Abstract
Wireless implants for interaction with the cortex have developed rapidly over the last decade and increasingly meet demands of clinical brain–computer interfaces. For such applications, well-established technologies are available, suitable for recording of neural activity at different spatial scales and adequate for modulating brain activity by cortical electrical stimulation. The incorporation of recording and stimulation into closed-loop systems is a major aim in active, fully implantable medical device design. To reduce clinical long-term implantation risk and to increase the spatial specificity of epicortical recordings and stimulation, micro-electrocorticography is a promising technology. However, currently there is a lack of implants suitable for chronic human clinical applications that utilize micro-electrocorticography and possess closed-loop functionality. Here, we describe the clinical importance of cortical stimulation, give an overview of existing implants that use mainly epicortical recording methods, and present results of a closed-loop micro-electrocorticography system developed for clinical application within a collaborative framework. Finally, we conclude with our vision of future design options in the field of neuroprosthetic devices.
1. Introduction
Recently, there has been growing interest to build devices that can be implanted chronically without the use of connector solutions, with the aim of interacting with the central nervous system for research or clinical purposes, e.g. brain–computer/brain–machine interfacing (BCI/BMI). There exist different concepts to interface the cerebral cortex, from non-invasive, superficial techniques like electroencephalography (EEG [Citation1,2]), to invasive but epicortical techniques like electrocorticography (ECoG [Citation3–5]) to cortex-penetrating intra-cortical electrodes with large numbers of individual contacts [Citation6–9]. Of these methods, all have been shown to provide suitable electrophysiological signals for BCI/BMI applications.
Increased invasiveness leads to an increased selectivity down to the recording scale of single-unit and multi-unit activity and hence more fine-grained electrophysiology, albeit on a limited area of the cortex. On the other hand, due to its high invasivness, intra-cortical implantation generates a higher risk for the patient in the form of infection or possible biological rejection of the device. In addition, intra-cortical implantations can be associated with substantial signal loss, particularly during long-term implantations, which are of interest for clinical applications. For a more extensive review on non-invasive and intra-cortical neuroprosthetic approaches, see [Citation10,11].
One promising technology for long-term neural recordings on the mesoscopic scale is electrocorticography (ECoG). This technique provides detailed spatio-temporal information on cortical population activity beyond what can be achieved with non-invasive methods, with better signal quality [Citation12] and minimal harm to brain tissue. However, a better understanding of the characteristics and underlying mechanisms of ECoG signal changes in chronic long-term implantations would be desirable. The standard clinical ECoG electrode arrays used in previous studies have contacts with a diameter of several millimeters and an inter-contact distance on the order of centimeters. In the last few decades, however, there is growing interest in reducing the clinical risk for chronic implantations by decreasing the overall size of epicortical electrode arrays and to further increase the spatial resolution of ECoG recordings at once. To this end, novel micro-electrocorticography (μECoG) electrode arrays with a smaller contact size and higher electrode density than conventional ECoG have been developed for chronic implantation [Citation13,14]. These arrays may record even down to spike activity as measured with intra-cortical devices [Citation15], suggesting the information content that can be exploited for decoding applications to be closer to intra-cortical recordings [Citation16,17] than previously thought (compared to non-invasive and conventional ECoG recordings). Consequently, μECoG has already been utilized in BCI research [Citation17–20] as well. Besides basic research applications [Citation21–24], these μECoG properties might be useful for many clinical applications where detailed spatio-temporal information is required. These applications include the detection of epileptic tissue [Citation14], electrical stimulation mapping [Citation25–27], functional mapping of cortex [Citation28–30], or regeneration of neural connectivity [Citation31], e.g. after stroke or spinal cord injury [Citation32].
Cortical electrical stimulation (CES), which is involved in many of the aforementioned applications, is a useful tool for researchers, since it provides manifold possibilities to interact with the cerebral cortex. Aside from stimulation approaches in the auditory (e.g. cochlear implants), visual [Citation33,34] and language systems [Citation35,36], researchers only recently used CES on somatosensory and motor cortices to induce artificial somatosensory sensations. In the latter studies, researchers artificially evoked somatosensory perception in patients looking at a rubber hand foreign to the subjects’ body [Citation37], to restore sensations in patients while they used a BCI [Citation38,39] or restored sensation in patients with spinal cord injury [Citation40]. These researchers have been trying to combine the approaches of electrical recording and stimulation as closed-loop systems. Closed-loop systems react autonomously and flexibly to changes in brain activity. This makes them especially interesting for applications that demand stimulation with a high spatio-temporal accuracy. In addition, closed-loop systems can operate in an energy-saving manner and without the need of supervising human personnel. Examples are, amongst others, automatic epileptic seizure detection, tremor detection in Parkinson’s disease, or feedback stimulation as used in assistive BCIs as described above. To provide patients with the possibility of improving their quality of life on a long-term scale, researchers aim for the modularization and translation of invasive recording technologies into active implantable medical devices (AIMDs).
