate buffer as in the control group. Davis et al. [Citation36] was assessed the lethal dose of AE over 500 mg/kg/day
Group 3 (Diabetic): The rats were fasted for 12 hours, then injected intraperitoneally with a single dose of STZ (60 mg/kg in a buffer of citric acid at pH 4.5) [Citation37].
Diabetic and AE-treated group: The rats were injected with STZ as in group 3, followed by treatment with AE (350 mg/kg/day).
The fasting blood glucose (FBG) from a tail vein sample was measured using a blood glucose meter (LifeScan, CA, USA) three days after the administration of STZ in order to confirm the DM induction. Rats with FBG levels above 16.8 (mmol/L) for three consecutive days were considered diabetic [Citation38]. The experiment was extended for 4 weeks for groups (1–3) and 8 weeks for group 4. Following a 12-hour fast, the rats were weighed at the completion of the experiment, and each rat’s tail vein was used to collect blood. According to the biochemical parameter assay, blood samples were stored at −20°C after being centrifuged at 1300 g for serum separation. Finally, the animals were anaesthetized using 50 mg/kg 1% chloral hydrate (Catalogue No: En40–11/35-1-1-1997E), then dissected, and the kidneys were excised, weighed, and then processed for histopathological, immunohistochemical and antioxidant examinations.
Preparation of kidney homogenate
The kidneys were washed with isotonic saline. Each kidney was cut lengthwise into two halves. One half was processed for histopathological and immunohistochemical investigations. The other half was cut into slices and homogenized using a homogenizer with 10% (w/v) phosphate-buffered (0.1 M, pH 7.4). The centrifuged kidney homogenate was processed for estimation of antioxidant enzymes in the supernatant.
Biochemical assays
i. Glucose level
Serum glucose was measured spectrophotometrically based on the glucose oxidase protocol (Waco kits, Japan), using the blood glucose meter (Icare TD-4279) [Citation39].
ii. Neutrophil gelatinase-associated lipocalin (NGAL) and interleukin-1β (IL-1β)
The serum NGAL level was detected using ‘NGAL ELISA kit’, (BIOPORTO diagnostics, Denmark) (Catalog No: #DLCN20). IL-1β was estimated using ELISA Kit (Catalogue No: KE00021, Proteintech, China) based on the manufacturer’s protocol.
iii. Beta2-microglobulin (β2-MG)
A turbidimetric immunoassay technique has been employed to quantify the serum β2-MG level. The reagents were subjected to an automated biochemical analyzer (Beckman Dxl 800) using Beckman’s β2-MG kit according to instructions by the manufacturer.
iv. Serum ceruloplasmin
Serum ceruloplasmin level was tested using a nephelometric protocol (BN II System; Siemens, Marburg, Germany) [Citation40].
v. Kidney injury molecule 1 (KIM-1)
Following the manufacturer’s instructions, the KIM-1 levels in plasma were determined using the ELISA kit (MyBioSource, Catalogue No: MBS260195).
vi. Renal functions (creatinine, urea and uric acid)
The serum creatinine (Cr)and urea (Ur) levels were measured using creatinine and urea kits (Vitro Scient, Inshas Industrial Zone, Belbis, Sharkia, Egypt) [Citation41]. Using a uric acid (uricase/peroxidase) kit (BioSystems S.A. Quality System licensed according to EN ISO 13,485 and EN ISO 9001 standards, Costa Brava, 30. 08030 Barcelona, Spain), the serum uric acid(UA) level was calculated in accordance with the procedure outlined by Barhamet al [Citation42].
vii. Lipid profiles
Biuret, Bromocresol green, and GPO-HMMPS were used to determine serum total triglycerides (Ttr), and an automatic biochemistry analyzer (Hitachi 7180, Hitachi, Tokyo, Japan) was used to quantify total cholesterol (TC)using an enzyme reaction method. A direct technique was used to quantify the levels of high-density lipoprotein (HDL) and low-density lipoprotein (LDL) using an automatic analyzer (BioMajestyTM JCA-BM8000 series, Japan Electron Optics Laboratory, Tokyo, Japan).
viii. Evaluation of antioxidants in tissue homogenate
The estimation of superoxide dismutase (SOD) and catalase (CAT) activities in the kidney homogenate was carried out using the methodology outlined by Nishi Ahad and Kumar [Citation43]. The glutathione peroxidase (GPx) activity was tested using the RANSEL kit (Randox Crumlin, UK), according to Paglia and Valentine [Citation44]. Using the 5–5-dithiobis (2-nitrobenzoic acid) (DTNB) reagent, reduced glutathione (GSH) levels in tissue homogenates were tested; absorbance was seen at 412 nm. Thiobarbituric acid-reactive substances (TBARS) were used to measure the lipid peroxidation levels (MDA: malondialdehyde) in kidney supernatant. The reaction mixture’s absorbance was recorded at 535 nm [Citation45].
Kidney histopathological examination
Hematoxylin and eosin (H&E) staining was applied to kidney tissues after they were immediately fixed in 10% neutral buffered formalin, dried using ascending alcohol grades, embedded in paraffin, and cut into sections of 5.6–5.6 μm. The slides underwent a microscopic examination using light microscopy to investigate any potential pathological relevance [Citation46].
Immunohistochemical analysis of tumor necrosis factor –α (TNF–α) and proliferating cell nuclear antigen (PCNA)
Kidneys were sliced into five-micrometer thick paraffin sections, then mounted onto positively charged slides, deparaffinized, and rehydrated in a descending series of alcohols. Following this, endogenous peroxidase activity was blocked using 1% diluted H2O2 in PBS for 10 minutes. Powdered skim milk (3% in phosphate buffered saline) was used to suppress the background staining after the slides were washed with PBS containing 1% bovine serum albumin (BSA). For immunohistochemical labeling of TNF-α, the kidney sections were incubated overnight at °CC with anti-TNF-α (Santa Cruz Biotechnology, USA).
For PCNA labeling, the sections were incubated in anti-PCNA (monoclonal antibody against PCNA, diluted 1:50 in PBS; Dako, Denmark) for 10–12 h at 48°C. The sections were treated with biotinylated antibody for 45 minutes at room temperature after being rinsed three times for five minutes each in PBS plus 1% BSA.
The sections were incubated for 45 minutes using Vector Laboratories’ (USA) AB complex. Sections were washed again, and the reaction was revealed by DAB (Sigma-Aldrich, USA) and finally counterstained with Mayer’s hematoxylin. The slides were washed in PBS, counterstained with Mayer’s hematoxylin, and then examined and microphotographed using an Axioskop 2 Plus microscope (Zeiss, Germany) with a Leica DFC 320 digital camera (Leica, Germany) [Citation47].
Statistical analysis
Version 20.0 of the IBM SPSS software fits was used to input and analyze data. New York, Armonk: IBM Corp. The Shapiro-Wilk test was used to determine whether the continuous data were normal. The standard deviation and mean were used to express quantitative data. The four groups under study were compared using a one-way ANOVA test, and pairwise comparisons were conducted using the Post Hoc test (Tukey). The 5% level was used to assess the results’ significance.
Results
Body weight and kidney weight changes
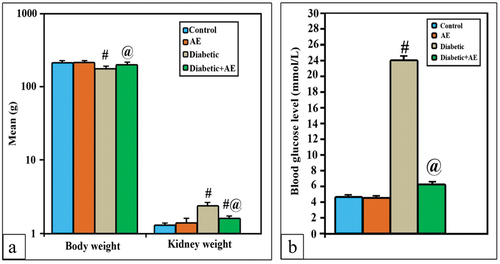
A comparison of the mean body weight (213.7 g) and kidney weight (1.4 g) of the rats given AE supplementation and the control group (211.7 g and 1.3 g, respectively) revealed no statistically significant difference. In STZ-treated rats, the body weight appeared significantly lower (175.8 vs. 211.7 g), while the kidney weight (2.4 g) appeared significantly higher (p < 0.001*) if compared with the control. Supplementation of AE (350 mg/kg) for consecutive 28 days was successful in returning the body weight near that of control (201.8 g vs. 213.7 g; p > 0.001). However, the kidney weight was significantly decreased if compared with diabetics (1.6 g vs. 2.4 g; p < 0.001), but still significantly higher than the kidney weight of control (1.6 g vs. 1.3 g; p < 0.001) ().
