Abstract
Kefir grains are multi-species natural starter culture consisting of lactic acid bacteria, acetic acid bacteria, and yeasts, creating complex symbiotic community and widely used in fermented dairy products. The microbiological and chemical composition of kefir grains indicate that they are very complex probiotic, with lactic acid bacteria, generally the predominant microorganisms. Therefore, kefir grains were usually used the starter in fermented dairy products. Our review provides an overview of microbiological characteristics, microstructure, chemical composition of the kefir grains and their use in fermented dairy products.
Public Interest Statement
Kefir grains play a natural starter culture role during the production of kefir and are recovered after the fermentation process by milk straining. These grains are composed of microorganisms immobilized on a polysaccharide and protein matrix, where several species of bacteria and yeast coexist in symbiotic association. In this ecosystem there is a relatively stable microorganism population, which interacts with and influences other members of the community. This review provides an overview of kefir grains’ microbiological characteristics, microstructure, chemical composition, and their fermented dairy products, which help industries to develop kefir-like products and also help researchers for further research.
Competing Interests
The authors declare no competing interest.
1. Introduction
Kefir is a traditional drink obtained via fermentation of milk by kefir grains. The name kefir is derived from the Turkish language word keyif, meaning “good feeling” for the feelings experienced after drinking it (Leite et al., Citation2015). Kefir grains are white to yellowish, cauliflower-like grains, with a slimy but firm texture (see Figure ). The grains are composed of an inert matrix made up of polysaccharides and proteins. The matrix is densely populated by lactic acid bacteria species, acetic acid bacteria, and yeasts (Kalamaki & Angelidis, Citation2016; Macuamule, Wiid, Helden, Tanner, & Witthuhn, Citation2015).
Figure 1. Macroscopic structure of kefir grains (Leite et al., Citation2013).

1.1. Microbiological characteristics
The microflora of kefir grains is remarkably stable, retaining activity for years if preserved and incubated under appropriate cultural and physiological conditions (O’Brien, Citation2012; Vardjan, Mohar Lorbeg, Rogelj, & Čanžek Majhenič, Citation2013). The grain-milk ratio, incubation time and temperature, sanitation during separation of kefir grains, washing of grains and cold storage all drastically affect the product quality and the microflora of the kefir grains (Guzel-Seydim, Wyffels, Seydim, & Greene, Citation2005). However, their complex microbiological association makes them difficult to obtain defined and constant kefir starter culture appropriate for industrial kefir production of conventional properties (Vardjan et al., Citation2013). The common microorganisms isolated from kefir grains at different regions have differences. The bacteria of the grains are usually various homo- and heterofermentative lactic acid bacteria species of Lactobacillus, Lactococcus, Leuconostoc and Streptococcus; acetic acid bacteria species of Acetobacter. In Taiwanese kefir grains, Lactobacillus was the most frequent genus detected, and Lb. kefiri was the most frequently detected species (Chen, Wang, & Chen, Citation2008). Lactobacilli was present in all kefir grains from Bulgaria indicating the importance of this group of bacteria in the production of the beverage (Simova et al., Citation2002). Mainville, Robert et al. by polyphasic characterization, identified the species Lb. heleveticus, Lb. kefir, Lb. parakefir in kefir grains from Russia (Mainville, Robert, Lee, & Farnworth, Citation2006) (see Table ).
Table 1. Microflora species reported in kefir and kefir grains
1.2. Microstructure
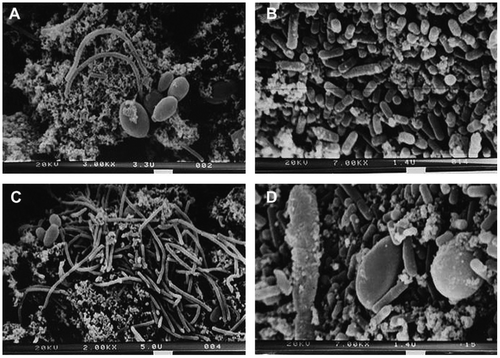
The exterior surfaces of the kefir grains looked smooth and shiny with the naked eye. However, the grain surfaces, under scanning electron microscopy, were revealed to be very rugged (Mei, Guo, Wu, & Li, Citation2014). In the inner portion of the grain, a variety of lactobacilli (long and curved), yeasts and fibrillar material were observed. The short lactobacilli and yeast were observed on the outer portion (Zhou, Liu, Jiang, & Dong, Citation2009). The density of microbial cell on the inner portion was less than that on the outer portion. No lactococcus was found on scanning electron micrographs, which may bedue to the bad attachment of lactococcus (see Figure ).