Here, we summarize recent advances in the development of fully implantable AIMDs (devices not relying on connector solutions), for interaction with the cerebral cortex. We present a state-of-the-art μECoG-based implant with closed-loop stimulation ability which was developed in a joint-venture between partners of the University of Freiburg, the University Medical Center Freiburg, and CorTec GmbH, Freiburg. Finally, we discuss future directions regarding the development of neuroprosthetic devices suitable for closed-loop human application in the clinical sector.
2. State of the art of active implantable systems for the cortex
There is a variety of existing AIMDs for interfacing the central nervous system, which are in different developmental stages. While some are currently in a prototype or bench-test phase with fast technological progress between two iterations, the ultimate aim for these devices are CE- or FDA-clearance. Other implants are approved for clinical utilization and have reached the necessary technological maturity. However, they are also more conservative in terms of technological sophistication: these devices utilize classical titanium housing, feedthrough, and electrode and cable technologies used in fabrication of conventional neuromodulator implants for decades.
Of these implantable devices, most utilize a large number of densely packed channels to perform neural recordings only. Implants [Citation41,42] rely on intra-cortical electrode technologies such as the popular Utah Array (Blackrock Microsystems) and have worked for quite some time in vivo. However, these electrode technologies may suffer from signal deterioration over time because of a structural mismatch between electrode material and brain tissue, electrode corrosion, or retraction of neuronal cells from the recording site [Citation6,43,44]. Devices focusing on ECoG technology [Citation45–47] may circumvent this problem by accumulating signals from larger neural populations, but comprehensive further studies on long-term implantations are also needed to investigate signal characteristics on a long-term scale. Nonetheless, these devices have a strong precedent for clinical applications, because of the likely lower surgical risk due to their non-penetrating electrode arrays and their technological relation to approved sub-chronic applications, e.g. seizure localization in epilepsy patients, using ECoG electrode arrays. However, several studies show that epicortical implantation may be accompanied by severe complications, amongst them, epi- and subdural hematoma, cerebral infarcts, increase of intracranial pressure, brain edema [Citation48–50]. The risk for such complications could be reduced by miniaturization of electrode arrays and devices. However, challenges in miniaturization, electrode development, and packaging seem to hinder some of the long-term experimental approaches. Implants with a large number of miniaturized electrode contacts require appropriate miniaturization of electronic components (amplifiers, etc.) and appropriate housing of miniaturized electronics. Furthermore, they require the ability to transfer large amounts of data via wireless transmission. As engineers overcome these issues, long-term implantation periods are expected to increase as the size of devices decreases.
However, research studies [Citation51,52] and the success of the two commercially available systems, Neuropace RNS® [Citation53] and Medtronic’s Activa PC+S® [Citation54], emphasize, despite their comparatively low channel count, progress of implantable devices, but also the need for miniaturization and closed-loop systems. Recently, researchers reported chronic implantation of an eight-channel version of Medtronic’s Activa PC+S® device in an amyotrophic lateral sclerosis (ALS) patient, which successfully operated several BCI applications, albeit the stimulation function was disabled in this implantation [Citation55]. Although all of these devices represent important contributions towards the optimization of neuroprosthetic implants, an overview in Table reveals that there is a lack of implant systems suitable for chronic human clinical application providing fully encapsuled high-channel μECoG electrode technology combined with closed-loop functionality.
Table 1. State-of-the-art AIMDs for interfacing the brain with evaluation of closed-loop suitability.
3. Fully implantable closed-loop devices developed in Freiburg
3.1. The first pre-clinical generation
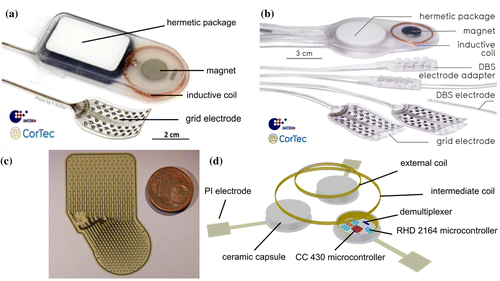
The implant system called Braincon (Figure (a)) was the first fully implantable, wireless AIMD, which was developed within the research collaboration in Freiburg and chronically utilized in animals (sheep model). The implant records from 16 electrode contacts (200x amplification, 1 kS/s at 16 bit) and is capable of voltage-controlled stimulation (max. voltage compliance: 18 V) through eight electrode contacts, all located on the same grid-type electrode array to be placed sub- or epidurally on the cortex. Electrode arrays suitable for μECoG recordings and equipped with 32 platinum-iridium electrode contact sites are manufactured in a customizable laser-based fabrication process [Citation56]. The recording and stimulation functionality is permanently assigned to the individual electrode contacts on the grid (16+8 out of 32 possible electrode contacts) during implant manufacturing. Each electrode contact is 1.1 mm in diameter with a center-to-center distance of 4 mm. The hermetically sealed three-part ceramic package hosts amplifiers, stimulation circuits, and infrared telemetry [Citation57]. An inductive coil for telemetric power reception (carrier frequency 16 MHz) is placed outside the package together with a permanent magnet in its center in order to allow alignment to the extracorporeal transceiver unit. This in turn is connected to a computer via universal serial bus (USB) 2.0, acting as communication and power interface. The computer runs proprietary software [Citation58], which permits recording, filtering, feature extraction, etc. of the cortical signals and generates commands to initiate electrical stimulation pulses. This can be accomplished either in open- or closed-loop mode. Latencies between recording and stimulation are in the range of tens of milliseconds, predominantly caused by latencies of up to 15 ms introduced by the operating system (in this case Windows 7), which manages the USB interface of the laptop PC that runs the implant application software. The latencies introduced by the implant itself are below 2 ms; additionally, up to 2 ms are introduced by the extracorporeal control unit.
