ABSTRACT
In this study, governance is understood as a process that guides technology through both promotion and regulation as a result of interaction, interdependence and confrontation between the government, research institutions, industry sector and social organizations. We analyze the National Standardization Technical Committee on Nanotechnologies (Comité Técnico Nacional de Normalización en Nanotecnologías, CTNNN), the entity responsible for developing voluntary standards for nanotechnology in Mexico. After identifying strategic, relevant and secondary actors, we find that technical standards are treated primarily as a competitiveness factor. We thus characterize the process of regulating nanotechnologies in Mexico as a case of “subordinated governance” in which international rules of operation and technical standards are reproduced in the narrow Mexican regulatory framework. The absence of regulation that could serve as a reference for identifying and managing risk thereby imposes greater responsibility on policy-makers.
Introduction
The governance of Science and Technology (S&T) evokes related policies and instruments to promote research and technological development. Its study includes, in general, the analysis of funding and scientific productivity, as well as interactions between the actors who generate and use scientific knowledge. Hagendijk et al. (Citation2005) define governance as the “processes of policy setting, implementation and assessment, which are not confined to government itself but which extend through a network of organizations and agencies and collective actors” (10). Felt and Wynne (Citation2007), for their part, distinguish two dimensions of governance of science. On the one hand, policy-related incentives that promote knowledge generation and the setting of research agendas. On the other hand, there is risk analysis and regulation. From this perspective, governance in this study is understood as a process that is intended to guide the trajectory of a technology either through promotional tools or by regulation (laws, records, guidelines for risk management), emphasizing that the instruments are a result of interaction, interdependence or confrontation between government, educational and research institutions, and industry and social organizations.
The standards are instruments of technology governance that are rarely studied. A standard is a document that provides requirements, specifications, guidelines or characteristics that can be used to ensure that materials, products, processes and services are fit for their purpose (http://www.iso.org/iso/home/standards.htm). Technical standards are considered a competitive factor because they provide consumers and manufacturers parameters to measure the quality, safety and interoperability of products or services (Hatto Citation2013, 6). In the UK, it is estimated that the implementation of standards generates 13% of growth in labor productivity and in Germany it represents approximately 1% of gross domestic product (Lucatero Citation2014). Those who study technology trajectories note that the emergence of technical standards is evidence that technology has emerged from an early stage to one of organized development (Huber Citation2004). In other words, new technology is being adopted by the industry and requires a common vocabulary and clear specifications to communicate within the whole value chain and interoperate.
Technical standards are an important stabilizing instrument of new technologies to generate the agreements between groups of stakeholders who stipulate the attributes of technology products and determine their effects on society. Thus, technical standards are a type of regulation that facilitate transactions around technology but also constitute the specifications that condition and determine public access to technology because they can favor or exclude use to certain companies or drive global market sharing.
At the same time, standards are emerging as an instrument of selection and control of technological risks, as defined by van de Poel and Fahlquist “Technical standards are usually recommendations rather than legal requirements that are written by engineering experts in standardization committees. Standards are usually more detailed than technical codes and may contain detailed provisions about how to design for safety” (Citation2012, 887). The ethical problem that arises in standardization processes according to these authors is of particular importance for new technologies where there are no regulations that serve as reference in the identification and management of risks, imposing greater moral responsibility for policy-makers, scientists, engineers, managers and regulators (van de Poel and Fahlquist Citation2012). Hence, this is how technical standards contribute to the modification or perpetuation of social relations, creating ethical dilemmas for which standardization is a matter of public interest because decisions are made on the impacts (benefits and risks) of technology.
Current development of nanotechnologies coincides with the aspects mentioned above as it is seen as a technology platform in full regalia, with a growing number of companies interested in its applications but also a growing number of initiatives and policy instruments. In this scenario, the creation of technical standards for nanotechnology is a field of regulation rarely studied. Related works, such as those of Meili and Widmer Citation2010 and Miles Citation2010 analyze the role of the International Standards Organization (ISO) as an important homogenization player in the production of nanostructures; and in the framework of ISO, studies examine the ethical integrity of the norms and question the transparency and legitimacy of the process by which they are promoted and adopted (Forsberg Citation2012).
Other studies have been commissioned to document the limited information given to consumers about the safety of products containing nano-objects through labeling or safety data sheets (Brown and Kuzma Citation2013; Lee et al. Citation2013). These studies were performed in high economic growth environments, where nanotechnologies and their applications are more widespread.
In Latin America, there is a growing number of studies on regulating nanotechnologies (Foladori Citation2009; Invernizzi Citation2011; Foladori Citation2012; Fonseca and Santos Pereira Citation2014), but these studies treat technical standards as tangential regulation issues, not deepening its process of creation. Part of the explanation comes from the fact that these new technologies do not arise from local need, but instead are the product of the inherent exchange with a globalized world and the international regulatory framework that is adopted, albeit with tensions and difficulties that reflect a weak or non-institutional structure. Additionally, as a result, in Latin America and Mexico, responsible innovation has not emerged as in the UK, Japan or the European Union, where it is part of research and development programs in nanotechnology (Guston et al. Citation2014).
In Mexico, examining and analyzing the technical standards elaboration process for nanotechnologies through its ad hoc committee is a regional level reference for decision-makers responsible for evaluating technologies and introducing new policies. This article’s main objective is to contributing with a recount of the lessons learned from the existing gaps in responsible innovation and governance. We define subordinated governance as the process by which undeveloped countries, with their inherent limitations and asymmetric global relations regarding the development and commercialization of emerging technologies, seamlessly inherit regulations that were previously standardized by hegemonic countries as convenient, without even considering to perform thorough and systematic assessment of their national and local needs. This article comprises six sections. After this introduction, methodological aspects are briefly described. The context for international regulations for nanotechnologies is presented in the third section, and continues in the fourth section with an analysis of the National Standardization Technical Committee on Nanotechnologies (CTNNN), an entity responsible for elaborating voluntary norms for nanotechnologies in Mexico. The fifth section is a reflection of the process and characterization of governance of this technology as a result of the investigation. Finally, the last section provides some reflections on the scope of standardization activities and the research areas that require further study.
