?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Secondary structure of protein, surface hydrophobicity, and functional properties of defatted Moringa olifeira seed and leaf flour were studied. Protein presented high β-sheet (32.77–41.11%) and β-turn (37.97–39.31%) structures. Water holding and oil absorption capacities of leaf flour were adequate for using in food formulations. Surface hydrophobicity of seed flour increased significantly at pH 9. Leaf protein was more soluble than the seed one, excepted at pH 4–5. Neutral and basic pH favoured emulsifying capacity (EC) of seed flour. The maximum EC was 52.17%. The emulsion stability (ES) was greater at acidic pH and seed flour concentration of 4% (w/v). The maximum ES (53.84%) was observed pH 4 and oil to water ratio of 1/2 (v/v). For applications where high foaming properties are required, it is important to use the seed flour at combined high pH and concentration. The maximum foam capacity and stability (87.50 and 82.50%, respectively) was observed at pH 9 and flour concentration of 4% (w/v).
Public Interest Statement
There is a need to explore the utilization of new protein ingredients in the formulation of new food products or to enrich the traditional formulations. Then, Moringa olifeira is an inexpensive protein source with good nutritional quality and pharmacological properties. The objective of this study was to examine the secondary structure of protein, surface hydrophobicity, solubility, water and fat holding capacities, finally effects of oil, concentration and pH on the emulsifying and foaming properties of defatted M. olifeira seed and leaf flour. This study showed that water holding and oil absorption capacities of leaf flour, and solubility of leaf protein were adequate for utilization in food formulations. Adequate combination of pH, flour concentration, and oil to water ratio was necessary to produce high emulsifying and foaming properties from M. olifeira seed and leaf flour. Finally, M. olifeira seed and leaf flour presented good functionality for utilization in food formulations.
Competing Interests
The authors declare no competing interest.
1. Introduction
Protein malnutrition is one of the major nutritional problems in developing countries. The most disastrous consequences occur in children where protein malnutrition manifests itself in forms of two notorious diseases: marasmus and kwashiorkor. An alternative for improving protein intake is to supplement the diet with plant proteins. Legumes are inexpensive source of proteins with high protein content (17–40%) and calories (Duke, Citation1981). Therefore, there is an urgent need to explore the utilization of legume proteins in the formulation of new food products or to enrich the traditional formulations.
Moringa oleifera Lamarck (fam. Moringaceae), is a perennial foliaged tree, widely cultivated due to its high adaptability to climatic conditions and dry soils (Okuda, Baes, Nishijima, & Okada, Citation2001). It is considered as one of the most useful trees in the world because almost all parts of this plant can be used as food, in medicines and for industrial purposes (Khalafalla & Abdellatef, Citation2010). M. oleifera has been used in several developing countries to prevent protein-energy malnutrition especially among children at an early age and pregnant women (Oduro, Ellis, & Owusu, Citation2008).
Plant proteins are now regarded as versatile functional ingredients more than as essential nutrients. This evolution towards functionality is mainly driven by the demand of consumers. Functional properties constitute the major criteria for the adoption and acceptability of proteins in food systems. Physicochemical and conformational properties of proteins such as surface hydrophobicity, ligand binding, secondary structure, molecular flexibility and structure stability, affect functional properties (Mune Mune & Sogi, Citation2016; Zayas, Citation1997). Hence, this study examined the secondary structure, surface hydrophobicity, solubility, water and fat holding capacities, finally effects of oil concentration and pH on the emulsifying and foaming properties of Moringa olifeira seed and leaf flour.
2. Materials and methods
2.1. Materials
M. oleifera seeds and leaves were purchased from Mokolo market (Yaoundé, Cameroon) in dried form. Dried seeds and leaves were hand-picked and stored in polyethylene bags in the refrigerator (~4°C) until used.
2.2. Methods
2.2.1. Preparation of M. oleifera seed and leaf flour
M. Oleifera seeds were dehulled manually, then seeds and leaves were ground into flour and passed through a 150 μm mesh sieve. The flours were extracted twice with the hexane/ethanol (1:1, v/v) solvent system in a 1/3 (w/v) ratio as described by Lu et al. (Citation2009). The seed and leaf flour contained 33.53 and 18.63% protein, 7.06 and 14.79% moisture and 3.16 and 11% ash, respectively, determined according to AOAC (Citation1990) methods.