Biochemical changes in blood
Urea, creatinine, and uric acids
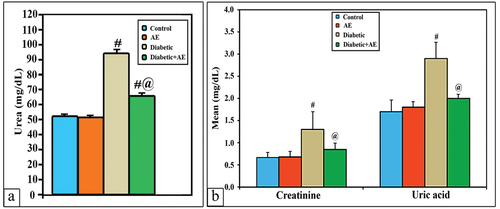
The levels of serum renal indices (Ur, Cr, and UA) showed a non-significant change (p > 0.001) between the AE group (51.9, 0.68, and 1.8 mg/dL) and the control group (50.7, 0.67, and 1.7 mg/dL), respectively. However, these levels appeared significantly higher (p < 0.001) than control in STZ-induced diabetic rats (93.8, 1.3, and 2.9 mg/dL, respectively). On the other hand, the diabetic rats treated with AE for 28 days revealed non-significant changes (p > 0.001) in the levels of serum Cr and UA (0.85, 2 mg/dL) if compared with control, while the level of Ur (64.9 mg/dL) seemed much higher (p < 0.001) than the control group but significantly lower than the diabetic group ().
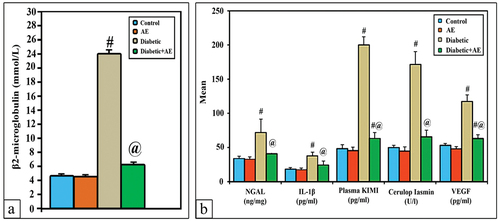
Serum β2-microglobulin, NGAL, IL-1β, ceruloplasmin, VEGF and plasma KIM-1
The mean levels of serum β2-microglobulin, NGAL, IL-1β, ceruloplasmin, VEGF, and plasma KIM-1 showed a non-significant change (p > 0.001) between the AE group (1.4, 33.1, 17.7, 44.8, 48.8, 45.4) and the control group (1.5, 34.1, 18.4, 50, 53.1, 48.2), respectively. However, these levels appeared significantly higher (p < 0.001) than control in STZ-induced diabetic rats (2.6, 72.1, 37.9, 65.7, 63.1, and 63.5), respectively. Treatment of diabetic rats with AE successfully ameliorated the levels of β2-microglobulin, NGAL, IL-1β, and ceruloplasmin near those of the control (p > 0.001). Nevertheless, the levels of VEG and plasma KIM-1 appeared significantly lowered in diabetic rats but still significantly higher (p < 0.001) than control ().
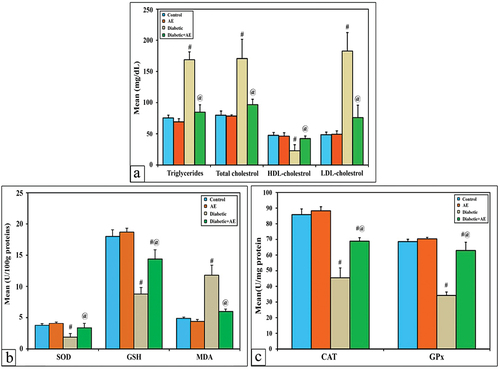
Lipid profiles (Ttr, TC, LDL and HDL)
The mean level of serum Ttr, TC, LDL-cholesterol, and HDL-cholesterol showed a non-significant change (p > 0.001) between the AE group (69.2, 78.5, 49.3, 46.2 mg/dL) and the control group (75.5, 79.8, 48.5, 47.5 mg/dL), respectively. In diabetic rats, the levels of these profiles appeared significantly different (p < 0.001) (168.7, 170.7, 182.7, and 22.9 mg/dL) than control. Treatment of diabetic rats with AE for consecutive 28 days successfully ameliorated the levels of lipid profiles near normal (84.7, 96.8,76, 42.3 mg/dL) but still significantly changed with control ().
Changes in the levels of kidney antioxidants (SOD, CAT, GSH and Gpx) and MDA
As shown in , the activities of SOD, CAT, GSH, GPx, and MDA in the kidney tissue homogenate of AE-supplemented rats (4.1, 88.4, 18.7, 70.4, and 4.4 U/mg protein) showed non-significant changes (p > 0.001) with control (3.8, 85.8, 18, 68.6, and 4.9 U/mg protein), respectively. In STZ-induced diabetic rats, the levels of SOD, CAT, GSH, and GPx (1.9, 45.5, 8.8, and 34.2 U/mg protein) appeared significantly lower (p < 0.001), while the MDA level (11.8 U/mg protein) showed a significant decrease if compared with the control. On the other hand, diabetic rats treated with AE showed remarkable amelioration in the levels of SOD, CAT, GSH, and MDA (3.4, 68.9, 14.4, and 6 U/mg protein), respectively, while the level of GPx was significantly increased compared with the diabetic group (62.9 U/mg protein) but still significantly lowered compared to the control.
Histopathological changes
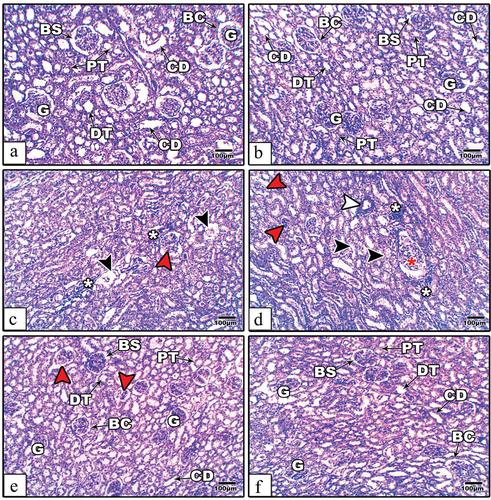
The kidney histological sections from the control and AE groups of rats appeared to have normal organized renal corpuscles and tubules (). In diabetic rats, some renal corpuscles appeared with hypertrophied, atretic, or atrophied glomeruli. Some of the renal tubules showed degenerated epithelium, a relatively dilated lumen, and scattered infiltrated cells (). Supplementation of AE to diabetic rats for 28 days successfully alleviated most of the kidney histological damage induced by diabetes, as noticed by the restoration of renal corpuscles and tubules near the normal architecture, while little atretic glomeruli were still present in some areas of the sections ().
Figure 5. Kidney histological images from the control (a), AE (b), STZ (c–d), and STZ+AE (e–f) groups of rats. In STZ-induced diabetic rats, the sections showing atretic or lytic (read arrow heads), hypertrophied (red star), damaged tubular epithelial cells with relatively wide lumens (black arrow heads), thickened wall capillaries (white arrow heads), and scattered infiltrated cells (white asterisks). In the diabetic and AE groups, the glomerular and tubular architectures show remarkable amelioration. Abbreviations: BC: Bowman’s capsule; BS: Bowman’s space; CD: collecting duct; G: glomerulus; DT: distal tubule; and PT: proximal tubule. (Stain: H&E, scale bar: 100µm).
Immunohistochemical changes
Pcna
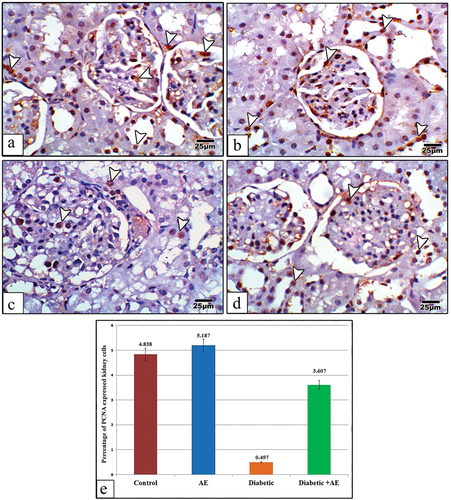
The renal cortical sections from the control and AE groups displayed strong positive immune expression for PCNA, especially in the nuclei of glomerular and tubular epithelial cells (). In diabetic rats, the kidney sections revealed weak immunohistochemical expression for PCNA if compared with the control (). In diabetic rats post-treated with AE, the kidney sections displayed positive expression for PCNA higher than the diabetic group but apparently with little expression compared with the control ().