Methodological aspects
This research is a result of participant observation in CTNNN, the only body in the country formally recognized by the Ministry of Economy to develop Mexican standards for nanotechnology. The CTNNN brings together experts from various sectors whose activities are coordinated by the Directorate General of Standards (Dirección General de Normas, DGN) of the Secretariat. Observations were conducted from March 2012 to December 2014 and consisted of attending monthly meetings and access to the minutes and working papers.
To review CTNNN activities, the Governance Analytical Framework proposed by political scientist Hufty (Citation2011) is used, and comprises the following analytical categories ().
Table 1. Governance analytical framework.
Hufty (Citation2011) proposes the concept of nodal points as central places to observe the processes of governance because, in these spaces, actors can be identified and realize their speech and influence on the definition of social norms and the governance process. For the analysis of the actors, this author uses Prats’ classification, which classifies them into three categories: strategic, relevant and secondary, and defines them as follows:
Strategic actors are “any individual, organization or group with sufficient power resources to hinder or disturb the functioning of the rules or procedures for decision-making and resolution of collective conflicts. Relevant actors are those who form part of the institutional fabric and have the necessary resources to be considered as strategic, but who do not use these resources or are dominated by others in the process. Secondary actors do not have sufficient power to change the rules of the game, or remain passive”. (Prats Citation2001; 120 cited by Hufty Citation2011, 412)
Given this analytical framework, it is proposed that CTNNN be the nodal point to study the regulation of nanotechnology in Mexico; it is the only body in the country where representatives of government, scientific and industrial sectors work on the development of technical regulations for this technology. Within this committee, the Mexican standards that make up the National Standardization Program and are subsequently issued and validated by the Ministry of Economy are proposed.
Another aspect that justifies CTNNN as a nodal point is where activities of its counterpart at the ISO, Technical Committee 229 hereinafter ISO/TC 229, are discussed and addressed, which means broadening the view of national governance processes to international standards and registering regulation issues but also indirectly observing, the interests of the actors involved.
Context of the international regulatory framework for nanotechnology
In 2003, the environmental organization ETC Group visualized environmental and health problems inherent to nanoscale materials; in its report The Big Down: Atomtech: Technologies Converging at the Nano-scale (ETC Citation2003), the organization noted that due to its size and physicochemical properties, nanoparticles are potentially toxic and that their production, use and trade was being conducted in a resounding regulatory vacuum. In that report, ETC Group called for a moratorium on commercial production of engineered nanomaterials and, although no government has prohibited the production or commercialization of nanomaterials, the warning launched by this environmental organization lead several pro-environment NGOs, such as the Center for Technology Assessment, Friends of the Earth, Beyond Pesticides, Corporate Watch, Greenpeace, and others, to join in the demand for the assessment of nanomaterials before they reach the market. Meanwhile, in Latin America Rel-UITA (The Latin American Regional Secretariat of International Union of Food, Agricultural, Hotel, Restaurant, Catering, Tobacco and Allied Workers’ Associations, a trade union with more than 12 million affiliates) launched a declaration in 2007 noting that products containing nanocomponents were being released into the market before civil society and social movements had a chance to assess their possible implications in economic, environmental, and social terms and their effect on human health (Foladori Citation2012). These movements contributed to a growth in discussion in S&T forums on the safety aspects of nanomaterials and the public and private resources that can be channeled to research on nanotoxicity. Since then, scientific organizations (RS&RAE Citation2004), governments (NSTC Citation2004) and international organizations (FAO/WHO Citation2010; OECD Citation2011) have inserted the item into their agendas. Although none of these actors supported a moratorium, it is through technical reports, project financing and organizing working groups that various actions that guide the overall course of this technology have been carried out. Indeed, in relation to nanomaterials there seems to be a consensus on the need for assessment on the impact on human health and the environment, but so far there is a lack of adequate methodologies to quantify the risk of nanomaterials in their various matrixes and applications.
In 2004, the Royal Society and the Royal Academy of Engineering published an influential report on implications and uncertainties in the development of nanotechnology. This report, published by one of the oldest and most prestigious scientific associations, symbolizes the scientific opinions around diverse implications of nanomaterials to society including its regulation. The report by RS&RAE points out that more research is needed to understand the behavior of nanomaterials but concludes that size and shape are two factors that make nanoparticles potentially toxic. However, shape and size are not the only risk factors. Chemical composition, surface charge, electric and magnetic properties, state of aggregation and agglomeration, and the matrix in which they are inserted, among others, may be factors that increase or decrease the toxicity of nanoparticles (RS&RAE Citation2004; Kumar Citation2006; Schierow Citation2008; FAO/WHO Citation2010). Meanwhile, research and development at the nanoscale requires dipping into those properties to create new materials and innovations. Additionally, dose and exposure time are other essential factors for determining risk. As the market for nano-enabled products expands, these factors can probably be expected to increase.
Given this context, the regulation of nanotechnology can be grouped into at least three issues: establishing terms and definitions to facilitate communication between those who design, produce and use nanomaterials (e.g. agree what is meant by nanoplate, nanotube or nanofiber); determining the usefulness of the current methods of risk assessment and management; assessing the levels of exposure to nanomaterials in the long term and at various stages of the life cycle of products. Further work is to review the regulatory framework governing chemical substances for each country and on a global scale. To illustrate these issues, some examples are given below.
With regard to the regulatory framework, the discussion focuses on nanomaterials as new chemicals, whether or not certain nanomaterials are nanoscale versions of already registered substances. The reasoning for this is that there is no certainty that the behavior of a substance at the nanoscale has the same toxicological effects on both health and the environment. Depending on the legal framework and risk culture in each country, this measure would mean that those who manufacture or market nanomaterials would have to demonstrate that their products are safe before going to market or would have indicated the safety measures taken when they present risks. Such a measure would likely face significant opposition insofar as it poses a barrier to trade and innovation. In general, mandatory regulation schemes would most likely be opposed because of the costs involved for manufacturers, who must comply with the tests, and for the regulators, who would have to monitor their compliance.