2.2.2. Surface hydrophobicity
Surface hydrophobicity was determined by the bromophenol blue (BPB) fixation method as described by Chelh, Gatellier, and Santé-Lhoutellier (Citation2006). BPB has been shown to bind to the same hydrophobic sites on proteins as fluorescent probes (Bertsch, Mayburd, & Kassner, Citation2003), and the absorption difference spectroscopy of BPB provides a valuable supplement to 1,8-anilinonaphthalenesulphonate (ANS), as a fluorescent probe, for determining in solution surface hydrophobicity (Chelh et al., Citation2006).
2.2.2. Secondary structure characterization
The secondary structures of protein concentrates were characterized using Fourier Transform Infrared (FTIR) spectra. The dried protein samples were put between two aluminium foils and pressed into a pellet. FTIR spectra were obtained in the wavenumber range of 400–4,000 cm−1 during 32 scans with 4 cm−1 resolution using a FTIR spectrometer (Perkin Elmer, Massachusetts, USA). Data were analyzed by means of the Spectrum software version 3.01 and Peakfit 4.12 (Systat Software, San Jose, USA). Secondary structural features were calculated from the amide I envelope by non-linear regression fitting of Gaussian peaks of the original spectra. Peaks assignments were made using the results of Farrell, Wickham, Unruh, Qi, and Hoagland (Citation2001).
2.2.3. Functional properties
2.2.3.1. Water holding capacity
Water holding capacity (WHC) was determined by using the method outlined by Beuchat (Citation1977).
2.2.3.2. Oil absorption capacity
The oil absorption capacity was determined using the method of Chakraborty (Citation1986) using soybean oil.
2.2.3.3. Protein solubility
The flours were mixed with water at the ratio of 1/100 (w/v), and pH of the mixtures adjusted to 2–10 if necessary with 1.0 M NaOH and HCl. The suspensions were hydrated overnight at 4°C, and stirred at room temperature for 10 min. The protein solutions were then centrifuged at 6,000 rpm for 20 min (Mune Mune & Sogi, Citation2016). Protein concentration in each supernatant was determined by the Kjeldahl method (AOAC, Citation1990). Protein solubility was calculated as , where W1 was the weight of protein in the supernatant (g), W0 was the weight of protein in the sample (g).
2.2.3.4. Emulsifying properties
Emulsifying capacity (EC) and emulsifying stability (ES) of M. Oleifera seed and leaf flour were measured as describe by Lawal (Citation2004). Aqueous dispersions of 2 and 4% (w/v) of flour were prepared and adjusted to pH 4, 7 and 9 with 0.3 M HCl or NaOH. A 3 ml dispersion volume was mixed with 1.5 or 3 ml of soybean oil to obtain a oil to water ratio (OWR) of 1/4 or 1/2 (v/v). The dispersion was mixed at high speed for 1 min at room temperature, using a Bioblock Scientific 35011 (Illkirch, France) magnetic stirrer, then centrifuge at 1,100 rpm for 5 min in an Eppendorf AG (Hamburg, Germany) centrifuge. The emulsifying activity was calculated as:
ES was determined by heating the emulsion at 80°C for 30 min before centrifuging at 1,100 rpm for 5 min.
2.2.3.5. Foaming properties
The foaming properties were determined in triplicate using the method described by Wu, Wang, Ma, and Ren (Citation2009). Concentrations of 2 and 4% (w/v) flour were prepared in distilled water and adjusted to pH 4, 7 and 9 with 0.6 M HCl or NaOH. Volumes of 100 ml (V1) of M. olifeira flour suspensions were agitated for 5 min using a magnetic agitator (Heidolph, Schwabach, Germany), then whipped at high speed for 1 min in a Faciclic blender (Bordeaux, France). The blend was then poured in 250 ml graduated cylinder and the volume of foam (V2) were immediately recorded at 0, 30 and 60 min. Foaming was calculated using the following equation: . Foaming capacity (FC) was determined at 0 min, and foam stability (FS) after 30 and 60 min.
2.2.4. Statistical analyses
Results are expressed as mean value ± standard deviation of three different determinations. Means were compared by the one-way ANOVA analysis followed by Duncan post hoc test. The probability p < 0.05 was considered statistically different. Data analysis and graphic plotting were done using STATISTICA™ (version 5.5, 2002; Statsoft Inc., USA) and SPSS (16.0 version 10.1, 2000, SPSS Inc., USA) softwares.