Figure 6. Images of kidney sections from control (a), AE (b), STZ (c), and STZ+AE (d) stained with PCNA antibody. The immunohistochemical expression of PCNA appears strong in the glomerular and tubular cells of the control and AE groups, very weak in the diabetic group, and moderately expressed in the diabetic and AE groups. Panel (e) indicates the quantitative analysis (using image analysis) for the percentage of PCNA expressed kidney cells among investigated groups. The arrowheads point to PCNA immunoreactivity. (PCNA antibody stain, scale bar: 25µm).
TNF-α
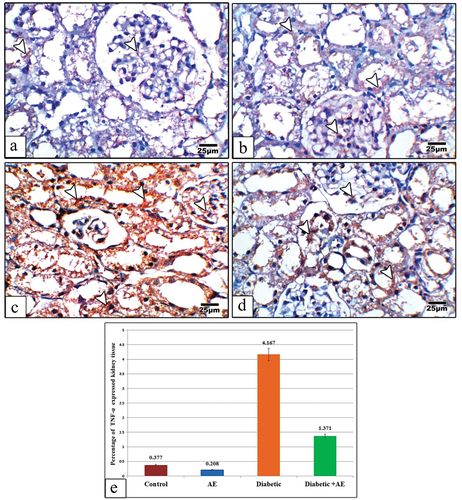
The kidney sections from control and AE group displayed negative or very weak immune reaction for TNF-α ()., however in diabetic rats this reaction appeared strongly expressed all-over the glomerular and tubular cells (). On the other hand, supplementation of diabetic rats with AE was markedly declined the degree of TNF-α immunoreactivity but still highly expressed than control ().
Figure 7. Images of kidney sections from control (a), AE (b), Diabetic (c), and Diabetic +AE (d) stained with TNF-α antibody. The immunohistochemical expression of TNF-α appears negative or very weak in the glomerular and tubular cells of the control and AE groups, strong in the diabetic group, and moderately expressed in the diabetic and AE groups. Panel (e) illustrates the quantitative analysis (using image analysis) for the percentage of TNF-α expressed kidney cells among investigated groups. The arrow heads point to the TNF-α immunoreactivity. (TNF-α antibody stain, scale bar: 25µm).
Discussion
Currently, insulin and traditional hypoglycemic medications are available to treat DM, yet continuous use of these medications might cause negative effects. Consequently, numerous studies have examined the therapeutic and preventive benefits of various natural therapies in the management of DM [Citation48–50]. Previous research has demonstrated the potential for Annona spp. to have hypoglycemic, antibacterial, hepatoprotective, antiplasmodial, cytotoxic, antioxidant, and anti-inflammatory properties [Citation51]. One prominent long-term microvascular consequence of DM is diabetic nephropathy. About one-third of diabetic patients have it recorded [Citation52]. Animal models of DM caused by streptozotocin are thought to be useful for studying hyperglycemia. Insulin secretion becomes impaired, and cytotoxicity is caused in the pancreatic β-cells by STZ [Citation53]. Because diabetic nephropathy is a prominent complication caused by STZ in male rats, the purpose of this study was to assess the hypoglycemic and ameliorative effects of A. squamosa fruit extract against this threat.
In the current work, the mean kidney weights were apparently increased in STZ-induced diabetic rats; however, on treatment with AE, the kidney weights were significantly lowered when compared with the diabetic group. This suggests that diabetes is implicated in the induction of kidney hypertrophy, and AE attenuates this hypertrophy. According to Zafar and Naqvi [Citation54], persistent hyperglycemia causes renal hypertrophy by increasing the production of proteins and/or slowing down the breakdown of extracellular components of the kidney. Another theory suggests that DM is a primary cause of the overexpression of transforming growth factor beta-1 in the kidney, particularly in glomerular mesangial cells and proximal convoluted tubule cells, which is associated with the development of renal hypertrophy [Citation55]. In STZ-diabetic rats, AE therapy decreased kidney weight, reversing kidney hypertrophy. Renal disorders have been discovered to be improved by polyphenols and their metabolites [Citation56]. It has been shown that the fruit of Annona squamosa is rich in essential flavonoids, such as acetogens, terpenes, and alkaloids, which assist in reducing renal inflammation [Citation57].
According to Mestry et al. [Citation58], diabetes mellitus (DM) is linked to a considerable drop in body weight because of hyperglycemia and hypoinsulinemia, which promote muscle atrophy and tissue protein loss. The body weight of the STZ-induced diabetic rats in the current study was much lower than that of the control rats; however, after receiving AE treatment, the body weight significantly became nearer to the control value. Research has shown that A. squamosa is a desirable dietary supplement for the management of type 2 diabetes mellitus (T2DM). It also contains rutine, which has been shown to enhance muscle absorption and promote proper digestion and weight maintenance [Citation25]. A comparable investigation found that after 28 days of treatment with AE, the body weight changes of STZ-induced diabetic rats significantly improved [Citation59].
The result of the present work revealed a significant increase in the level of blood glucose in STZ-treated rats but decreased near to the normal range on treatment with AE. It was established that in diabetic kidney damage models, STZ is used to induce inflammation, hyperlipidemia, and hyperuricemia [Citation34]. Furthermore, STZ inhibits insulin production and causes cytotoxicity in pancreatic β cells [Citation53]. Numerous phytochemicals, including flavonoids, alkaloids, phenols, saponins, tannins, glycosides, and diterpenes, are present in custard apples and have anti-diabetic and antioxidant qualities [Citation60]. According to earlier studies [Citation25,Citation61], A. squamosa has antidiabetic effects via increasing insulin secretion or action, blocking the digestion of starch and protein glycation, delaying the absorption of glucose, and reducing Dipeptidyl Peptidase IV (DPP-IV) enzyme activity. According to a different theory, A. squamosa may have a hypoglycemic effect by either improving blood glucose transport to peripheral tissues or potentiating the pancreatic release of insulin from islet β-cells [Citation62]. Moreover, phytochemicals can have strong anti-diabetic benefits by improving insulin secretion and sensitivity, enhancing muscle and adipose tissue’s absorption of glucose, and supporting pancreatic β-cells [Citation50].
One of the most significant effects of diabetes is nephropathy. Serum urea, creatinine, and uric acid concentrations are frequently used as common indicators of renal function [Citation63]. According to our research, the concentrations of Ur, Cr, and UA markedly increased in the diabetic rats whereas they declined in the animals that received AE treatment. The data about the notable increases in serum urea, creatinine, and uric acid caused by STZ is consistent with the findings of Kumar et al. [Citation64]. According to studies, diabetes mellitus (DM) can cause renal damage because of abnormal glucose regulation, which can lead to increased levels of glucose and glycosylated protein, changes in renal tissue hemodynamics, and increased oxidative stress, which raises levels of Cr, Ur, and UA [Citation65].
The data concerned with a significant reduction in the concentrations of urea, creatinine, and uric acid in diabetic rats given custard apples go in parallel with the findings of Adil [Citation66], who found a remarkable reduction in the concentration of urea, creatinine, and uric acid after treatment of diabetic rats with 300 mg/kg AE for a consecutive 30-day period. Although the exact mechanism is unknown, A. squamosa antioxidant activity might be relevant [Citation65]. Consistent results from another study further support the renoprotective effect of A. squamosa in renal failure caused by animal models with 5/6 nephrectomies [Citation67].
Serum β2 microglobulin has been proposed as a stronger glomerular filtration rate marker compared to serum creatinine [Citation68,Citation69] discovered a correlation between various phases of chronic kidney disease (CKD) and serum β2 microglobulin levels. Moreover, high β2 microglobulin levels have been linked to proliferative lymphatic disorders, chronic renal failure, dialysis patients, inflammation, infection, and other conditions [Citation70]. According to Kim et al. [Citation71], there is a higher risk of diabetic nephropathy among individuals with high serum levels of β2 microglobulin as opposed to those with low levels and normal renal function. In the present study, the level of β2 microglobulin was much higher in diabetic rats, but it markedly diminished after receiving AE therapy, matching that of normal control rats. This finding suggests that STZ may contribute to the development of DN, but AE may help to mitigate this concern. It is still unclear how A. squamosa can lower serum β2 microglobulin levels in diabetic nephropathy, but earlier research suggested that phytochemical components like tannins and saponins play a potent antioxidant role in combating diabetic oxidative stress, which improves renal function by increasing glomerular filtration rate [Citation72,Citation73].