So far, the closest thing to a mandatory regulatory framework is the obligatory records that some European countries have implemented. In 2012, France issued a national decree (Décret n ° 2012–232 du 17 février 2012) that requires manufacturers, importers, distributors, professional users and scientists to submit an annual statement of nanomaterials used above 100 grams to the ministry of Ecology, Sustainable Development and Energy (http://www.safenano.org/news/intheknow/in-the-know-onthe-french-decree/). This register is intended to gather information on the properties, applications and toxicology of nanomaterials circulating in the French market. Meanwhile, Belgium (Bergeson Citation2014a) and Denmark (Bergeson Citation2014b) also issued regulations with features similar to the French decree. Furthermore, it is noteworthy that The European Parliament has adopted three directives in cosmetics, biocides and food which have specific provisions for nanomaterials in terms of labeling and notification or registration procedures. These directives are: the Regulation (EC) No 1223/2009 of the European Parliament and of the Council on Cosmetic products, the Regulation 1169/2011 on the provision of Food Information to Consumers and the Regulation on Biocidal products (EU) No 528/2012.
Another important regulatory agenda topic is the safety of workers handling nanomaterials, when they are unaware of the risks represented by materials. In 2004, the Royal Society recommended in its report on nanotechnology that workers should not be intensively exposed to nanostructured substances (RS&RAE Citation2004, 86). This is because, although industries have implemented the required safety and hygiene measures, there are uncertainties about protective equipment, safety guidelines or whether exposure limits currently known work for nanomaterials. For example, a recent study on TiO2 (a compound widely used in products such as paints, plastics, cosmetics and food), published by the National Institute for Occupational Safety and Health in the United States, concluded that under the established exposure limits, there was insufficient evidence to classify this material as a potential occupational carcinogen; however, there is insufficient information in this regard for nanoscale particles, so the agency recommends that worker exposure to this substance be reduced to the lower limits (NIOSH Citation2011, viii). There are likely to be numerous substances that may be in this category and are not being reviewed by national health and environment agencies.
Finally, an emerging concern is the handling of nanomaterials at the waste stage; given the increasing use of nano products, one may wonder what happens to the waste generated in research labs and nano products after use (tires, batteries, electronics, auto parts, cosmetics, textiles, disinfectants and paintings)? On this subject, another abyss of uncertainty opens regarding the behavior of nanomaterials at this stage depending on the mechanisms of waste, either by incineration, chemical recycling or mechanical recycling (OECD Citation2012).
It is important to recognize that the management of risks to human health and the environment, are subordinate to commercialization. The objectives of regulations that are promoted through international organizations and government actors are primarily concerned with maintaining the pace of scientific research, accelerating the commercialization of laboratory results, and designing strategies to promote industries using nanomaterials. ISO is a promoter, and in 2005 created the ISO/TC 229 precisely to formulate technical standards to the uncertainties that would limit the full diffusion of nanotechnologies into industrial sectors. The committee’s business plan indicates the following priorities for standardization and regulation:
Priority has been given to developing horizontal standards for terminology and nomenclature, measurement and characterization, health, safety and the environmental (HSE) and nanomaterial characterization. These priorities are designed to support research, commercialization and trade in materials and products at the nanoscale. In particular, and responding to societal concerns about the safety of nanotechnology, the HSE standards will support the development of appropriate national and international regulatory regimes, including guidance documents, in the fields of occupational and environmental health and safety, promoting good practice in the production, use and disposal of nano-materials, nanotechnology products and nanotechnology enabled systems and products. These regimes will provide certainty and confidence for workers, consumers, manufacturers and users alike. (ISO Citation2011, 7)
In very simple terms, ISO/TC 229 operates as follows: it comprises 34 countries, including Mexico, who through their Metrology Institutes propose standardization projects and participate on the decisions committee; there are 14 other member countries that participate only as observers. Each member country has a group of experts who attend the standardization work of the committee and it is within these groups that draft standards are voted on and decisions on the scope and content are made. Briefly, the experts attend the national working groups that design the technical content of products, services or technology that is subject to standardization. National groups are made up of representatives from various sectors, including scientists from public and private institutions, regulators, representatives of large companies and law firms specializing in risk analysis and technology litigation.
Additionally, ISO regulations support some degree of involvement from other organizations, called liaison members. These members can be other ISO technical committees, civil society or international organizations; 26 ISO technical committees and 11 international organizations participate in ISO/TC 229, among which the Organization for Economic Co-operation and Development (OECD), the European Association for the Co-ordination of Consumer Representation in Standardization (ANEC), the European Commission, the European Environmental Citizens’ Organization for Standardization (ECOS) and the Nanotechnology Industries Association, which has among its members companies such as 3M, BASF, Promethean Particles and Nanogap (http://www.iso.org/iso/home/standards_development/list_of_iso_technical_committees/iso_technical_committee.htm?commid=381983).
Another notable feature of the role of ISO is its level of influence on the regulation of member countries because, after this organization publishes these standards, they tend to be more easily adopted into national legislation. The adoption of ISO standards in developed countries is not usually problematic because they are steeped in the interests of government agencies and companies who have the resources to directly participate in the working groups that formulate the rules. The demands of economic globalization facilitate the adoption of international standards because they benefit from the free flow of goods; recall that the goal of ISO/TC 229 is to produce international standards that reduce the technical constraints that hinder the market (ISO Citation2011).
The mosaic of actors involved in the formulation of international technical standards is illustrative of the process that Beck (Citation1998) called “forms of organized irresponsibility” where the number of actors involved in decision-making is such that it is difficult to attribute responsibility because they are the result of the actions of various actors, institutional policies and a combination of other factors.
In summary, the regulatory landscape of nanotechnology is characterized by (i) the lack of laws that allow tracking manufacturing and the use of nanomaterials in the markets, and information necessary to identify and manage risks in the international order; (ii) the abundance of technical reports on the toxicity of nanomaterials, some showing adverse effects, others safety and (iii) the proliferation of voluntary self-regulation schemes, such as codes of conduct, guidelines for best practices in risk management and technical standards, except in some European countries such as Belgium, Denmark and France as we have mentioned before.
Let us now turn to the CTNNN study to characterize the governance of nanotechnology regulation in Mexico.