Figure 2. Scanning electron micrographs of Tibetan kefir grains. (A, C) The inside surface of Tibetan kefir grain. (B, D) The outside surface of Tibetan kefir grain (Zhou et al., Citation2009).
1.3. Chemical composition
Kefir grains are soft, gelatinous white biological mass, comprised of protein, lipids and soluble polysaccharide, the kefiran complex. Kefiran is a water souble glucogalactian produced by Lb. kefiranofaciens, Lc. plantarum (Ahmed, Wang, Anjum, Ahmad, & Khan, Citation2013; Hamet, Piermaria, & Abraham, Citation2015; Wang, Xiao, Zheng, Yang, & Yang, Citation2015), and so on. In general, kefir grains increase their weight with subcultures in milk due to the increase in microorganism biomass together with an increase in amount of the matrix that composed by protein and polysaccharide (Garrote, Abraham, & De Antoni, Citation2001).
1.4. Fermented dairy products
1.4.1. Cheese
The kefir culture has gained researchers’ attention with regarding to cheese manufacturing due to its potential effect on quality, health, and safety properties of the product. Kefir grains or kefir has been used as a starter in many types of cheese. Goncu and Alpkent used kefir, yoghurt or a commercial cheese culture as a starter in the white pickled cheese production (Goncu & Alpkent, Citation2005). During 120 days ripenning, scores for appearance, structure, and odour were rated highest in white cheese samples produced by using kefir culture. Kefir can be successfully used as a starter culture in production of white pickled cheese. However, for the commercial production of cheese, direct use of kefir grains is impractical regarding transportation, storage, and cell dosage. Freeze-dried or thermally-dried is a solution for long-term preservation of microorganisms and convenience for shipping (Morgan, Herman, White, & Vesey, Citation2006). Dimitrellou et al. (Citation2010) evaluated the use of a freeze-dried kefir culture in the production of a novel type of whey-cheese similar to traditional Greek Myzithra-cheese. The use of kefir culture as a starter led to increased lactic acid concentrations and decreased pH values in the final product compared with whey-cheese without starter culture. The degree of proteolysis were significantly higher in cheeses produced by freeze-dried kefir culture during the later stages of ripening. The cheeses produced were characterized as high-quality products during the preliminary sensory evaluation. The freeze-dried kefir culture added in the cheese seemed to suppress growth of pathogens and increased preservation time. Besides, Dimitrellou, Kourkoutas, Koutinas, and Kanellaki (Citation2009), Dimitrellou et al. (Citation2010) also evaluated the use of thermally-dried immobilized kefir on casein as a starter culture for protein-enriched dried whey cheese. Thermally-dried immobilized kefir starter culture resulted in an improved profile of aroma-related compounds. The preliminary sensory evaluation ascertained the soft, fine taste and the overall improved quality of cheese produced with the thermally-dried immobilized kefir. The free or immobilized freeze-dried kefir cells was used as a starter culture in hard-type cheese production. The freeze-dried kefir culture improved aroma, taste, and texture characteristics while increasing the degree of openness in comparison to traditional hard-type cheese products (Katechaki, Panas, Rapti, Kandilogiannakis, & Koutinas, Citation2008). Then the thermally-dried free and immobilized kefir cells were compared in the hard-type cheese production (Katechaki, Panas, Kourkoutas, Koliopoulos, & Koutinas, Citation2009). Both free and immobilised cells of kefir culture led to the production of improved cheese products as regards preservation time, sensory and textural characteristics. Thermal drying contributed to the volatile composition of the final product when compared to cheeses made with the alternate method of freeze-drying. The thermal drying process was simple, and of low cost, lower than that of freeze drying. A freeze-dried Tibetan kefir co-culture was used in the Camembert-type cheese production (Mei, Guo, Wu, Li, & Yu, Citation2015; Mei et al., Citation2014). A total of 45 compounds were detected during ripenning. Volatile carboxylic acids were abundant in the headspace of the cheese. A total of 147 bacteria and 129 yeasts were obtained from the cheese during ripening. Lactobacillus paracasei represents the most commonly identified lactic acid bacteria isolates, with 59 of a total of 147 isolates.