Ceruloplasmin is one of the recognized indicators of oxidative stress. The oxidation of Fe2+ into Fe3+ is linked to the copper-dependent oxidase activity considered by ceruloplasmin. There have been reports of elevated serum ceruloplasmin levels in type 2 diabetes [Citation74]. Ceruloplasmin also plays a significant role in the regulation of membrane lipid oxidation under physiological conditions [Citation75]. Elevated ceruloplasmin levels observed in DM might represent a defensive mechanism [Citation76]. In the current investigation, serum ceruloplasmin levels were significantly higher in rats treated with STZ than in controls; however, after treatment with AE, serum ceruloplasmin levels were significantly lower in rats treated with diabetes but still significantly higher than in controls. These results imply that DM is a condition of elevated oxidative stress [Citation13,Citation77] had recorded significant increase in patients with diabetic foot and patients with retinopathy [Citation77] and Diabetic nephropathy [Citation78]. The significant decline in the levels of serum ceruloplasmin in this study may be attributed to the antioxidants activity of A. squamosa by preventing free radical formation, blocking the chain reactions or repairing the oxidative damage biomolecules [Citation79,Citation80].
Several renal tubular markers, such as NGAL and KIM-1 May 2001act as clinical indicators of the onset and progression of DN [Citation81,Citation82]. In our study, four weeks after STZ induction in rats, we found a statistically significant increase in serum NGAL and plasma Kim-1 values. But after receiving AE therapy, their values dramatically declined. Increased levels of NGAL can be considered a protective response to oxidative stress caused by diabetes as well as a predictor for DN. On the other hand, a strong correlation between serum KIM-1 and a decreased GFR was observed by Hammoud et al. [Citation83]. Additionally, clinical research has shown that patients with diabetes mellitus have progressively higher KIM-1 tests [Citation14]. The role of AE in attenuation of diabetic nephropathy through lowering of NGAL and Kim-1 is still unknown; however, previous studies have proven that the high contents of gallic acid, vitamin C and flavonoids, and cyclosquamosin D play a major and effective role in combating diabetic nephropathy [Citation84,Citation85].
DM causes hypertriglyceridemia, hypercholesterolemia, and a fatty liver. Moreover, diabetic nephropathy and high TC are associated [Citation58]. The current study showed a noticeable increase in the levels of TC, Ttr, and LDL but a decrease in the level of HDL in the group of STZ-induced diabetic rats, while these levels improved toward normalcy after treatment with AE. Studies have shown promising outcomes for the effect of A. squamosa on experimental diabetic animals. For instance, in diabetic rats and rabbits caused by alloxan and streptozotocin (STZ), the ethanolic extract of A. squamosa enhanced glucose tolerance and reduced LDL, Ttr, and TC levels [Citation59]. Furthermore, in rats with high-fat diet-induced obesity, 15 days of administration of the aqueous extract of A. squamosa reduced the levels of Ttr, total LDL, TG, glycosylated hemoglobin level, and elevated HDL [Citation86]. The strong antihyperlipidemic effect of AE could also be due to its control of hyperglycemia [Citation87].
Reactive oxygen species (ROS) are generated at higher levels in chronic hyperglycemia and are implicated in the development of several diabetic complications, including DN [Citation10,Citation88]. The cell becomes more vulnerable to oxidative damage as a result of the ROS depleting its antioxidant capabilities. The oxidation of proteins, lipids, and DNA that it targets further alters the structure and function of cells. GSH is essential for maintaining the antioxidant status of the cells and for scavenging free radicals; however, GPx protects the cells from oxidative damage [Citation89]. Additionally, the biological role of GPx is to convert free hydrogen peroxide to water [Citation90]. SOD catalyzes the dismutation of the superoxide anion radical into hydrogen peroxide and ordinary molecular oxygen. Consequently, catalase assists SOD in completely suppressing ROS [Citation91]. MDA is an indication of late-stage lipid oxidation and a crucial marker of lipid peroxidation caused by free radicals [Citation88]. Animals with diabetes had higher amounts of MDA in their plasma, renal cortex, proximal tubule cells, and mesangial cells [Citation92]. Many antioxidant enzymes, such as SOD and GPx, are less synthesized and active when blood glucose levels are elevated, most likely due to glycation [Citation93]. Moreover, individuals with diabetes exhibit reduced levels of antioxidant defenses, enzymatic (SOD, CAT, and GPx) and non-enzymatic (free radical scavengers, vitamin C, E, or A) [Citation93]. In STZ-induced diabetic rats in this study, renal MDA levels were elevated while SOD, CAT, GSH, and GPx levels declined. However, after receiving AE treatment for a consecutive 28 days, MDA levels were significantly lowered and SOD, CAT, GSH, and GPx levels were noticeably elevated when compared to the diabetic group. In addition, angiotensin-converting enzyme, protein kinase C, and mitogen-activated protein kinase (MAPK) activation may be synthesized more quickly in diabetic oxidative stress, which could potentially aggravate diabetic neuropathy [Citation91,Citation94].
The data concerned with the restoration antioxidant enzymes after treatment with AE in this study may be attributed to the presence of high levels of flavonoids, tannins, and polyphenols in A. squamosa [Citation10,Citation95]. Furthermore, it is well recognized that phenolic compounds, such as flavonoids, have anti-inflammatory, antioxidant, and antidiabetic properties [Citation96,Citation97]. Previous studies have shown that A. squamosa pulp and leaves contain a high percentage of phenolic compounds (75.8 ± 1.31 mg/g) [Citation98,Citation99]. The mechanism of action of A. squamosa as an antioxidant has been previously explained in several ways. According to Silva et al. [Citation100], the primary cause of the antioxidant activity of phenolic compound molecules is the presence of hydroxyls, which also contribute to the elimination of reactive species and free radicals. Additional research has demonstrated that carotenoids, which are present in A. squamosa, also participate to the antioxidant activity because of their mechanism of action, which involves both inactivating molecules that are in an excited state, such as those resulting from photosensitive reactions, and suppressing superoxide O2−•, a highly reactive form that can cause cell damage [Citation100,Citation101].
Similarly, in the present study, the histopathological diabetic kidney tissues in rats displayed atretic and hypertrophied glomeruli, damaged tubular epithelial cells with relatively wide lumens, thickened wall capillaries and scattered infiltrated cells. These changes suggested the degeneration of renal cells. On the other hand, there was a noticeable improvement in the histological structure of the kidneys after treatment with the aqueous extract of AE at standard doses of 350 mg/kg; also, the results are in good agreement with the control. The renal histopathological signs can be explained because of an increase in the release of free radicals, which consequently leads to a deficiency in antioxidants, especially GSH and GPx, leading to cell damage. In addition, the oxidative stress caused by diabetes led to an increase in NAG and KIM-1 levels, which in turn led to the emergence of these histopathological signs. The ameliorative role of AE extract against kidney histological damage induced by diabetes may be due to the presence of bioactive antioxidant compounds like acetogenins, quercetin, and gallic acid, which play a role as free radical scavenging elements to relieve cell injury [Citation85,Citation102]. A. squamosa alkaloids, flavonoids, total phenols, annonacin, and ascorbic acid contents indicated their capacity to scavenge free radicals and ROS to prevent tissue damage [Citation103]. Besides, gallic acid presence helps in restoring GSH levels [Citation104].