CTNNN as a nodal point for the regulation of nanotechnology in Mexico
CTNNN was established by an initiative of the Mexican Institute of Standardization and Certification on 18 May 2007 at a meeting on nanotechnology at the Autonomous University of Puebla. The National Center of Metrology (CENAM)Footnote1 was a prominent member at the meeting (IMNC Citation2010). CTNNN is a group of experts authorized by the Ministry of Economy to “create Mexican Standards (NMX) for products, equipment, test methods, health issues and environmental practices in nanotechnology” (SE Citation2013, 1). NMXs are voluntary federal technical specifications on Metrology and Standardization. Although they are not binding, the approval process is similar to mandatory standards because they have to be prepared by a national standards organization with the participation of various sectors and are subject to public consultation before being published in the Official Journal. One differentiating feature is that NMXs are based on international standards, which expands their ability to be a reference for determining the quality or safety of products, in the public and private sectors. Additionally, as already mentioned, CTNNN has another function; it acts as a national mirror committee to address the standardization work on nanotechnologies in ISO/TC229, which means that its members must contribute technically to the formulation of international standards through a national interest lens (SE Citation2012).
For a long time, CTNNN operated under quite ambiguous conditions because the Ministry of Economy took six years to issue operating rules to regulate their operation and empower them to propose draft standards in this area. Thus, under current regulations, the functional structure of CTNNN is as follows: the Directorate General of Standards belonging to the Ministry of Economy is the technical secretariat that oversees its operation and serves as liaison between CTNNN and ISO/TC 229; CENAM holds the presidency; and representatives of the sectors, which can be technical staff from government agencies, the academic sector, industry organizations or consumers. By 2014, the committee brought together 34 institutions, almost 5 times the number it had in 2007.
From here, CTNNN will be analyzed as a nodal point. First, the actors will be grouped by section and described, then the roles of these actors within the committee will be examined to determine their influence on the governance of nanotechnology regulation in Mexico.
Academic sector actors: Academics from 12 university research institutions and public research centers represent 35% of the committee members. Participants are active in scientific research and the development of nanomaterials; however, this group lacks specialists on nanotoxicology, and only one of them is familiar with the analysis of health risks. This group includes two specialists in the social analysis of nanotechnology, which is rare in committees dominated by “hard” scientists; these are usually unreachable for social scientists. It is fair to say that during the course of this research, we did not observe any controversy about regulation of nanotechnologies between hard scientists and social scientists, there is little for discussion, due to the tight time table of the committee to accomplish the ISO/TC 229 agenda (for instance, analyze lengthy documents, prepare national voting to new item proposals, translate technical standards from English to Spanish, etc.).
Industry actors: This sector represents 35% of the committee; its members have technical profiles and managerial positions in both the business side and in the R&D laboratories of companies. Business groups such as the National Chamber of Cosmetic Industry (CANIPEC), Cosmetologists Chemical Society (SQC) and Nanotechnology Cluster of Nuevo Leon are included. The CANIPEC brings together 66 of the leading companies, producers and distributors of cosmetics in the country and in addition to Mexican companies, subsidiaries of multinationals such as L’Oreal, Revlon, Avon and Procter & Gamble participate. Nuevo Leon’s Nanotechnology Cluster is an association of governmental institutions, research centers and companies linked to the promotion of this technology, including the major companies in the country, such as Cemex, Alfa Group, Vitro, Cydsa and Proeza.
CANIPEC and SQC’s incorporation is no coincidence considering that cosmetic products incorporating nanomaterials are already being regulated in some areas of the world, such as the European Union, which issued Regulation No. 1223/2009 in November 2009 requiring companies that manufacture cosmetics to notify the use of nanomaterials in their formulation and to identify them in the list of ingredients on the product label (Official Journal of the European Union Citation2009).
Other companies represented in this sector are Farma Quimia, a Mexican company dedicated to the development of specialty bismuth chemicals for cosmetic and pharmaceutical applications; and Gresmex, a Mexican company in the chemical sector that manufactures cleaning products, personal care products and disinfectants, developing a line of products with nanotechnology called N-Belyax.Footnote2 Lotto Nano Bio Laboratories, is a company focused on the use of silver, gold and magnetite nanoparticles for biomedical applications; VIRETEC, a start-up that supports other companies in the use of nanomaterials; Nanomaterials is another company participating in the committee, it develops nanoparticle additives for the ceramic industry and finally, FEI, Zeiss and Micra Nanotecnología, companies engaged in the sale of scientific equipment, are CTNNN members.
Government sector actors: This sector represents 21% of the committee and is composed of middle-ranking officials with technical profiles. Member institutions are the DGN of the Ministry of Economy as already mentioned, coordinating the operation of CTNNN without affecting the technical discussion; and CENAM is a sectored body to the Ministry of Economy and chairs the committee. CENAM is also a tie to nanotechnologies with various international organizations such as the OECD, ISO, the Versailles Project on Advanced Materials and Standards (VAMAS) and the United States. The National Institute of Ecology and Climate Change (INECC), the Mexican Petroleum Institute (IMP), the National Institute of Neurology and Neurosurgery and the National Institute for Nuclear Research (ININ) are also part of this sector. These institutions perform regulatory science in environmental, health and energy policy, respectively. The National Health Service, Food Safety and Quality (SENASICA) is the only formal regulatory agency member, but we will later explain the informal intervention of other agencies.
National Standard Bodies: National Standard Bodies are private organizations that are legally recognized to develop Mexican standards and occupy 3% of the membership of CTNNN. The National Association for Standardization and Certification of the Electrical Sector (ANCE) and the Mexican Institute of Standardization and Certification (IMNC) both participate. The ANCE generates rules for the electricity sector and the IMNC generates rules for quality systems, metrology, environmental management and safety. A member of the National Mirror Committee ISO/TC 262 Risk management, also joined the committee due to their interest in managing occupational hazards related to the handling of nanomaterials.
summarizes the composition of CTNNN indicating the profile of its members and the motivation for their membership on the committee. Weight changes in the sectorial distribution of the actors, noting increasing interest and the consolidation of nanotechnologies in the Mexican business sector are shown graphically in .