3. Results and discussion
3.1. FTIR spectra analysis
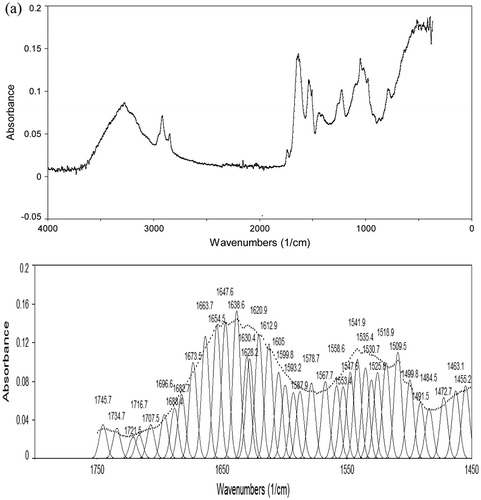
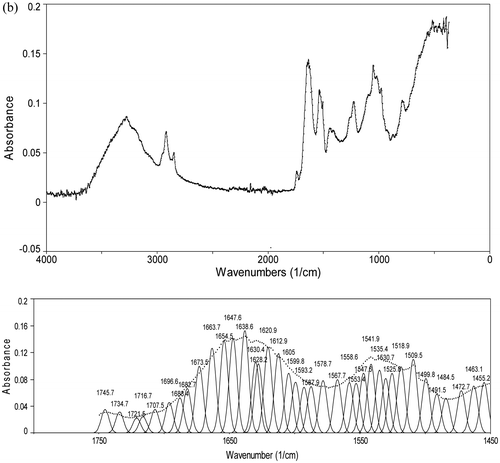
FTIR analysis was used in this study to assess the conformation of M. olifeira seed and flour proteins. The original spectra showed two major bands, the amide I at 1,633 cm−1 and the amide II band at 1,550 cm−1 (Figure (a)), indicating the typical structure of protein. The samples also contained water (3,200–3,600 cm−1), carbohydrates (2,800–3,000 cm−1), and fats (2,800–3,000 cm−1; 970 cm−1) (Nawrocka & Lamorska, Citation2013). The amide I band was further resolved by Fourier self-deconvolution and second derivative employing a Gaussian fit of the peaks (Figure (b)). The analysis clearly resolves bands which have been assigned on the basis of data available in literature. The bands were easily assigned as follows: ß-turn: 1,660–1,700 cm; α-helix: 1,650–1,656 cm−1; irregular structure: 1,640–1,644 cm; and ß-sheet or extended structure: 1,620–1,640 cm−1. (Farrell et al., Citation2001). According to the calculation of the individual component peak area, results on the secondary structure composition of M. olifeira protein is given as follows: α-helix: 10.27 and 13.49%; β-sheet: 41.11 and 32.77%; β-turn: 37.97 and 39.31% and irregular structure: 10.65% and 14.43%, respectively for seeds and leaves. Generally plant protein showed high levels of β-sheet secondary structure (Mune Mune & Sogi, Citation2016). In addition, rice dreg protein showed high β-turn (43.04%), low irregular structure (10.0%) and no α-helix contents.
Figure 1. Original and self-deconvoluted FTIR spectrum and their curve fitting results of amide I and II of Moringa olifeira seed (a) and leaf (b) flour.
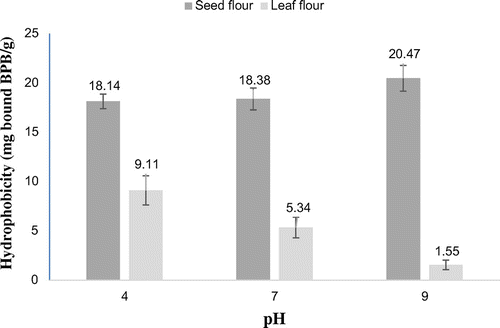
3.2. Surface hydrophobicity
Surface hydrophobicity of seed flour was significantly (p < 0.05) higher than that of leaf flour at pH 4–9 (Figure ). It was observed that variation in pH 4–7 did not affect surface hydrophobicity (18.34 mg BPB/g) of M. olifeira seed protein, while a significant (p < 0.05) increase was noticed at pH 9 (20.47 mg BPB/g). Also, surface hydrophobicity of leaf flour decreased with increasing pH. Mune Mune and Sogi (Citation2016) demonstrated that partial unfolding and hydrophobicity of Bambara bean protein was related to decrease in β-sheets and increase in β-turn and irregular structure contents.