One pro-inflammatory cytokine that is crucial to the pathophysiology and clinical course of diabetic nephropathy is TNF-α. Nephron damage has been observed in association with NF-α up-regulation [Citation105]. In this study, we observed an elevated NF-α expression in the untreated diabetic rats, while the diabetic rats administered with AE showed reduced expression of NF-α further depicting that aqueous extracts of AE has anti-inflammatory potential against diabetic induced inflammation. This means that diabetes caused inflammation, while AE reduced such inflammation. According to a meta-analysis conducted by Qiao et al., diabetic patients exhibit considerably higher levels of TNF-α in comparison to healthy controls [Citation106]. Moreover, Mitrović et al. [Citation107] found that increased TNF-α has been linked to both the prediction of DN and microvascular and macrovascular complications in diabetic individuals. Another related study on experimental animals has indicated the key role of TNF-α in mediating the pathogenesis of diabetic peripheral neuropathy [Citation108]. The role of AE in reducing the activity of TNF-α may be due to the presence of anti-inflammatory compounds in this extract, such as caryophyllene oxide [Citation109], gallic acid [Citation110], alkaloids, flavonoids, phenols, terpenoids, tannins, saponins, and quinones [Citation111].
PCNA plays a vital role in the proliferation and replacement of dead cells [Citation112]. Indeed, STZ-induced diabetic rats showed a dramatic down-regulation of PCNA expression. The combination of proteins and replication factors involved in DNA replication, cell cycle regulation, and repair may become dysfunctional as a result of PCNA down-regulation, ultimately leading to an apparent reduction in cell differentiation. Here, there was a significant decrease in the expression of PCNA of glomerular and tubular cells in diabetic rats, which was consistent study of Khamis et al. [Citation113]. After the treatment with AE, the expression of PCNA was observably up-regulated, this indicated that AE may improve DM-induced kidney dysfunctions potentially via up-regulating the activity of PCNA. It has been proven that ethanolic extracts from different species of Annona have a vital and effective role against apoptotic cell death in rat kidneys induced by ifosfamid [Citation85].
Conclusion
Based on our findings, aqueous extract from Annona squamosa pulp has a powerful ameliorative impact against diabetes mellitus and its consequence, diabetic nephropathy, due to the antioxidant and anti-inflammatory properties of this extract. This was achieved via modulation of diabetic nephropathy markers, including β2 microglobulin, ceruloplasmin, NGAL, KIM-1, lipid profiles, and antioxidant enzymes. To fully understand how Annona squamosa is used to treat diabetic nephropathy, more research is necessary.
Author contributions
GA, NA, and GM designed the work protocol. MA and AFB follow up the practical technique and provide comments. ES gives the statistical analysis of the results. All authors contribute to the writing and revision of the paper.
Ethics approval and consent to participate
All procedures were performed in accordance with the guidelines of the bioethics committee of faculty of Science, Damanhour University (DMU-SCI-CSRE- 2 February 2024).
Acknowledgments
The authors express their gratitude to the biochemical lab staff at the science faculty, Damanhour University for their crucial help in accomplishing the practical protocol.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Singh S, Kushwah V, Agrawal AK, et al. Insulin- and quercetin-loaded liquid crystalline nanoparticles: implications on oral bioavailability, antidiabetic and antioxidant efficacy. Nanomedicine (Lond). 2018;13(5):521–537. doi: 10.2217/nnm-2017-0278
- Banday MZ, Sameer AS, Nissar S. Pathophysiology of diabetes: An overview. Avicenna J Med. 2020;10(4):174–188. doi: 10.4103/ajm.ajm_53_20
- Asmat U, Abad K, Ismail K. Diabetes mellitus and oxidative stress-A concise review. Saudi Pharm J. 2016;24(5):547–553. doi: 10.1016/j.jsps.2015.03.013
- Xue R, Gui D, Zheng L, et al. Mechanistic Insight and Management of Diabetic Nephropathy: Recent Progress and Future Perspective. J Diabetes Res. 2017;2017:(1839809–09). doi: 10.1155/2017/1839809
- Thrailkill KM, Nimmo T, Bunn RC, et al. Microalbuminuria in type 1 diabetes is associated with enhanced excretion of the endocytic multiligand receptors megalin and cubilin. Diabetes Care. 2009;32(7):1266–1268. doi: 10.2337/dc09-0112
- Habib SL. Kidney atrophy vs hypertrophy in diabetes: which cells are involved? cell cycle (Georgetown, Tex. Cell Cycle. 2018;17(14):1683–1687. doi: 10.1080/15384101.2018.1496744
- Charles MA, Leslie RD. Diabetes: concepts of β-cell organ dysfunction and failure would lead to earlier diagnoses and prevention. Diabetes. 2021;70(11):2444–2456. doi: 10.2337/dbi21-0012
- de Boer IH, Khunti K, Sadusky T, et al. Diabetes management in chronic kidney disease: A consensus report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Diabetes care. 2022;45(12):3075–3090. doi: 10.2337/dci22-0027
- Fayfman M, Pasquel FJ, Umpierrez GE. Management of hyperglycemic crises: diabetic ketoacidosis and hyperglycemic hyperosmolar state. The medical clinics of North America. Med Clin North Am. 2017;101(3):587–606. doi: 10.1016/j.mcna.2016.12.011
- Sahakyan G, Vejux A, Sahakyan N. The role of oxidative stress-mediated inflammation in the development of t2dm-induced diabetic nephropathy: possible preventive action of tannins and other oligomeric polyphenols. Molecules (Basel, Switzerland), 2022;27(24):9035. doi: 10.3390/molecules27249035
- Gomes IBS, Porto ML, Santos MCLFS, et al. Renoprotective, anti-oxidative and anti-apoptotic effects of oral low-dose quercetin in the C57BL/6J model of diabetic nephropathy. Lipids Health Dis. 2014;13(1):13(184–84. doi: 10.1186/1476-511X-13-184
- Park S, Lim W, Bazer FW, et al. Quercetin inhibits proliferation of endometriosis regulating cyclin D1 and its target microRnas in vitro and in vivo. J Nutr Biochem. 2019;63(87):87–100. doi: 10.1016/j.jnutbio.2018.09.024
- Sharma D, Gondaliya P, Tiwari V, et al. Kaempferol attenuates diabetic nephropathy by inhibiting RhoA/Rho-kinase mediated inflammatory signalling. Biomedicine & Pharmacotherapy. 2019;109(1610–19). doi: 10.1016/j.biopha.2018.10.195 109
- de Carvalho JAM, Tatsch E, Hausen BS, et al. Urinary kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin as indicators of tubular damage in normoalbuminuric patients with type 2 diabetes. Clin Biochem. 2016;49(3):232–236. doi: 10.1016/j.clinbiochem.2015.10.016
- He P, Bai M, J-P H, et al. Significance of neutrophil gelatinase-associated lipocalin as a biomarker for the diagnosis of diabetic kidney disease: a systematic review and meta-analysis. Kidney Blood Pressure Res. 2020;45(4):497–509. doi: 10.1159/000507858
- Nithya R, Subramanian S. Sinapic acid, a naturally occurring carboxylic acid derivative ameliorates hyperglycemia in high fat diet-low dose stz induced experimental diabetic rats. Int J Sci Eng Tech Res. 2015:4(5746–50).