Figure 1. State of the art implant system and future design options for closed-loop neuroimplants developed in Freiburg, Germany. (a) The Braincon μECoG-based implant system utilized in former animal studies consisting of a hermetic package, which hosts internal electronic components, a magnet for proper alignment between inductive coil and an extracorporeal powering device and one electrode array. (b) The BrainInterchange implant system supporting two μECoG electrode arrays and two DBS leads for neural recordings and stimulation. (c) 246-channel μECoG electrode array for future high-resolution neural recordings. Upper (rectangular) part of the array: area with electrode contacts. Lower (circular) part of the array: area connected to electronic parts. (d) Option for future brain implants utilizing highly efficient multi-coil inductive powering technology for a modular device design.
Overall, three Braincon devices were implanted in three animals for 98, 463, and 575 days. All animal experiments in the study were approved by the Animal Committee of the University of Freiburg and the Regierungspräsidium Freiburg, Baden-Wuerttemberg, and EU Directive 2010/63/EU was followed for all experiments. The electrode arrays were placed beneath the dura mater on the somatosensory cortex and the hermetic package carrying the electronics was located in the back of the sheep.
In acute experiments with peripheral electrical stimulation in, the Braincon system could compete with commercial neural recording systems (e.g. the gUSBamp from gtec, Schiedlberg, Austria) in terms of signal quality and resolution despite its small dimensions and location of operation in a living organism (Figure ). The presented results are based on data from 34 (gUSBamp) and 27 (BrainCon) recording sessions respectively. Each recording session comprised ca. 360 trials that were recorded during electrical stimulation in the orofacial area of the acutely implanted (somatosensory cortex) and anesthetized sheep. Additional results from recordings in acute implantations of the electrode array and chronic implantation of the Braincon system showed that electrical stimulation in the orofacial region of the sheep elicited clear somatosensory evoked potentials in a somatotopic manner which were accompanied by significant changes in the spectral domain in the acute setting [Citation23]. Results from stimulation experiments with the Braincon device are the main topic of a paper currently under review featuring this closed-loop system. In it we show that closed-loop stimulation with different stimulation frequencies elicits spectral changes with distinct spatio-temporal patterns. Despite these encouraging results, the limited amount of channels (16 for recording and 8 for stimulation) and the challenge to maintain strictly low humidity levels within the hermetic encapsulation over time demanded a successive generation device. This new device also underwent miniaturization for compatible implantation in the human skull.
Figure 2. Signal quality comparison between commercial amplifier and the BrainCon system. (a) Somatosensory evoked potential (SEP) for the μECoG electrode array manufactured by CorTec connected either to the gUSBamp (blue trace) or to the BrainCon system (magenta trace). The black rectangle at the SEP peak depicts the time window for which signal strength, noise strength and signal-to-noise ratio were calculated. The shown SEP was recorded at the electrode contact with maximum response to orofacial electrical stimulation. (b) Signal strength (SEP, averaged potential across trials), noise strength (interquartile range across trials), and signal-to-noise ratio (SNR, signal strength divided by interquartile range) for the two different measurement types. In these experiments, no significant differences in signal characteristics between the two amplifier systems could be observed (Wilcoxon rank sum test).
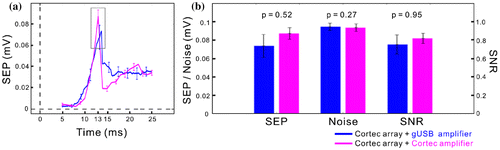
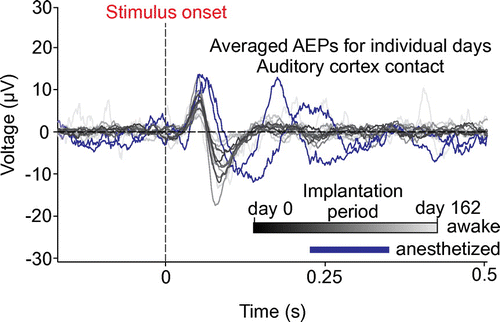
Figure 3. Summary of recording experiment for the second generation device (BrainInterchange). Gray-scaled traces represent the averaged AEP from individual recording days and one contact on the auditory cortex electrode array while the animal was awake (from black = day 0 to light-gray = day 162 after implantation) and blue traces represent measurement under general anesthesia (each measurement consisted of 121 to 300 trials). Dashed lines indicate the stimulus onset (vertical) and the zero potential (horizontal).