Figure 1. Sectorial distribution of participant actors in the National Standardization Technical Committee on Nanotechnologies in 2007 and 2014.
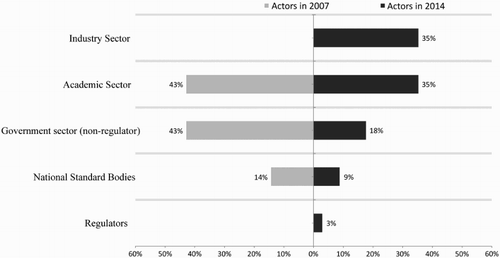
Table 2. Actors of the National Standardization Technical Committee on nanotechnologies in 2014.
We now turn to the assessment of the actors. The academic sector is a relevant actor in regulation because it has the technical resources to influence norms; however, scientists on the committee showed little interest in influencing content or proposing new topics for standardization. The fact that committee membership is an activity in addition to their teaching and research responsibilities and an honorable activity that integrates reward and stimulus practices in the Mexican research system, reduces the importance of standardization activity. As a block, this sector is the most involved in the committee’s work, from the physical meetings, responding to the work of ISO/TC 229, to the translation of ISO standards to be published in Mexico. However, it was noted that the participation of scientists is limited to their personal capability because the institutions they represent do not assume the commitments to support the institutions they represent and do not assume the commitments acquired by belonging to the committee (e.g. travel expenses to attend international ISO/TC229 plenary meetings). Representatives of government offices also serve in their individual capacity.
The industrial sector is a strategic actor because it has economic and cognitive resources with which to influence national standardization. This is the only sector that has presented two concrete regulation proposals, the first one related to measurement of nanoparticles in liquid media and the second one to measure the antibacterial activity of nanoparticles on ceramic surfaces. Although overall participation of representatives of this sector is low, committee membership has increased because they have privileged access to information that circulates there, such as documents on nanoscale methods and manufacturing processes, contacts with the leaders in the development of nanomaterials and knowledge of international regulations and technical specification industries. Membership on the committee supports technological strategic monitoring of companies because it gives them the most recent development information regarding nanomaterials as committee documents cite the most recent patents and scientific literature.
The government sector, analyzed as a whole, is a relevant actor because it can influence the standardization process through economic and political resources; however, the Ministry of Economy, the department at the highest institutional level in the group, has not shown a willingness to mobilize its resources to create public policy to regulate nanomaterials. CTNNN, within the government sector, is highly dependent on the science sector to discuss nanotechnology applications and to deal with challenges imposed by the risk assessment of nanomaterials. This means that the institutional capacities of the Mexican State are insufficient to anticipate the adverse effects of nanomaterials or to monitor their safe use.
The absence of regulating entities in the CTNNN shows the lack of interest in regulating nanotechnology in Mexico; for instance, the Ministry of Labor and the Federal Commission for the Protection from Sanitary Risks (COFEPRIS) are not included. The participation of both institutions is necessary because within the committee various safety standards for the work environment and standards for labeling of products with nanomaterials are discussed. COFEPRIS has remained close to the Committee’s work and is no stranger to the subject of the risks, but this organization maintains that the current regulatory framework is sufficient to meet the challenges related to the products containing nanomaterials.Footnote3
Civil society organizations are also absent from the CTNNN, despite the fact that their intervention is covered by the rules of operation. The development of nanotechnology has drawn the attention of NGOs in several countries, but has not become part of the agenda of Mexican civil society organizations. Some possible reasons for this lack of participation are the lack of knowledge related to this technology and its discussions, the narrowness of organized civil society as well as the incidence of other national issues that capture the national agenda. The few consumer organizations which exist in the country have focused on problems such as obesity in children because of junk food, and poor regulation in the telecommunications sector (costs of wireless media and Internet).
The involvement of civil society is a way to make the content of the rules more reflective by inserting the concerns of non-experts into standardization. The engagement of many actors concerns what Jasanoff (Citation2003) has called the participatory turn in the relationship between science, technology and society, which focuses on the growing demand for transparency and accountability in the activities and results experts offer, particularly in risk assessment and the assessment of costs, benefits and uncertainties of new technologies. This also relates to the shared responsibility of those involved in the creation of technical standards and, in the case of CTNNN, the issue of occupational risk management and the labeling of products with nanomaterials.
This type of analysis, by aggregating sectors only partially, reflects the involvement of each actor at a lower level of governance. Because of their resources, capacity and willingness to influence the regulatory tasks, several actors have proved strategic in the standardization process. These actors are CENAM and the Center for Research in Advanced Materials (CIMAV).
CENAM is a strategic actor that concentrates and controls privileged information on trends in the regulation of nanotechnologies. They are legally entitled to be a point of contact for various national and international bodies concerned with measurements. As a result, this body represents Mexico at global nodal points of governance in nanotechnologies including the Working Group on Manufactured Nano-materials of the OECD (OECD-WPMN), VAMAS and in the work of the High-Level Regulatory Cooperation Council between Mexico and the United States, the latter being a mechanism for harmonization of regulations launched by the United States in 2010 as mentioned below. CENAM has a limited measurement infrastructure, limited financial resources and is limited in terms of its ability to summon senior government and business officials.
CIMAV, meanwhile, is a public research center that has adapted to the settings of the C&T policy in recent years, and has been emphasizing the marketing knowledge generated in these institutions. This center is one of the main promoters of nanotechnologies in Mexico because its geographical location in the industrial north of Mexico has given it great capacity for dialogue with industry and the scientific sectors. CIMAV has a place on the Cluster Board of Nanotechnology and leads several national initiatives. The center allocates intellectual work to their experts in many activities including conducting experiments for the technical review of ISO standards, and provides the technology infrastructure for regular meetings.Footnote4
Given the above situation, the development of standards by the CTNNN has been slow and most of the rules deal with terminology. When this article was written, the Mexican government had published seven voluntary standards, highlighting the recent approval of the technical specification for labeling of products with nano-objects ().
Table 3. Voluntary Standards published by the Mexican government.