- Klein G, Kim J, Himmeldirk K, et al. Antidiabetes and anti-obesity activity of Lagerstroemia speciosa. Evid Based Complement Alternat Med. 2007;4(4):401–407. doi: 10.1093/ecam/nem013
- Spiller HA. Toxicology of oral antidiabetic medications. Am J Health Syst Pharm. 2006;63(10):929–938. doi: 10.2146/ajhp050500
- Shokeen P, Anand P, Murali YK, et al. Antidiabetic activity of 50% ethanolic extract of Ricinus communis and its purified fractions. Food Chem Toxicol. 2008;46(11):3458–3466. doi: 10.1016/j.fct.2008.08.020
- Alam MM, Meerza D, Naseem I. Protective effect of quercetin on hyperglycemia, oxidative stress and DNA damage in alloxan induced type 2 diabetic mice. Life Sci. 2014;109(1):8–14. doi: 10.1016/j.lfs.2014.06.005
- Stenvinkel P, Painer J, Kuro-O M, et al. Novel treatment strategies for chronic kidney disease: insights from the animal kingdom. Nat Rev Nephrol. 2018;14(4):265–284. doi: 10.1038/nrneph.2017.169
- Ansari P, Hannan JMA, Seidel V, et al. Polyphenol-rich leaf of annona squamosa stimulates insulin release from brin-bd11 cells and isolated mouse islets, reduces (ch(2)o)(n) digestion and absorption, and improves glucose tolerance and glp-1 (7-36) levels in high-fat-fed rats. Metabolites. 2022;12(10):995. doi: 10.3390/metabo12100995
- Arumugam G, Manjula P, Paari N. A review: anti diabetic medicinal plants used for diabetes mellitus. J Acute Dis. 2013;2(3):196–200. doi: 10.1016/s2221-6189(13)60126-2
- Tran N, Pham B, Le L. Bioactive compounds in anti-diabetic plants: from herbal medicine to modern drug discovery. Biology (Basel). 2020;9(9):252. doi: 10.3390/biology9090252
- Ansari P, Flatt PR, Harriott P, et al. Evaluation of the Antidiabetic and Insulin Releasing Effects of A. squamosa, Including Isolation and Characterization of Active Phytochemicals. Plants (Basel, Switzerland), 2020;9(10):1348. doi: 10.3390/plants9101348
- Pandey K, Sinha A, Perween Z. Important medicinal plants with their medicinal uses from Jharkhand State. Int J Of Research In Engineering, Science And Management. 2020;3(8):532–542.
- Mariod AA, Abdelwahab SI, Elkheir S, et al. Antioxidant activity of different parts from Annona squamosa, and Catunaregam nilotica methanolic extract. Acta Sci Polonorum Technologia Aliment. 2012;11(3):249–258.
- Saelee C, Thongrakard V, Tencomnao T. Effects of Thai medicinal herb extracts with anti-psoriatic activity on the expression on NF-κB signaling biomarkers in HaCaT keratinocytes. Molecules (Basel, Switzerland), 2011;16(5):3908–3932. doi: 10.3390/molecules16053908
- Tomar RS, Sisodia SS. Antidiabetic activity of Annona squamosa L. in experimental induced diabetic rats. Int J PharmInt J Pharm Biol Arch. 2012;3:1492–1495.
- Sangala R, Kodati D, Burra S, et al. Evaluation of antidiabetic activity of Annona squamosa Linn Seed in alloxan–induced diabetic rats. Diabetes. 2011;2(1):100–106.
- Uduman TS, Sundarapandian R, Muthumanikkam A, et al. Protective effect of methanolic extract of Annona squamosa Linn in isoniazid-rifampicin induced hepatotoxicity in rats. Pak J Pharm Sci. 2011;24(2):129–134.
- Gupta RK, Kesari AN, Diwakar S, et al. In vivo evaluation of anti-oxidant and anti-lipidimic potential of Annona squamosa aqueous extract in type 2 diabetic models. J Ethnopharmacol. 2008;118(1):21–25. doi: 10.1016/j.jep.2008.03.008
- Bhat R, Paliyath G. Fruits of tropical climates: Dietary importance and health benefits. Encycl Food Health. 2016, Elsevier. p. 144–149.
- Tong F, Liu S, Yan B, et al. Quercetin nanoparticle complex attenuated diabetic nephropathy via regulating the expression level of ICAM-1 on endothelium. IJN. 2017;12(7799–813):7799–7813. doi: 10.2147/IJN.S146978
- Shehata MG, Abu-Serie MM, Abd El-Aziz NM, et al. Nutritional, phytochemical, and in vitro anticancer potential of sugar apple (Annona squamosa) fruits. Sci Rep. 2021;11(1):6224–24. doi: 10.1038/s41598-021-85772-8
- Davis JA, Sharma S, Mittra S, et al. Antihyperglycemic effect of Annona squamosa hexane extract in type 2 diabetes animal model: PTP1B inhibition, a possible mechanism of action? Indian J Pharmacol. 2012;44(3):326–332. doi: 10.4103/0253-7613.96304
- Lu Q, Ji X-J, Zhou Y-X, et al. Quercetin inhibits the mTorc1/p70s6k signaling-mediated renal tubular epithelial–mesenchymal transition and renal fibrosis in diabetic nephropathy. Pharmacol Res. 2015;99(237–47). doi: 10.1016/j.phrs.2015.06.006
- Yue T, Xu H-L, Chen P-P, et al. Combination of coenzyme Q10-loaded liposomes with ultrasound targeted microbubbles destruction (UTMD) for early theranostics of diabetic nephropathy. Int J Pharmaceut. 2017;528(1–2):664–674. doi: 10.1016/j.ijpharm.2017.06.070
- Rahmati M, Keshvari M, Mirnasouri R, et al. Exercise and Urtica dioica extract ameliorate hippocampal insulin signaling, oxidative stress, neuroinflammation, and cognitive function in STZ-induced diabetic rats. Biomed Pharmacother. 2021;139(111577):111577. doi: 10.1016/j.biopha.2021.111577
- Vaidya VS, Waikar SS, Ferguson MA, et al. Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin Transl Sci. 2008;1(3):200–208. doi: 10.1111/j.1752-8062.2008.00053.x
- Jaffe M. About the rainfall, which picric acid in normal urine generated and a new reaction of creatinine. Z PhysZ Physiol Chern. 1986;10(391–400).
- Barham D, Trinder P. An improved colour reagent for the determination of blood glucose by the oxidase system. Analyst. 1972;97(1151):142. doi: 10.1039/an9729700142
- Nishi AA, Kumar P. Protective effect of chlorogenic acid against diabetic nephropathy in high fat diet/streptozotocin induced type-2 diabetic rats. Int J Pharm Pharm Sci. 2013;5:489–495.
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70(1):158–169. Available from: https://www.ncbi.nlm.nih.gov/pubmed/6066618
- Halliwell B, Chirico S. Lipid peroxidation: its mechanism, measurement, and significance. Am J Clin Nutr. 1993;57(5):715S–725S. doi: 10.1093/ajcn/57.5.715s
- Suvarna KS, Layton C, Bancroft JD. Bancroft’s theory and practice of histological techniques E-Book. Elsevier health sciences; 2018.
- Hussein AM, Ahmed OM. Regioselective one-pot synthesis and anti-proliferative and apoptotic effects of some novel tetrazolo[1,5-a]pyrimidine derivatives. Bioorg Med Chem. 2010;18(7):2639–2644. doi: 10.1016/j.bmc.2010.02.028
- Watal G, Dhar P, Srivastava SK, et al. Herbal medicine as an alternative medicine for treating diabetes: the global burden. Evid Based Complement Alternat Med. 2014;2014(596071–71):1–2. doi: 10.1155/2014/596071
- Nasiri A, Ziamajidi N, Abbasalipourkabir R, et al. Beneficial effect of aqueous garlic extract on inflammation and oxidative stress status in the kidneys of type 1 diabetic rats. Ind J Clin Biochem. 2017;32(3):329–336. doi: 10.1007/s12291-016-0621-6
- Alam S, Sarker MMR, Sultana TN, et al. Antidiabetic Phytochemicals from medicinal plants: Prospective candidates for new drug discovery and Development. Front Endocrinol. 2022;13:800714–14. doi: 10.3389/fendo.2022.800714
- Marahatta AB, Aryal A, Basnyat RC, et al. The phytochemical and nutritional analysis and biological activity of Annona squamosa Linn. Int J Herb Med. 2019:7(19–28).