3.2. The second chronically implantable generation
The next development of the implant system, named BrainInterchange (Figure (b)), utilizes an extended hermetic packaging concept for improved long-term reliability by the use of a double-sealing methodology for reduced water leakage into the implant to protect its electronics. A circular-shaped alumina housing is soldered onto a ceramic substrate (ø36 mm), which has been functionalized with screen-printed tracks, isolation layers, and a solderable thick film ring. Additionally, an internal epoxy seal provides protection of the electronics against potentially corrosive fumes from the sealing procedure [Citation59]. The BrainInterchange uses its own application-specific integrated circuit (ASIC [Citation60]), allowing for each of the 32 channels to be used, for electrical stimulation as well as for neural recording (1 kS/s, 12 bit) and complex impedance measurements. Electrical stimulation is done using current-controlled, biphasic pulses with amplitudes up to 6 mA and pulse widths of up to 1 ms. The compliance voltage is 18 V. The implant provides eight individual current sources, which permit current steering for effective and more localized stimulation. The implant is inductively powered by the extracorporeal transceiver unit using a reduced carrier frequency of 250 kHz compared to the former generation’s 16 MHz, since lower inductive carrier frequencies allow for more efficient power transmission. Communication to the extracorporeal transceiver unit is still done via infrared link and data storage is achieved via direct connection to the computer using a USB cable. Up to two electrode arrays, which can be customized according to the application and shape of the cortex, can be hard-wired to the implant. Furthermore, the device provides adapter solutions to connect up to two four-channel deep brain stimulation electrodes by Medtronic Inc. (e.g.e.g. 3387 or 3389 leads). An initial 162-day sheep study has been performed with the new implant to demonstrate its in vivo functionality and reliability. In short, we were able to record auditory evoked potentials (AEPs) across the complete implantation period in a robust manner using tones with a duration of 3 s, a center frequency of 8 kHz and a noise bandwidth of 125 Hz. Tones were delivered to the awake animal and AEPs could be recorded using a μECoG electrode array that was chronically implanted on the auditory cortex of the sheep. However, amplitudes decreased over time (Figure ), showing loss of signal strength as it can also occur in the case for intra-cortical long-term recordings. However, reduced signal amplitudes do not necessarily imply relevant reductions in the signal-to-noise ratio of relevant signal components, particularly if the main source of noise would also be brain signals, possibly decrementing in parallel to the ‘signal’. To address these issues, we performed a more comprehensive analysis of signal, noise, and signal-to-noise characteristic for long-term implantations of both device generations to find the origin of this signal decay. The corresponding analysis and outcomes would be out of scope for the present paper and are hence under preparation for a separate study.
While the next steps certainly are long-term animal applications with a strong focus on closed-loop utilization, the implant is mainly developed for human application. Its dimensions (60 × 38 × 7 mm3), comparable to the NeuroPace RNS® and some cochlear implants, allow for implantation in the human skull. Furthermore, safety measures in compliance with AIMD directive 90/385/ECC ensuring safe stimulation have been implemented.
4. Design directions
The development and efficacy assessment of medical implants for closed-loop interaction with the human cortex are still in their beginnings, yet researchers envision next-generation devices for even more sophisticated recording and stimulation that also harness highly efficient powering options for wireless brain implants. The design options envisioned in the Freiburg initiative have two key features: high-density μECoG arrays and the modular design of implantable devices.
Although existing μECoG electrode arrays may have several advantages compared with clinical standard ECoG arrays regarding the spatial specificity of neural recordings [Citation16] and stimulation on the cortex, further development of miniaturized arrays with even smaller electrode contacts is subject to current engineering efforts. Such next-generation arrays may be beneficial for several clinical application areas, including BCIs meant for the control of artificial limbs, spatially specific recordings in epilepsy patients to further localize the areas involved in seizure generation, and the design of closed-loop systems using electrical cortical stimulation. Higher spatial specificity may be crucial for systems aiming at the induction or strengthening of micro-scale neuronal connectivity. To this end, the Freiburg initiative works on high-density μECoG electrode arrays of which an example is shown in Figure (c). The presented array has a maximum of 246 recording or stimulation electrode contacts on an area of approximately 20 mm2, which can be attached to a hermetic ceramic package housing the electronics. The main components are a digitization unit (RHD2164, Intan Technologies, Los Angeles, USA), which supports up to 64 recording channels per unit and provides input for a microcontroller (CC430, Texas Instruments Inc., Dallas, USA) with built-in sub-GHz RF communication link. Several of these modular units can be inductively powered by a multi stage coil system, consisting of an external coil, positioned outside the skull, and an intermediate coil positioned inside the skull. This technology, described recently [Citation61, 62], ensures an inductive powering with one power source for all modules instead of individual powering. Furthermore, this technology may improve inductive coupling, which allows for smaller coils and therefore a more flexible positioning of the implant. An example of such a modular brain implant is modeled in Figure (d). This modular design is highly beneficial for neural recordings on more than two cortical areas, for example when investigating stroke-induced connectivity changes within the cortical sensorimotor system or possibly the treatment of multi-focal epilepsy. Both examples may also benefit from the closed-loop stimulation system incorporated in such a wireless brain implant.