Reflection on the governance process and its characterization
In Mexico, there is increasing interest in studying multiple dimensions of the regulation of nanotechnologies. Some of the literature (Delgado-Ramos Citation2014; Foladori and Záyago Citation2014; Anzaldo and Herrera-Basurto Citation2015) focuses on analyzing one of the few regulatory instruments issued by the Mexican government, the “Guidelines for regulations on nanotechnology to boost competitiveness and protect the environment, health and safety of consumers” (SE Citation2012). This document, signed within the framework of a regulatory harmonization agreement between Mexico and the United States, is the country’s accession to commercial values that prioritized the reduction of barriers to trade and innovation, above the protection of health and the environment. Another topic addressed by academics in Mexico is the vulnerability of the workers who handle nanomaterials (Foladori Citation2009; Foladori and Záyago Citation2011).
This article provides a description of the way technical standards on nanotechnologies have been shaped and who the major and minor players in various stages have been. The focus will now shift to the analysis of the process.
The regulation of nanotechnology in Mexico is subordinate governance, in part because of authorities’ belated efforts – over a period of six years – to conform to the rules of operation and fit the technical standards of the ISO into the narrow Mexican regulatory framework. The absence of anticipatory governance (Guston Citation2013) arises from this dependence on international regulations and the lack of an official stance from which to respond to the development of this technology and making it more sensitive to the concerns of society. This situation is symptomatic of Mexican underdevelopment and is consistent with the mainstream neoliberal model that Mexico and other developing nations have increasingly adopted. Further, it highlights the lack of qualified human resources for standardization activities, both inside governmental offices and within the scientific community. As mentioned in the analysis of CTNNN, the State is a minor player when it should be a strategic one. Indeed, “globalization has unleashed market forces so powerful that states, especially from developing countries often cannot control” (Stiglitz Citation2006, 47).
The CTNNN dynamics, in conjunction with CTI policy governance of nanotechnologies in Mexico, coincides with some features of market governance (Hagendijk et al. Citation2005) because it is the State that decides the ideological framework and objectives of policies at CTI in a delegation relationship with scientists’ isolated decisions keeping them from public debate. The characteristics of a market mode of governance are observed in how the financing of nanotechnologies is conditioned on a close relationship between private companies and the scientific sector. In market governance, “the value of science comes from the surplus value created through its commercialization and the general contribution to the generation of wealth in society” (Citation2005, 18). For the public, this refers to a person’s rights as a consumer or user, not as a citizen with rights and obligations to influence decision-making. In market governance, decisions are kept away from both the government and citizens.
Subordination to the norms dictated by ISO in the case of nanotechnology raises serious concerns because there are many examples of failed exercises of self-regulation. Examples include companies such as Enron, Carlsberg, WorldCom and the most recent case of Volkswagen’s avoidance of environmental standards, where failed self-regulation led to financial ruin and in some cases job loss for thousands of workers. However, in the case of nanotechnology, poor voluntary regulation can lead to irreversible consequences for society and the environment.
Another important aspect is that interactions between the participants of the committee are outlined by interests and values that do not include responsible innovation, therefore social participation is excluded from the agenda, and the regulation of nanotechnology is not a public concern. Sustained arguments in these areas are common; it is assumed that the public is ignorant of scientific facts, and therefore, needs science education so that they can participate (van Oudheusden Citation2014).
Conclusions
This study is illustrative of the conditions of subordinate governance in a Latin American country when facing highly controversial technologies. Research shows that the governance of nanotechnologies in Mexico is doubly subordinate. Mexican regulatory policy is subordinate to the decisions of international bodies such as the ISO and the regulatory policy and commercial interests of its northern neighbor, the United States through NAFTA. It is urgent for Mexico to move toward a governance scheme that institutionalizes an early technology assessment on par with the funding policy to meet the regulatory challenges of nanotechnology. CTNNN reflects at least two widespread problems in Mexican politics for new technologies, it presents a democratic deficit by not including civil society organizations that could add knowledge and perspective and by including only a few institutions in its meetings. In ethical terms, the current standards development process has the Problem of Many Hands described by philosopher Dennis Thompson, 1980 (as quoted in van de Poel and Fahlquist Citation2012, 898), who describes the difficulties in assessing responsibility for the effects of technological developments, in this case the formulation of a standard, when it is difficult to know who did what and who is responsible for the effects.
Scientists are a strong component of the committee; however, the scientists’ actual influence in the committee is minimal because their scope has more to do with the generation of knowledge than with regulation. Furthermore, there are no mechanisms or the political will to create human resources in this field, which fosters a vacuum that triggers subordinated governance and the blind adoption of international technical standards. If we add that international regulations tend toward self-regulation, it is clear that Mexico is in a very vulnerable situation and risks irreversible damage.
Two existing Mexican norms emerged from the private sector. Will this sector also dictate regulatory changes? Moreover, if foreign companies that dominate key sectors, such as cosmetics or pharmaceuticals are incorporated they would worry that the regulations were unfavorable to them.
The axis of the paper is to illustrate that standardization is not a neutral activity, but one which reflects and materialices the interests and values of those who participate in its elaboration. Therefore, the final purpose of this paper is to illustrate how in the context of CTNNN – the collegiate space which we have identified as the nodal point of governance of nanotechnologies– México has been limited to creating ad hoc standards for the interests of some of the actors meeting there. One reason why Mexican norms have not been established is because standardization is an activity which requires participating institutions and companies to dedicate part of their human and economic resources for the writing of documents, the purchase of equipment and testing. In this aspect the committee’s capacities have been overstretched and it has found itself concentrating on international norms, leaving aside the national context. For example, up to this moment the committee has failed to publish two norms proposed by actors in the business sector, one related to measurement of nanoparticles in liquid media and the second one to measure the antibacterial activity of nanoparticles on ceramic surfaces, as mentioned above. Another reason is that this activity is not contemplated as a constitutive part of the formulation of economic, commercial or scientific policies. This is evident from the fact that Mexico has so far not published standards in nanotechnology for the protection of human health and the environment.