- Chamberlain JJ, Rhinehart AS, Shaefer CF, et al. Diagnosis and management of diabetes: synopsis of the 2016 American Diabetes Association standards of medical care in diabetes. Ann internal med. 2016;164(8):542. doi: 10.7326/m15-3016
- Rezagholizadeh L, Pourfarjam Y, Nowrouzi A, et al. Effect of Cichorium intybus L. on the expression of hepatic NF-κB and IKKβ and serum TNF-α in STZ− and STZ+ niacinamide-induced diabetes in rats. Diabetol Metab Syndr. 2016;8(1):11–11. doi: 10.1186/s13098-016-0128-6
- Zafar M, Naeem-Ul-Hassan NS. Effects of STZ-Induced diabetes on the relative weights of Kidney, Liver and Pancreas in Albino Rats: A comparative study. Int J Morphol. 2010;28(1). doi: 10.4067/s0717-95022010000100019
- Zhao L, Zou Y, Liu F. Transforming Growth Factor-Beta1 in diabetic kidney disease. Front Cell Dev Biol. 2020;8(187–87). doi: 10.3389/fcell.2020.00187
- Guerreiro Í, Ferreira-Pêgo C, Carregosa D, et al. Polyphenols and their metabolites in renal diseases: An overview. Foods (Basel, Switzerland), 2022;11(7):1060. doi: 10.3390/foods11071060
- Costa EV, Pinheiro MLB, de Souza ADL, et al. Trypanocidal activity of oxoaporphine and pyrimidine-β-carboline alkaloids from the branches of Annona foetida Mart. (Annonaceae). Molecules (Basel, Switzerland), 2011;16(11):9714–9720. doi: 10.3390/molecules16119714
- Mestry SN, Dhodi JB, Kumbhar SB, et al. Attenuation of diabetic nephropathy in streptozotocin-induced diabetic rats by Punica granatum Linn. leaves extract. J Tradit Complement Med. 2016;7(3):273–280. doi: 10.1016/j.jtcme.2016.06.008
- Gupta RK, Kesari AN, Murthy PS, et al. Hypoglycemic and antidiabetic effect of ethanolic extract of leaves of Annona squamosa L. in experimental animals. J Ethnopharmacol. 2005;99(1):75–81. doi: 10.1016/j.jep.2005.01.048
- Kumar M, Changan S, Tomar M, et al. Custard Apple (Annona squamosa L.) Leaves: Nutritional Composition. Phytochem Profile Health-Promoting Biol Act Biomol. 2021;11(5):614. doi: 10.3390/biom11050614
- Onwusonye J, Uwakwe A, Patrick A, et al. Acute and sub-acute toxicity studies of methanol leaf extracts of Annona squamosa Linn. in mice. Sky J Biochem Res. 2014;3(7):53–59.
- Rout SP, Kar DM, Maharana L. Anti-hyperglycemic effect of different fractions of annona reticulata leaf. Asian J Pharm Clin Res. 2016:256. doi: 10.22159/ajpcr.2016.v9s2.13710
- Sivakumar S, Palsamy P, Subramanian SP. Impact of d-pinitol on the attenuation of proinflammatory cytokines, hyperglycemia-mediated oxidative stress and protection of kidney tissue ultrastructure in streptozotocin-induced diabetic rats. Chem Biol Interact. 2010;188(1):237–245. doi: 10.1016/j.cbi.2010.07.014
- Kumar S, Mondal H, Lata M, et al. Correlation of serum uric acid with lipid profile in patients with type 2 diabetes mellitus with normal creatinine level: Report from a tertiary care hospital in India. J Family Med Prim Care. 2022;11(6):3066–3070. doi: 10.4103/jfmpc.jfmpc_2131_21
- Kaleem M, Medha P, Ahmed Q, et al. Beneficial effects of Annona squamosa extract in streptozotocin-induced diabetic rats. Singapore Med J. 2008;49(10):800. Available from: https://www.ncbi.nlm.nih.gov/pubmed/18946614
- Adil M. Qualitative features and therapeutic value of custard apple fruit. Int J Food And Allied Sci. 2019;4(1):12–17.
- Deshmukh AB, Patel JK. Aqueous extract of Annona squamosa (L.) ameliorates renal failure induced by 5/6 nephrectomy in rat. Indian J Pharmacol. 2011;43(6):718–721. doi: 10.4103/0253-7613.89834
- Bianchi C, Donadio C, Tramonti G, et al. REAPPRAISAL of SERUM β2-MICROGLOBULIN as MARKER of GFR. Ren Fail. 2001;23(3–4):419–429. doi: 10.1081/jdi-100104725
- Liabeuf S, Lenglet A, Desjardins L, et al. Plasma beta-2 microglobulin is associated with cardiovascular disease in uremic patients. Kidney Int. 2012;82(12):1297–1303. doi: 10.1038/ki.2012.301
- Wu H-C, Lee L-C, Wang W-J. Associations among serum beta 2 Microglobulin, malnutrition, inflammation, and advanced cardiovascular event in patients with chronic kidney disease. J Clin Lab Analysis. 2017;31(3):e22056. doi: 10.1002/jcla.22056
- Kim MK, Yun KJ, Chun HJ, et al. Clinical utility of serum beta-2-microglobulin as a predictor of diabetic complications in patients with type 2 diabetes without renal impairment. Diabetes & Metabolism. 2014;40(6):459–465. doi: 10.1016/j.diabet.2014.08.002
- Mwagandi Chimbevo L, Essuman S. Preliminary Screening of Nutraceutical Potential of Fruit Pulp, Peel and Seeds from Annona Squamosa (L.) and Annona Muricata (L.) Growing in Coast Region of Kenya. AJBIO. 2019;7(3):58. doi: 10.11648/j.ajbio.20190703.11
- Korah MC, Rahman J, Rajeswari R, et al. Evaluation of diuretic efficacy and antiurolithiatic potential of ethanolic leaf extract of Annona squamosa Linn. In experimental animal models. Indian J Pharmacol. 2020;52(3):196–202. doi: 10.4103/ijp.IJP_92_18
- Satyanarayana G, Keisham N, Batra HS, et al. Evaluation of serum ceruloplasmin levels as a biomarker for oxidative stress in patients with diabetic retinopathy. Cureus. 2021;13(2):e13070–e70. doi: 10.7759/cureus.13070
- Nowak M, Wielkoszyński T, Marek B, et al. Antioxidant potential, paraoxonase 1, ceruloplasmin activity and C-reactive protein concentration in diabetic retinopathy. Clin Exp Med. 2009;10(3):185–192. doi: 10.1007/s10238-009-0084-7
- Inoue K, Sakano N, Ogino K, et al. Relationship between ceruloplasmin and oxidative biomarkers including ferritin among healthy Japanese. J Clin Biochem Nutr. 2013;52(2):160–166. doi: 10.3164/jcbn.12-122
- Mohora M, Vîrgolici B, Coman A, et al. Diabetic foot patients with and without retinopathy and plasma oxidative stress. Rom J Intern Med. 2007;45(1):51–57.
- Lee MJ, Jung CH, Kang YM, et al. Serum ceruloplasmin level as a predictor for the progression of diabetic nephropathy in Korean Men with type 2 diabetes mellitus. Diabetes Metab J. 2015;39(3):230–239. doi: 10.4093/dmj.2015.39.3.230
- Kothari V, Seshadri S. Antioxidant activity of seed extracts of Annona squamosa and Carica papaya. Nutr Food Sci. 2010;40(4):403–408. doi: 10.1108/00346651011062050
- Albuquerque TG, Santos F, Sanches-Silva A, et al. Nutritional and phytochemical composition of Annona cherimola Mill. fruits and by-products: potential health benefits. Food Chem. 2016;193(187–95). doi: 10.1016/j.foodchem.2014.06.044
- Jana S, Mitra P, Roy S. Proficient novel biomarkers guide early detection of acute kidney injury: A review. Diseases (Basel, Switzerland), 2022;11(1):8. doi: 10.3390/diseases11010008
- Joshi A, Mathur A, Parashar R, et al. A Study of Role of Ngal in Diagnosis and Staging the Severity of Diabetic Nephropathy in Type 2 Diabetes Mellitus Patients in Sms Medical College, Jaipur. J Assoc Physicians India. 2022;70(4):11–12. Available from: https://www.ncbi.nlm.nih.gov/pubmed/35443326
- Hammoud MS, Baban RS, Ali SH. EVALUATION of URINARY KIDNEY INJURY MOLECULE-1 (KIM-1) as PROGNOSTIC BIOMARKER in CHILDREN with TYPE-1 DIABETIC NEPHROPATHY. Biochemical & Cellular Archive. 2021;21(1):715–719.