5. Requirements and challenges for the next decade and beyond
From the technological point of view, important five-year goals for the development of implantable devices for closed-loop interaction with the cerebral cortex are: (1) improved telemetric powering and data transmission; (2) the use of implantable low-profile reversible connectors suitable for electrode arrays with high electrode contact counts; and (3) the development of smaller electrode contacts for improved stimulation and recording. Electrode size does not correlate with electrochemical safety issues if recording is performed only. Stimulation, on the other hand, is strongly entangled with the question of electrode contact miniaturization and linked to the challenge of safe and sufficient charge injection for interaction with the cortex [Citation63]. The important topic here is the so-called charge injection density, which is an electrical charge (current amplitude multiplied by the pulse width) per electrode contact surface area. The charge injection density is the dominating property that sets the safety limits of electrical stimulation, below which the risk of electrode contact corrosion and tissue irritation can be neglected. It is specific for a given material, e.g. for platinum-iridium, literature describes typical values around 90μC/cm2 [Citation64]. Since this value needs to be multiplied with the electrode contact area in order to obtain the actual charge (stimulation intensity), the maximum charge that can safely be injected without risking electrode contact corrosion is proportional to the electrode contact diameter squared.
Additional challenges exist in improving and miniaturizing battery and energy storage technology, which could solve power-supply problems linked to extensive power consumption of, e.g. stimulation strategies directly linked to continuous adaptive signal processing. Even intracorporeal energy harvesting devices could be employed to power at least parts of implantable devices to maintain low-power sensor functionalities [Citation65, 66]. Overall device miniaturization in general could reduce the extent of surgical intervention, while other challenges arise from this aim. Smaller devices should provide the same or a higher degree of technological sophistication, while maintaining reliability for safe and efficient recording and stimulation. However, reliability is always at risk if a technology is miniaturized without knowing its long-term performance. Despite all accelerated aging concepts, thorough testing and clinical approval of significantly smaller, high channel count commercial devices could take roughly a decade. However, these and associated questions remain to be investigated more thoroughly, especially regarding the clinical approval of new technologies and materials, which is of course a persistent topic on all time and spatial scales.
Aside from technological challenges, the patient’s willingness to undergo surgery for implantation is a limiting factor for the testing and further development of implantable devices to be used for clinical purposes. In two recent studies, 50% of respondents suffering from various neurological diseases (stroke, amyotrophic lateral sclerosis (ALS), spinal cord injury, muscular dystrophy and neuropathies) [Citation67] and 72% of patients suffering from ALS [Citation68] were willing to undergo surgery if the devices would considerably improve their communication ability and mobility or if implant performance reaches an acceptable degree. This acceptance rate could potentially be increased as well by a reduced extent of the surgical intervention, e.g. by device miniaturization as described above and hence risk for the patient.
On the time scale of the next 10 years, we envision a focus on: (1) smart switches to be employed in high channel count electrode arrays for a more flexible way of interacting with the cortical surface; (2) implantable BCIs designed to be compatible with magnetic resonance imaging (MRI) to monitor position of the systems in vivo and eventually their performance by means of fMRI; (3) the development of distributed systems with implant-implant intracorporeal communication, potentially including BCIs linked to peripheral nervous system implants as well as multi-modal implants (chemical, optical, etc.); (4) understanding and optimizing the implant-tissue interactions, e.g. to reduce connective tissue growth and improved signal stability for neural recordings in long-term implantations; (5) improved links to assistive devices, such as robotic prostheses or electric wheelchairs as reliable low-latency BCIs become available; and (6) the appropriate control of such BCIs using algorithms capable of processing high-dimensional data in a real-time closed-loop fashion.
Predictions even further into the future are increasingly difficult, particularly in a fast-evolving field such as neurotechnology. Nevertheless, as a major, overarching aim and perspective, one might expect that in the next 15 to 20 years neurotechnology will indeed enter broad clinical application and start changing the way treatment and diagnostics are routinely performed in many major neurologic disorders.
Funding
This work was supported by the German Federal Ministry of Education and Research, BMBF, [01GQ1510 OPTISTIM and 13GW0053D MotorBIC] and by the Deutsche Forschungsgemeinschaft, DFG, [EXC1086 BrainLinks-BrainTools].
Disclosure statement
Fabian Kohler, Joerg Fischer, Christian Stolle, Joern Rickert, and Martin Schuettler hold positions at CorTec GmbH, Freiburg Germany. Joern Rickert, Martin Schuettler, and Thomas Stieglitz hold shares at CorTec GmbH, Freiburg, Germany, and have financial interest in the developments described in this work which may be licensed to CorTec GmbH, Freiburg, Germany.