For developing countries, ISO standards are considered a technical reference and are adopted almost automatically because these countries lack the technical and organizational capabilities to develop their own standards.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Mónica Anzaldo Montoya is an Outreach Director at El Colegio de San Luis A.C. in Mexico; she has a Ph.D. from the Science, Technology and Society program at CINVESTAV. Her research focuses on the governance of nanotechnologies in Mexico, promotion and regulation. She is a member of the National Standardization Technical Committee on Nanotechnologies, an entity that served as a case study for a chapter of her Ph.D.
Michelle Chauvet is a Professor in the Sociology Department at the Universidad Autónoma Metropolitana, Campus Azcapotzalco, Mexico. She has a Ph.D. and a Master’s degree in Economics and a Bachelor’s degree in Journalism and Communications from the Universidad Nacional Autónoma de Mexico. Since 1990, she has been researching the socioeconomic impacts of biotechnology on agriculture and the environment. She is the author of several publications related to regulation of new technologies that focus on biosafety in agricultural issues. Her current research interests include nanotechnologies in the food sector and the governance of new technologies.
Additional information
Funding
Notes
1. CENAM is the governmental organization responsible for establishing and maintaining national measurement standards, metrological services such as calibration of instruments and standards, certification and the development of reference materials, specialized courses in metrology and consulting.
2. According to company information, the N-Byelax biocatalyst is an intelligent product that eliminates viruses, bacteria, fungi and protozoa in a safe manner without creating resistance.
3. This conclusion has been reached and was recorded in an interview with José Jesus Herrera Bazan, Manager of Sampling and Monitoring COFEPRIS on 3 September 2012.
4. These include: the Punto Nacional de Contacto Sectorial en Nanotecnología y Nuevos Materiales, the Laboratorio Nacional de Nanotecnología, the Centro Virtual Brasileño-Mexicano de Nanotecnología and the Pan-American Nanotechnology Network (http://www.cimav.edu.mx/investigacion/institucionales/programa-nano).
References
- Anzaldo, Mónica, and Raúl Herrera-Basurto. 2015. “Actores, Visiones y Perspectivas de la Gobernanza de la Regulación de las Nanotecnologías en México.” In Nanotecnologías en América: Trabajo y Regulación, coordinated by Guillermo Foladori, Anwar Hasmy, Noela Invernizzi, and Edgar Záyago, 25–39. México: Universidad Autónoma de Zacatecas/ReLans/Porrúa.
- Beck, Ulrich. 1998. La Sociedad del Riesgo. Hacia una Nueva Modernidad. Translated by Jorge Navarro, Daniel Jiménez and Ma. Rosa Borrás. Barcelona: Paidós Básica.
- Bergeson, Lynn L. 2014a. “Belgium Publishes Decree on the Nanomaterial Register.” Nano and Other Emerging Chemical Technologies Blog, October 28. http://nanotech.lawbc.com/2014/10/belgiumpublishesdecreeonthenanomaterialregister/.
- Bergeson, Lynn L. 2014b. “Denmark Order on Nano Products Register Enters Into Force.” Nano and Other Emerging Chemical Technologies Blog, June 18. http://nanotech.lawbc.com/2014/06/denmarkorderonnanoproductsregisterentersintoforce/.
- Brown, Jonathan, and Jennifer Kuzma. 2013. “Hungry for Information: Public Attitudes toward Food Nanotechnology and Labeling.” Review of Policy Research 30 (5): 512–548. doi:10.1111/ropr.12035.
- Delgado-Ramos, Gian Carlo. 2014. “Nanotechnology in Mexico: Global Trends and National Implications for Policy and Regulatory Issues.” Technology in Society 37: 4–15. doi: 10.1016/j.techsoc.2013.09.005
- ETC. (2003). The Big Down: Atomtech Technologies Converging at the Nano Scale. Action Group on Erosion, Technology and Concentration: Winnipeg, Canada. www.etcgroup.org.
- Felt, Ulrike, and Brian Wynne. 2007. “Taking European Knowledge Society Seriously.” Report of the Expert Group on Science and Governance to the Science, Economy and Society Directorate, Directorate-General for Research. Brussels: European Commission. http://bookshop.europa.eu/en/taking-european-knowledge-society-seriously-pbKINA22700/.
- Foladori, Guillermo. 2009. “La Gobernanza de las Nanotecnologías.” Sociológica 24 (71): 125–153.
- Foladori, Guillermo. 2012. “Riesgos a la Salud y al Medioambiente en las Políticas de Nanotecnología en América Latina.” Sociológica 27 (77): 143–180.
- Foladori, Guillermo, and Edgar Záyago. 2011. “The Role of Organized Workers in the Regulation of Nanotechnologies.” In Nanotechnology and the Challenges of Equity, Equality and Development Volume 2 of the series Yearbook of Nanotechnology in Society, edited by Susan Cozzens, and Jameson Wetmore, 181–198. New York: Springer. doi:10.1007/978-90-481-9615-9.
- Foladori, Guillermo, and Edgar Záyago. 2014. “The Regulation of Nanotechnologies in Mexico.” Nanotechnology Law & Business 11 (2): 164–171.
- Fonseca, Paulo, and Tiago Santos Pereira. 2014. “The Governance of Nanotechnology in the Brazilian Context: Entangling approaches.” Technology in Society 37: 16–27. doi: 10.1016/j.techsoc.2013.07.003
- FAO/WHO (Food and Agriculture Organization of the United Nations/World Health Organization). 2010. “ Expert Meeting on the Application of Nanotechnologies in the Food and Agriculture Sectors: Potential Food Safety Implications. Meeting Report. Rome: FAO/WHO.
- Forsberg, Ellen-Marie. 2012. “Standardisation in the Field of Nanotechnology: Some Issues of Legitimacy.” Science and Engineering Ethics 18 (4): 719–739. doi:10.1007/s11948-011-9268-0.
- Guston, David. 2013. “Understanding ‘Anticipatory Governance’.” Social Studies of Science 44 (2): 218–242. doi:10.1177/0306312713508669.
- Guston, David, Erik Fisher, Armin Grunwald, Richard Owen, Tsjalling Swierstra, and Simone van der Burg. 2014. “Responsible Innovation: Motivations for a New Journal.” Journal of Responsible Innovation 1 (1): 1–8. doi:10.1080/23299460.2014.885175.