- Dellai A, Maricic I, Kumar V, et al. Parallel synthesis and anti-inflammatory activity of cyclic peptides cyclosquamosin D and met-cherimolacyclopeptide B and their analogs. Bioorg Med ChemBioorganic & Medicinal Chemistry Letters. 2010;20(19):5653–5657. doi: 10.1016/j.bmcl.2010.08.033
- Abd-Elrazek A, Shapana H, Shukry W, et al. Comparison between Annona squamosa, Annona cherimolia and Annona atemoya ethanolic extracts extenuative impact against oxidative stress, inflammation and apoptosis in rat kidney induced by Ifosfamid. Toxicol Res (Camb). 2021;10(4):947–958. doi: 10.1093/toxres/tfab078
- Alkhalidy H, Al-Nabulsi A, Mhawish R, et al. Low-dose of phenolic rich extract from Annona squamosa Linn leaves ameliorates insulin sensitivity and reduces body weight gain in HF diet-induced obesity. Front Nutr. 2023;10:10(1146021–21. doi: 10.3389/fnut.2023.1146021
- Aderibigbe K, Komolafe OA, Adewole OS, et al. Anti hyperglycemic activities of Annona muricata (Linn). Afr J Trad Compl Alt Med. 2009;6(1). doi: 10.4314/ajtcam.v6i1.57075
- Cheng D, Liang B, Li Y. Antihyperglycemic effect of Ginkgo biloba extract in streptozotocin-induced diabetes in rats. Bio Med Res Int. 2013;2013:162724–24. doi: 10.1155/2013/162724
- Muthukumar K, Nachiappan V. Cadmium-induced oxidative stress in Saccharomyces cerevisiae. Indian J Biochem Biophys. 2010;47(6):383–387. Available from: https://www.ncbi.nlm.nih.gov/pubmed/21355423
- Muthukumar K, Rajakumar S, Sarkar MN, et al. Glutathione peroxidase3 of Saccharomyces cerevisiae protects phospholipids during cadmium-induced oxidative stress. Antonie Van Leeuwenhoek. 2011;99(4):761–771. doi: 10.1007/s10482-011-9550-9
- Morsy MD, Hassan WN, Zalat SI. Improvement of renal oxidative stress markers after ozone administration in diabetic nephropathy in rats. Diabetol Metab Syndr. 2010;2(1):29–29. doi: 10.1186/1758-5996-2-29
- Darenskaya M, Kolesnikov S, Semenova N, et al. Diabetic nephropathy: significance of determining oxidative stress and opportunities for antioxidant therapies. Int J Mol Sci. 2023;24(15):12378. doi: 10.3390/ijms241512378
- Yoshida S-I, Hashimoto T, Kihara M, et al. Urinary oxidative stress markers closely reflect the efficacy of candesartan treatment for diabetic nephropathy. Nephron Exp Nephrol. 2008;111(1):e20–e30. doi: 10.1159/000178764
- Lu Y, Xing C, Lv X, et al. Changes of ACE2 in different glucose metabolites and its relationship with COVID-19. Medicine (Baltimore). 2022;101(41):e31102–e02. doi: 10.1097/MD.0000000000031102
- Kalidindi N, Thimmaiah NV, Jagadeesh NV, et al. Antifungal and antioxidant activities of organic and aqueous extracts of Annona squamosa Linn. Leaves J Food Drug Anal. 2015;23(4):795–802. doi: 10.1016/j.jfda.2015.04.012
- de Freitas Laiber Pascoal G, de Almeida Sousa Cruz MA, de Abreu J P, et al. Evaluation of the antioxidant capacity, volatile composition and phenolic content of hybrid vitis vinifera L. varieties sweet sapphire and sweet surprise. Food Chem. 2022;366(130644):130644. doi: 10.1016/j.foodchem.2021.130644
- Sawczuk R, Karpinska J, Filipowska D, et al. Evaluation of total phenols content, anti-DPPH activity and the content of selected antioxidants in the honeybee drone brood homogenate. Food Chem. 2022;368(130745):130745. doi: 10.1016/j.foodchem.2021.130745
- Varadharajan V, Janarthanan UK, Krishnamurthy V. Physicochemical, phytochemical screening and profiling of secondary metabolites of Annona squamosa leaf extract. World J Pharm Res. 2012;1(4):1143–1164.
- Dubey S, Ojha K, Chandrakar J, et al. Assessment of total phenolic content and antioxidant potentiality of selected Indian folk medicinal plants by spectrophotometric method. Plant Sci Today. 2020;7(3):383–390. doi: 10.14719/pst.2020.7.3.765
- Kdrr S, Sirasa MSF. Antioxidant properties of selected fruit cultivars grown in Sri Lanka. Food Chem. 2018;238(203–08):203–208. doi: 10.1016/j.foodchem.2016.08.102
- Leite DOD, Camilo CJ, Nonato C, et al. Chemical Profile and evaluation of the antioxidant and anti-acetylcholinesterase activities of Annona squamosa L. (Annonaceae) extracts. Foods (Basel, Switzerland), 2021;10(10):2343. doi: 10.3390/foods10102343
- Neelima S, Dwarakanadha Reddy P, Kothapalli Bannoth CS. Nephroprotective activity of Annona Squamosa leaves against paracetamol-induced nephrotoxicity in rats: in vitro and in vivo experiments. Future J Pharm Sci. 2020;6(1). doi: 10.1186/s43094-020-00149-4
- Wen W, Ti Z, Ti Z. Antidiabetic, antihyperlipidemic, antioxidant, anti-inflammatory activities of ethanolic seed extract of Annona reticulata L. in streptozotocin induced diabetic rats. Front Endocrinol. 2019;10(438167). doi: 10.3389/fendo.2019.00716
- Ahmadvand H, Mahdavifard S. Protective effect of thioctic acid on renal ischemia–reperfusion injury in rat. Int J Prev Med. 2019;10(1):176–76. doi: 10.4103/ijpvm.IJPVM_396_17
- Kanasaki K, Taduri G, Koya D. Diabetic nephropathy: the role of inflammation in fibroblast activation and kidney fibrosis. Front Endocrinol (Lausanne). 2013;4:7–7. doi: 10.3389/fendo.2013.00007
- Qiao Y-C, Chen Y-L, Pan Y-H, et al. The change of serum tumor necrosis factor alpha in patients with type 1 diabetes mellitus: A systematic review and meta-analysis. PloS one. PLoS One. 2017;12(4):e0176157–e57. doi: 10.1371/journal.pone.0176157
- Mitrović M, Popović Đ, Naglić D, et al. Markers of inflammation and microvascular complications in type 1 diabetes. Open Med. 2014;9(6):748–753. doi: 10.2478/s11536-013-0335-6
- Shi G-J, Li Y, Cao Q-H, et al. In vitro and in vivo evidence that quercetin protects against diabetes and its complications: A systematic review of the literature. Biomedicine & Pharmacotherapy. 2019;109(1085–99). doi: 10.1016/j.biopha.2018.10.130 1085–1099
- Chavan MJ, Wakte PS, Shinde DB. Analgesic and anti-inflammatory activity of caryophyllene oxide from Annona squamosa L. bark. Phytomedicine. 2010;17(2):149–151. doi: 10.1016/j.phymed.2009.05.016
- Zhu L, Gu P, Shen H. Gallic acid improved inflammation via NF-κB pathway in TNBS-induced ulcerative colitis. Int Immunopharmacol. 2019;67(129):129–137. doi: 10.1016/j.intimp.2018.11.049
- Awada N, Ayoub A, Jaber A, et al. Evaluation of the Anticancer, Anti-Inflammatory, and Antioxidant properties of various extracts of Annona Squamosa L. Pharm Sci. 2023;29(3):384–394. doi: 10.34172/ps.2023.5
- Tabara M, Shiraishi K, Takii R, et al. Testicular localization of activating transcription factor 1 and its potential function during spermatogenesis. Biol Reprod. 2021;105(4):976–986. doi: 10.1093/biolre/ioab099
- Khamis T, Abdelkhalek A, Abdellatif H, et al. BM-MSCs alleviate diabetic nephropathy in male rats by regulating ER stress, oxidative stress, inflammation, and apoptotic pathways. Front Pharmacol. 2023;14(1265230–30). doi: 10.3389/fphar.2023.1265230