3.3. Water and oil binding properties
WHC of M. olifeira seed flour (1.03 g/g) was significantly (p < 0.05) lower than that of leaf flour (3.17 g/g). WHC of M. olifeira leaf flour was within the range of recommended WHC values, i.e. 1.49–4.72 g/g for viscous foods (Aletor, Oshodi, & Ipinmoroti, Citation2002). Polar amino acid residues of proteins which have affinity with water molecules and carbohydrate composition are the major factors affecting WHC (Sreerama, Sashikala, Pratape, & Singh, Citation2012).
Oil absorption capacity (OAC) of M. olifeira seed flour (1.01 mL/g or 0.91 g/g) was significantly (p < 0.05) lower than that of leaf flour (1.6 mL/g or 1.46 g/g). OAC of leaf flour was in the same range than that of commercial soy protein concentrate (ISOPRO) (1.33 mL/g) as reported by Lin, Humbert, and Sosulski (Citation1974). Mune Mune and Sogi (Citation2016) demonstrated that high OAC of legume proteins was related to high β-sheet secondary structure content.
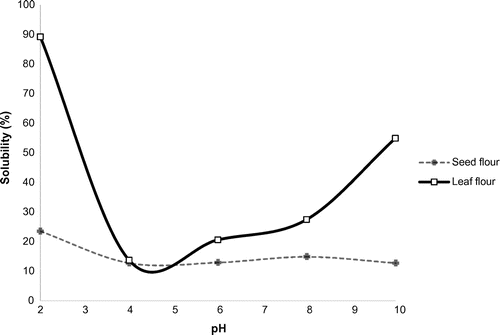
3.4. Protein solubility
Protein solubility of leaf protein followed a U-shape trend with minimum solubility at pH 4–5 (12%), and maximum solubility at pH 2 (89%) and pH 10 (54%) (Figure ). Solubility of seed protein was low (<15%) at pH 4–10 and maximum at pH 2 (25.52%). Similar results were obtained by AL-Kahtani and Abou-Arab (Citation1993) for Moringa Peregrina flour. High protein solubility was attributed to the increased net negative/positive charge, which dissociates the protein aggregates.
3.5. Emulsifying capacity and Emulsion stability
Effects of flour concentration, pH and oil to water ratio (OWR) on the EC and ES of M. olifeira seed and leaf flour are presented on Table . Generally, emulsifying properties of M. olifeira seed flour was significantly (p < 0.05) higher than that of leaf flour, excepted for EC at pH 4, flour concentration 2% (w/v) and OWR 1/4 (v/v). EC of seed flour increased significantly (p < 0.05) with the pH 4–9 at 2% (w/v) flour concentration. At 4% (w/v) concentration, non significant (p > 0.05) difference in EC was found at pH 7–9, and EC increased from pH 4 to 7. High EC could be obtained at pH 9; OWR of 1/2 (v/v) and seed flour concentration of 2% (w/v), and EC was not significantly (p > 0.05) different at pH 7 and 9 (50.53 and 52.17%, respectively); OWR of 1/2 (v/v) and flour concentration of 4% (w/v). Increase in EC with the pH probably reflected the exposure of hydrophobic amino acid residues previously buried due to the partial unfolding of protein (Zayas, Citation1997). Different behaviour of EC was observed with increasing flour concentration 2 to 4% (w/v). Hence, a non significant (p > 0.05) difference in EC was observed at pH 7 (OWR 1/4, v/v), and at pH 9 (OWR 1/2, v/v). Also, EC decreased at pH 9 (OWR 1/4, v/v) and increased at pH 7 (OWR 1/2, v/v). Accumulation of protein in the aqueous phase increased the protein-protein interaction at the expense of protein-oil interaction, and then reduced emulsifying properties (Lawal, Citation2004). Finally, EC of M. olifeira seed flour increased with OWR 1/4 to 1/2 (v/v) at pH 7–9 as also noticed by Cano-Medina et al. (Citation2011) for sesame and soy protein concentrates.