References
- Wolpaw JR, McFarland DJ. Control of a two-dimensional movement signal by a noninvasive brain-computer interface in humans. Proc Nat Acad Sci. 2004 Dec 21;101(51):17849–17854. 10.1073/pnas.0403504101
- Wolpaw JR, Birbaumer N, McFarland DJ, et al. Brain–computer interfaces for communication and control. Clin Neurophysiol. 2002 Jun;113(6):767–791. 10.1016/S1388-2457(02)00057-3
- Milekovic T, Fischer J, Pistohl T, et al. An online brain–machine interface using decoding of movement direction from the human electrocorticogram. J Neural Eng. 2012 Aug 1;9(4):046003. 10.1088/1741-2560/9/4/046003
- Hammer J, Fischer J, Ruescher J, et al. The role of ECoG magnitude and phase in decoding position, velocity, and acceleration during continuous motor behavior. Front Neurosci [Internet]. 2013 [cited 2016 Dec 22];7. Available from: http://journal.frontiersin.org/article/10.3389/fnins.2013.00200/abstract
- Hammer J, Pistohl T, Fischer J, et al. Predominance of movement speed over direction in neuronal population signals of motor cortex: intracranial EEG data and a simple explanatory model. Cereb Cortex. 2016 Jan 6;26(6):2863–2881. 10.1093/cercor/bhw033
- Rousche PJ, Normann RA. Chronic recording capability of the Utah Intracortical Electrode Array in cat sensory cortex. J Neurosci Methods. 1998 Jul 1;82(1):1–15. 10.1016/S0165-0270(98)00031-4
- Hochberg LR, Serruya MD, Friehs GM, et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006 Jul 13;442(7099):164–171. 10.1038/nature04970
- Kennedy PR, Bakay RAE. Activity of single action potentials in monkey motor cortex during long-term task learning. Brain Res. 1997 Jun 20;760(1-2):251–254. 10.1016/S0006-8993(97)00051-6
- Bartels J, Andreasen D, Ehirim P, et al. Neurotrophic electrode: method of assembly and implantation into human motor speech cortex. J Neurosci Methods. 2008 Sep 30;174(2):168–176. 10.1016/j.jneumeth.2008.06.030
- Lebedev MA, Nicolelis MAL. Brain–machine interfaces: past, present and future. Trends Neurosci. 2006 Sep;29(9):536–546. 10.1016/j.tins.2006.07.004
- Homer ML, Nurmikko AV, Donoghue JP, et al. Implants and decoding for intracortical brain computer interfaces. Annu Rev Biomed Eng. 2013;15:383–405. 10.1146/annurev-bioeng-071910-124640
- Ball T, Kern M, Mutschler I, et al. Signal quality of simultaneously recorded invasive and non-invasive EEG. NeuroImage. 2009 Jul 1;46(3):708–716. 10.1016/j.neuroimage.2009.02.028
- Henle C, Raab M, Cordeiro JG, et al. First long term in vivo study on subdurally implanted Micro-ECoG electrodes, manufactured with a novel laser technology. Biomed Microdevice. 2011 Feb 1;13(1):59–68. 10.1007/s10544-010-9471-9
- Viventi J, Kim D-H, Vigeland L, et al. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nat Neurosci. 2011 Dec;14(12):1599–1605. 10.1038/nn.2973
- Khodagholy D, Gelinas JN, Thesen T, et al. NeuroGrid: recording action potentials from the surface of the brain. Nat Neurosci. 2015 Feb;18(2):310–315.
- Kellis S, Sorensen L, Darvas F, et al. Multi-scale analysis of neural activity in humans: Implications for micro-scale electrocorticography. Clin Neurophysiol [Internet]. [cited 2015 Jul 6]; Available from: http://www.sciencedirect.com/science/article/pii/S1388245715006306
- Rouse AG, Williams JJ, Wheeler JJ, et al. Spatial co-adaptation of cortical control columns in a micro-ECoG brain–computer interface. J Neural Eng. 2016;13(5):056018. 10.1088/1741-2560/13/5/056018
- Kellis S, Miller K, Thomson K, et al. Decoding spoken words using local field potentials recorded from the cortical surface. J Neural Eng. 2010 Oct;7(5):056007. 10.1088/1741-2560/7/5/056007
- Kellis S, Hanrahan S, Davis T, et al. Decoding hand trajectories from micro-electrocorticography in human patients. In: 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). 2012. p. 4091–4094.