- Hagendijk, Rob, Peter Healey, Maja Horst, and Alan Irwin. 2005. Science, Technology and Governance in Europe: Challenges of Public Engagement. Brussels: University of Copenhagen.
- Hatto, Peter. 2013. Standards and Standarisation. A Practical Guide for Researchers. Directorate-General for Research. Luxembourg: European Commission. http://ec.europa.eu/research/industrial_technologies/pdf/handbook-standardisation_en.pdf.
- Huber, Joseph. 2004. New Technologies and Environment Innovation. Cheltenham: Edward-Elgar.
- Hufty, Marc. 2011. “Investigating Policy Processes: The Governance Analytical Framework (GAF).” In Research for Sustainable Development Foundations, Experiences, and Perspectives, edited by Urs Wiesmann and Hans Hurni with an international group of co-editors, 403–424. Bern, Switzerland: NCCR North-South.
- IMNC (Instituto Mexicano de Normalización y Certificación). 2010. IMNC/CTNN 13 Nanotecnologías. México: IMNC.
- Invernizzi, Noela. 2011. “Nanotechnologies between de Lab and the Shop Floor: What are the Effects on Labor?” Journal of Nanoparticle Research 13 (6): 2249–2268. doi: 10.1007/s11051-011-0333-z
- ISO (International Organization for Standardization). 2011. Business Plan ISO/TC 229 Nanotechnologies. ISO/TC 229 N 791. ISO. http://isotc.iso.org/livelink/livelink/fetch/2000/2122/687806/ISO_TC_229__Nanotechnologies_.pdf?nodeid=6507632&vernum=-2.
- Jasanoff, Sheila. 2003. “Technologies of Humility: Citizen Participation in Governing Science.” Minerva 41: 223–244. doi: 10.1023/A:1025557512320
- Kumar, Challa, ed. 2006. Nanomaterials-Toxicity: Health and Environmental Issues. Weinheim: Wiley-VCH.
- Lee, J. H., W. K. Kuk, M. Kwon, J. H. Lee, K. S. Lee, and I. J. Yu. 2013. “Evaluation of Information in Nanomaterial Safety Data Sheets and Development of International Standard for Guidance on Preparation of Nanomaterial Safety Data Sheets.” Nanotoxicology 3 (7): 338–345.
- Lucatero, Jesús. 2014. “Retos y Oportunidades para México para Armonizar la Regulación con el Resto de América del Norte.” Power point presentation, México, July 9.
- Meili, Christoph, and Markus Widmer. 2010. “Voluntary Measures in Nanotechnology Risk Governance: The Difficulty of Holding the Wolf by the Ears.” In International Handbook on Regulating Nanotechnologies, edited by Graeme A. Hodge, Diana M. Bowman, and Andrew D. Maynard, 446–461. Cheltenham: Edward Elgar.
- Miles, John. 2010. “Nanotechnology Captured.” In International Handbook on Regulating Nanotechnologies, edited by Graeme A. Hodge, Diana M. Bowman, and Andrew D. Maynard, 83–106. Cheltenham: Edward Elgar.
- NIOSH (National Institute for Occupational Safety and Health). 2011. Occupational Exposure to Titanium Dioxide. Cincinnati, OH: Department of Health and Human Services.
- NSTC (National Science and Technology Council). 2004. Nanotechnology Initiative. Strategic Plan. Washington, DC: NSTC.
- OECD (Organisation for Economic Co-operation and Development). 2011. Nanosafety at the OECD: The First Five Years 2006–2010. Paris: OECD.
- OECD (Organisation for Economic Co-operation and Development). 2012. “Safe Management of Nanowaste”. Draft Agenda of the Workshop of Safe Management of Nanowaste, Munich, Germany, May 2012. http://nanotech.lawbc.com/wp-content/uploads/sites/539/2012/03/00092177.pdf.
- Official Journal of the European Union. 2009. Regulation (EC) No 1223/2009 on Cosmetic Products. Brussels: The European Parliament and the Council of the European Union. http://europa.eu/legislation_summaries/consumers/product_labelling_and_packaging/co0013_en.htm.
- Prats, Joan. 2001. “Gobernabilidad democrática para el desarrollo humano: marco conceptual y analítico.” In Revista Instituciones y Desarrollo, 10, 103–148. http://www.red-redial.net/referencia-bibliografica-48332.html.
- RS&RAE (Royal Society and Royal Academy of Engineering). 2004. Nanoscience and nanotechnologies: Opportunities and uncertainties. London: Royal Society.
- Schierow, Linda-Jo. 2008. “ Engineered Nanoscale Materials and Derivative Products: Regulatory Challenges.” Washington, DC: Congressional Research Service Reports. Paper 50. http://digitalcommons.unl.edu/crsdocs/50.
- SE (Secretaría de Economía). 2012. Lineamientos para regulaciones sobre nanotecnologías para impulsar la competitividad y proteger al medio ambiente, la salud y la seguridad de los consumidores [Guidelines for regulations on nanotechnology to boost competitiveness and protect the environment, health and safety of consumers]. Mexico, DF: Secretaría de Economía.
- SE (Secretaría de Economía). 2013. Reglas de Operación del Comité Técnico de Normalización Nacional en Nanotecnologías. Mexico, DF: Secretaría de Economía. http://www.economia.gob.mx/files/comunidad_negocios/competitividad/lineamientos_regulaciones_nanotecnologias_261112.pdf.
- Stiglitz, Joseph E. 2006. Cómo hacer que funcione la globalización. Mexico, DF: Taurus.
- Van de Poel, Ibo, and Jessica Nihlén Fahlquist. 2012. “Risk and Responsibility.” In Handbook of Risk Theory. Epistemology, Decision Theory, Ethics, and Social Implications of Risk, edited by Sabine Roeser, Rafaela Hillerbrand, Per Sandin, and Martin Peterson, 878–907. Berlin: Springer.
- Van Oudheusden, Michiel. 2014. “Where are the Politics in Responsible Innovation? European Governance, Technology Assessments, and Beyond.” Journal of Responsible Innovation 1 (1): 67–86. doi:10.1080/23299460.2014.882097.