Table 1. EC and ES of Moringa olifeira seed and leaf flour at different concentrations (2 and 4%, w/v), pH and oil to water ratio (OWR)
ES of M. olifeira seed flour was significantly (p < 0.05) higher than that of leaf flour. High ES of M. olifeira seed flour was observed at flour concentration 4% (w/v) and pH 4 (39.67–53.84%), and at pH 9. It is well known that High ES required molecular rearrangement of the adsorbed proteins at the oil-water interface to form a thick layer which prevents coalescence.
3.6. Foaming capacity and foam stability
Foaming properties of M. olifeira seed flour depending on pH and flour concentration are presented in Table . Foaming properties of M. olifeira seed flour were significantly (p < 0.05) higher than that of leaf flour (excepted at pH 4). It was noticed a significant (p < 0.05) increase in FC with seed flour concentration and pH. FC of leaf flour increased with the concentration, and maximum FC was observed at pH 9. The high FC at pH 10 may be due to an increase in the net charge of the protein molecules, which weakens hydrophobic interactions and increases protein flexibility. This allows them to spread to the air water interface more quickly, thus encapsulating air particles, and increasing foam formation (Lawal, Citation2004). FS after 30 and 60 min followed the same tendency as FC for seed flour. Non significant difference (p > 0.05) in FS of leaf flour was noticed at pH 4–7, and the maximum FS was observed at pH 9 and flour concentration 4% (w/v). Lawal (Citation2004) reported that increase in foaming properties with increase in protein concentration is a result of increase in the viscosity which facilitates formation of a multiplayer cohesive protein film at the interface. Moreover, Panyam and Kilara (Citation1996) indicated that the molecular properties of proteins that are relevant for foaming include solubility to enable rapid diffusion at the interface, amphipathicity to enhance interfacial interactions, segmental flexibility to facilitate unfolding at the interface and molecular rearrangement to prevent close approach of bubbles.
Table 2. FC and FS of Moringa olifeira seed and leaf flour at different concentrations (2 and 4%, w/v) and pH
4. Conclusion
This study revealed M. olifeira seed and leaf protein presented high β-sheet and β-turn secondary structures and low α-helix and irregular structure. The leaf flour showed high water holding and oil absorption capacities. Protein solubility of leaf flour was lower at pH 4–5, and maximum at pH 2 and 10. The surface hydrophobicity of seed flour was significantly higher at pH 9 compared to pH 7 and 4. The variation of pH, oil to water ratio and flour concentration could be useful to influence emulsifying properties of M. olifeira seed flour. High emulsifying activity could be obtained at pH 7, oil to water ratio 1/2 (v/v) and flour concentration 4% (w/v), while high emulsifying capacity and emulsion stability was observed at pH 9, and flour concentration of 4% (w/v). For applications where high foaming properties are required, it is important to use M. olifeira seed flour at high combined pH and concentration.
Additional information
Funding
Notes on contributors
Martin Alain Mune Mune
Martin Alain Mune Mune is Senior Lecturer at the University of Maroua, Cameroon, since 2010. He got a PhD in Biochemistry in 2009 at the University of Yaoundé I, and postdoctoral in India and Germany. He is presently working on protein and peptide Biochemistry, functional properties and bioactivities, with the major aim of designing new functional products. This study is a contribution on the potential utilization of underutilized plant proteins as food ingredients.
References
- Aletor, O., Oshodi, A., & Ipinmoroti, K. (2002). Chemical composition of common leafy vegetables and functional properties of their leaf protein concentrates. Food Chemistry, 78, 63–68.10.1016/S0308-8146(01)00376-4
- AL-Kahtani, H. A., & Abou-Arab, A. (1993). Comparison of physical, and functional properties of Moringa Peregrina (AL-Yassen or Al-Ban) and soybean proteins. Cereal Chemistry, 70, 619–626.
- AOAC (Association of Official Analytical Chemists). (1990). Official methods of analysis (15th ed.). Arlington, TX: Author.