- Rouse AG, Williams JJ, Wheeler JJ, et al. Cortical Adaptation to a Chronic Micro-Electrocorticographic Brain Computer Interface. J Neurosci. 2013 Jan 23;33(4):1326–1330. 10.1523/JNEUROSCI.0271-12.2013
- Gierthmuehlen M, Ball T, Henle C, et al. Evaluation of μECoG electrode arrays in the minipig: experimental procedure and neurosurgical approach. J. Neurosci. Methods. 2011 Oct 30;202(1):77–86. 10.1016/j.jneumeth.2011.08.021
- Escabi MA, Read HL, Viventi J, et al. A high-density, high-channel count, multiplexed μECoG array for auditory-cortex recordings. J Neurophysiol. 2014 Sep 15;112(6):1566–1583. 10.1152/jn.00179.2013
- Gierthmuehlen M, Wang X, Gkogkidis A, et al. Mapping of sheep sensory cortex with a novel microelectrocorticography grid. J Comp Neurol. 2014 Nov 1;522(16):3590–3608. 10.1002/cne.23631
- Zippo AG, Romanelli P, Torres Martinez NR, et al. A novel wireless recording and stimulating multichannel epicortical grid for supplementing or enhancing the sensory-motor functions in monkey (Macaca fascicularis). Front Syst Neurosci [Internet]. 2015 May 12 [cited 2016 Jun 29];9. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4429233/
- Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. 10.1093/brain/60.4.389
- Molina-Luna K, Buitrago MM, Hertler B, et al. Cortical stimulation mapping using epidurally implanted thin-film microelectrode arrays. J Neurosci Methods. 2007 Mar 30;161(1):118–125. 10.1016/j.jneumeth.2006.10.025
- Hosp JA, Molina-Luna K, Hertler B, et al. Thin-film epidural microelectrode arrays for somatosensory and motor cortex mapping in rat. J Neurosci Methods. 2008 Jul 30;172(2):255–262. 10.1016/j.jneumeth.2008.05.010
- Crone NE, Miglioretti DL, Gordon B, et al. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain. 1998 Dec 1;121(12):2301–2315. 10.1093/brain/121.12.2301
- Crone NE, Miglioretti DL, Gordon B, et al. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. I. Alpha and beta event-related desynchronization. Brain. 1998 Dec 1;121(12):2271–2299. 10.1093/brain/121.12.2271
- Crone NE, Sinai A, Korzeniewska A. High-frequency gamma oscillations and human brain mapping with electrocorticography. In: Christa Neuper and Wolfgang Klimesch, editor. Progress in Brain Research [Internet]. Elsevier; 2006 [cited 2013 Jan 22]. p. 275–295. Available from: http://www.sciencedirect.com/science/article/pii/S0079612306590193
- Jackson A, Mavoori J, Fetz EE. Long-term motor cortex plasticity induced by an electronic neural implant. Nature. 2006 Nov 2;444(7115):56–60. 10.1038/nature05226
- Nishimura Y, Perlmutter SI, Eaton RW, et al. Spike-timing-dependent plasticity in primate corticospinal connections induced during free behavior. Neuron. 2013 Dec 4;80(5):1301–1309. 10.1016/j.neuron.2013.08.028
- Schmidt EM, Bak MJ, Hambrecht FT, et al. Feasibility of a visual prosthesis for the blind based on intracortical micro stimulation of the visual cortex. Brain. 1996 Apr 1;119(2):507–522. 10.1093/brain/119.2.507
- Lewis PM, Rosenfeld JV. Electrical stimulation of the brain and the development of cortical visual prostheses: an historical perspective. Brain Res. 2016 Jan;1630(1630):208–224. 10.1016/j.brainres.2015.08.038
- Matsumoto R, Nair DR, LaPresto E, et al. Functional connectivity in the human language system: a cortico-cortical evoked potential study. Brain. 2004 Jan 10;127(10):2316–2330. 10.1093/brain/awh246
- Ojemann G, Ojemann J, Lettich E, et al. Cortical language localization in left, dominant hemisphere. Collections. 2009 May 8;112(2):316–326.
- Collins KL, Guterstam A, Cronin J, et al. Ownership of an artificial limb induced by electrical brain stimulation. Proc Natl Acad Sci. 2016 Dec;19:201616305.
- Tabot GA, Dammann JF, Berg JA, et al. Restoring the sense of touch with a prosthetic hand through a brain interface. Proc Nat Acad Sci. 2013 May 11;110(45):18279–18284. 10.1073/pnas.1221113110
- Tabot GA, Kim SS, Winberry JE, et al. Restoring tactile and proprioceptive sensation through a brain interface. Neurobiol Dis. 2015 Nov;83:191–198. 10.1016/j.nbd.2014.08.029
- Flesher SN, Collinger JL, Foldes ST, et al. Intracortical microstimulation of human somatosensory cortex. Sci Transl Med. 2016 Oct 19;8(361):361ra141–361ra141. 10.1126/scitranslmed.aaf8083
- Rizk M, Bossetti CA, Jochum TA, et al. A fully implantable 96-channel neural data acquisition system. J Neural Eng. 2009 Apr 1;6(2):026002. 10.1088/1741-2560/6/2/026002
- Borton DA, Yin M, Aceros J, et al. An implantable wireless neural interface for recording cortical circuit dynamics in moving primates. J. Neural Eng. 2013 Apr 1;10(2):026010. 10.1088/1741-2560/10/2/026010
- Barrese JC, Rao N, Paroo K, et al. Failure mode analysis of silicon-based intracortical microelectrode arrays in non-human primates. J Neural Eng. 2013 Dec;10(6):066014. 10.1088/1741-2560/10/6/066014
- Jorfi M, Skousen JL, Weder C, et al. Progress towards biocompatible intracortical microelectrodes for neural interfacing applications. J Neural Eng. 2015;12(1):011001. 10.1088/1741-2560/12/1/011001
- Hirata M, Yoshimine T. Electrocorticographic Brain–Machine Interfaces for Motor and Communication Control. In: Kansaku K, Cohen LG, Birbaumer N, editors. Clinical systems neuroscience [Internet]. Springer Japan; 2015 [cited 2015 Jun 10]. p. 83–100. Available from: http://link.springer.com/chapter/10.1007/978-4-431-55037-2_5
- Mestais CS, Charvet G, Sauter-Starace F, et al. WIMAGINE: wireless 64-channel ECoG recording implant for long term clinical applications. IEEE Trans Neural Syst Rehabil Eng. 2015 Jan;23(1):10–21. 10.1109/TNSRE.2014.2333541
- Piangerelli M, Ciavarro M, Paris A, et al. A fully integrated wireless system for intracranial direct cortical stimulation, real-time electrocorticography data transmission, and smart cage for wireless battery recharge. Front Neurol [Internet]. 2014 Aug 25 [cited 2015 May 4];5. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4142710/
- Wyler AR, Ojemann GA, Lettich E, et al. Subdural strip electrodes for localizing epileptogenic foci. J Neurosurg. 1984 Jun 1;60(6):1195–1200. 10.3171/jns.1984.60.6.1195
- Wong CH, Birkett J, Byth K, et al. Risk factors for complications during intracranial electrode recording in presurgical evaluation of drug resistant partial epilepsy. Acta Neurochir. 2009 Jan 1;151(1):37. 10.1007/s00701-008-0171-7
- Morrell MJ. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011 Sep 27;77(13):1295–1304. 10.1212/WNL.0b013e3182302056
- Sun FT, Morrell MJ. The RNS system: responsive cortical stimulation for the treatment of refractory partial epilepsy. Expert Rev Med Devices. 2014 Aug 21;11(6):563–572. 10.1586/17434440.2014.947274
- Rouse AG, Stanslaski SR, Cong P, et al. A chronic generalized bi-directional brain–machine interface. J Neural Eng. 2011 Jun 1;8(3):036018. 10.1088/1741-2560/8/3/036018
- NeuroPace. RNS® system user manual. DN 1015882 Rev 2. 2015. [cited 2015 Jun 09]. Available from: http://www.neuropace.com/product/pdfs/UserManual.pdf.
- Medtronic. Activa® PC 37601 multi-program neurostimulator─ implant manual. M929110A017. [cited 2015 Jun 09]. Available from: http://manuals.medtronic.com/wcm/groups/mdtcom_sg/@emanuals/@era/@neuro/documents/documents/contrib_210669.pdf.
- Vansteensel MJ, Pels EGM, Bleichner MG, et al. Fully implanted brain-computer interface in a locked-in patient with ALS. N Engl J Med. 2016 Nov 24;375(21):2060–2066. 10.1056/NEJMoa1608085
- Schuettler M, Kohler F, Ordonez JS, et al. Hermetic electronic packaging of an implantable brain-machine-interface with transcutaneous optical data communication. In: 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). 2012. p. 3886–3889.
- Schuettler M, Stiess S, King BV, et al. Fabrication of implantable microelectrode arrays by laser cutting of silicone rubber and platinum foil. J Neural Eng. 2005 Mar 1;2(1):S121. 10.1088/1741-2560/2/1/013
- Fischer J, Milekovic T, Schneider G, et al. Low-latency multi-threaded processing of neuronal signals for brain-computer interfaces. Front Neuroeng [Internet]. 2014 Jan 28 [cited 2014 Sep 12];7. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3904078/
- Kohler F, Kiele P, Ordonez JS, et al. A polymer-metal two step sealing concept for hermetic neural implant packages. In: 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). 2014. p. 3981–3984.
- Bihr U, Anders J, Rickert J, et al. A neural recorder IC with HV input multiplexer for voltage and current stimulation with 18 V compliance. In: European Solid State Circuits Conference (ESSCIRC), ESSCIRC 2014 - 40th. 2014. p. 103–106.
- Volk T, Bentler C, Stocklin S, et al. Novel concept for a wireless and batteryless brain implant array. In: 2015 12th International Multi-Conference on Systems, Signals Devices (SSD). 2015. p. 1–5.
- Volk T, Yousaf A, Albesa J, et al. Wireless power distribution system for brain implants. In: Instrumentation and Measurement Technology Conference (I2MTC), 2015 IEEE International. 2015. p. 1249–1254.
- Green RA, Toor H, Dodds C, et al. Variation in performance of platinum electrodes with size and surface roughness. Sens Mater. 2012;24(4):165–180.
- Cogan SF, Troyk PR, Ehrlich J, et al. In vitro comparison of the charge-injection limits of activated iridium oxide (AIROF) and platinum-iridium microelectrodes. IEEE Trans Biomed Eng. 2005 Sep;52(9):1612–1614. 10.1109/TBME.2005.851503
- Kerzenmacher S, Ducrée J, Zengerle R, et al. Energy harvesting by implantable abiotically catalyzed glucose fuel cells. J Power Sources. 2008 Jul 15;182(1):1–17. 10.1016/j.jpowsour.2008.03.031
- Zebda A, Cosnier S, Alcaraz J-P, et al. Single Glucose Biofuel Cells Implanted in Rats Power Electronic Devices. Sci Rep. 2013 Mar;3(1):26. 10.1038/srep01516
- Lahr J, Schwartz C, Heimbach B, et al. Invasive brain–machine interfaces: a survey of paralyzed patients’ attitudes, knowledge and methods of information retrieval. J Neural Eng. 2015;12(4):043001. 10.1088/1741-2560/12/4/043001
- Huggins JE, Wren PA, Gruis KL. What would brain-computer interface users want? Opinions and priorities of potential users with amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2011 Sep;12(5):318–324. 10.3109/17482968.2011.572978