- Bertsch, M., Mayburd, A. L., & Kassner, R. J. (2003). The identification of hydrophobic sites on the surface of proteins using absorption difference spectroscopy of bromophenol blue. Analytical Biochemistry, 313, 187–195.10.1016/S0003-2697(02)00590-0
- Beuchat, L. R. (1977). Functional and electrophoretic characteristics of succinylated peanut flour protein. Journal of Agricultural and Food Chemistry, 25, 258–261.10.1021/jf60210a044
- Cano-Medina, A., Jiménez-Islas, H., Dendooven, L., Herrera, R. P., González-Alatorre, G., & Escamilla-Silva, E. M. (2011). Emulsifying and foaming capacity and emulsion and foam stability of sesame protein concentrates. Food Research International, 44, 684–692.10.1016/j.foodres.2010.12.015
- Chakraborty, P. (1986). Coconut protein isolate by ultrafiltration. In M. LeMeguer & P. Jelen (Eds.), Food engineering and process applications, (Vol. 2, pp. 308–315). New York, NY: Elsevier.
- Chelh, I., Gatellier, P., & Santé-Lhoutellier, V. (2006). Technical note: A simplified procedure for myofibril hydrophobicity determination. Meat Science, 74, 681–683.10.1016/j.meatsci.2006.05.019
- Duke, J. A. (1981). Handbook of LEGUMES of world economic importance (pp. 307–310). New York, NY: Plenum Press.10.1007/978-1-4684-8151-8
- Farrell, Jr., H. M., Wickham, E. D., Unruh, J. J., Qi, P. X., & Hoagland, P. D. (2001). Secondary structural studies of bovine caseins: Temperature dependence of β-casein structure as analyzed by circular dichroism and FTIR spectroscopy and correlation with micellization. Food Hydrocolloids, 15, 341–354.10.1016/S0268-005X(01)00080-7
- Khalafalla, M., & Abdellatef, E. (2010). Active principle from Moringa oleifera Lam leaves effective against two leukemias and a hepatocarcinoma. African Journal of Biotechnology, 9, 8467–8471.
- Lawal, O. (2004). Functionality of African locust bean (Parkia biglobossa) protein isolate: Effects of pH, ionic strength and various protein concentrations. Food Chemistry, 86, 345–355.10.1016/j.foodchem.2003.09.036
- Lin, M. J. Y., Humbert, E. S., & Sosulski, F. W. (1974). Certain functional properties of sunflower meal products. Journal of Food Science, 39, 368–370.10.1111/jfds.1974.39.issue-2
- Lu, Q., Zhang, Y., Wang, Y., Wang, David, Lee, Ru-po, Gao, Kun, ... Heber, D. (2009). California Hass avocado: Profiling of carotenoids, tocopherol, fatty acid, and fat content during maturation and from different growing areas. Journal of Agricultural and Food Chemistry, 57, 10408–10413.10.1021/jf901839h
- Mune Mune, M. A., & Sogi, D. S. (2016). Emulsifying and Foaming Properties of Protein Concentrates Prepared from Cowpea and Bambara Bean Using Different Drying Methods. International Journal of Food Properties, 19, 371–384.10.1080/10942912.2015.1023399
- Nawrocka, A., & Lamorska, J. (2013). Determination of food quality by using spectroscopic methods. In S. Grundas & A. Stepniewski (Eds.), Advances in agrophysical research (pp. 347–367). Croatia: In Tech.
- Oduro, I., Ellis, W. O., & Owusu, D. (2008). Nutritional potential of two leafy vegetables: Moringa oleifera and Ipomoea batatas leaves. Scientific research and essay, 3, 57–60.
- Okuda, T., Baes, A. U., Nishijima, W., & Okada, M. (2001). Isolation and characterization of coagulant extracted from moringa oleifera seed by salt solution. Water Research, 35, 405–410.10.1016/S0043-1354(00)00290-6
- Panyam, D., & Kilara, A. (1996). Enhancing the functionality of food proteins by enzymatic modification. Trends in Food Science and Technology, 7, 120–125.10.1016/0924-2244(96)10012-1
- Sreerama, Y. N., Sashikala, V. B., Pratape, V. M., & Singh, V. (2012). Nutrients and antinutrients in cowpea and horse gram flours in comparison to chickpea flour: Evaluation of their flour functionality. Food Chemistry, 131, 462–468.10.1016/j.foodchem.2011.09.008
- Wu, H., Wang, Q., Ma, T., & Ren, J. (2009). Comparative studies on the functional properties of various protein concentrate preparations of peanut protein. Food Research International, 42, 343–348.10.1016/j.foodres.2008.12.006
- Zayas, J. F. (1997). Functionality of proteins in food (pp. 1–373). New York, NY: Springer.