1.4.2. Kefir
Kefir is a self-carbonated, refreshing fermented yogurt which has a unique flavor due to a mixture of lactic acid, carbon dioxide, acetaldehyde, acetoin, slight alcohol, and other fermentation flavor products (Guzel-Seydim, Seydim, & Greene, Citation2000; Nielsen, Gürakan, & Ünlü, Citation2014). Kefir typically contains 89–90% moisture, 0.2% lipid, 3.0% protein, 6.0% sugar, 0.7% ash and 1.0% each of lactic acid and alcohol. Kefir has been reported to contain 1.98 g/L of CO2 and 0.48% alcohol (Beshkova, Simova, Frengova, Simov, & Dimitrov, Citation2003), and the content of carbon dioxide (201.7–277.0 ml/L) positively correlated with the concentration (10–100 g/L) of kefir grains (Arslan, Citation2015). One feature of kefir that differs from other fermented yogurt products is that starter kefir grains are recovered after fermentation. The biomass of kefir grains slowly increases during the process of kefir fermentation (Guzel-Seydim, Kok-Tas, Greene, & Seydim, Citation2011). Beyond its inherent high nutritional value as a source of protein and calcium, kefir has a long tradition of being regarded as good for health in countries where it is a staple in the diet (Vinderola et al., Citation2005). Though cow’s milk is most common, kefir can be made from any type of milk. For dairy kefir, cow, goat, or sheep milk are all commonly used (Otles & Cagindi, Citation2003). Tratnik used the goat’s milk to produced the kefir. When the goat’s milk was fortified with 2/100 g skimmed milk powder, whey protein concentrate and inulin, the acidity level remained very stable in all the samples during the storage period. Goat’s samples have significantly lower viscosity and slightly lower sensory profiles (Tratnik, Božanić, Herceg, & Drgalić, Citation2006). The pasteurised goat milk and goat milk kefir prepared using different amount of Indonesian kefir grains. The best chemical characteristics (pH: 4.37; ethanol content: 0.91%; titratable acidity number: 0.76%; and lactose content: 4.23%) was obtained from goat milk kefir prepared with 7% (w/v) kefir grains and incubation time of 24 h (Chen, Liu, Lin, & Yeh, Citation2005). Varieties of kefir were made from bovine, caprine and ovine milk, using kefir grains and two direct-to-vat inoculation starter cultures (Wszolek, Tamime, Muir, & Barclay, Citation2001). Lactic acid bacteria and yeasts were the predominant flora in fresh and stored kefirs. The firmness and all the sensory attributes of the product were influenced by the type of milk used (ovine > bovine > caprine). Storage influenced mouth-feel characters (serum separation, chalky, mouth-coating, and slimy). In general, the type of milk had greater influence on product characteristics than that of starter cultures. Kefir is best made with milk containing fat. As there is an established relationship between many health problems and the consumption of saturated fats and cholesterol, a non-fat choice in kefir is desirable; however, non-fat milk makes a kefir with significantly lower quality (Nielsen et al., Citation2014). Ertekin and Guzel-Seydim experimented with non-fat milk supplemented with the fat substitutes inulin and Dairy-Lo@ to improve the quality of kefir made with skim milk. They found that while kefir grains fermenting whole fat milk resulted in the best quality kefir, Dairy Lo@ and inulin could be used without any adverse effect for the production of non-fat kefir (Ertekin & Guzel-Seydim, Citation2010).
1.4.3. Kefir beverage
Kefir grains successfully ferment the milk from most mammals and continue to grow in such milk. In addition, kefir grains ferment milk substitutes such as soy milk, rice milk and coconut milk, as well as other sugary liquids including fruit juice, coconut water, beer wort and ginger beer (Gaware et al., Citation2011). Carrot, fennel, melon, onion, tomato and strawberry juices underwent backslopping fermentations, could be carried out by water kefir microorganisms. Results indicated that lactic acid bacteria and yeasts were capable of growing in the juices tested. After fermentation, there was observance of a decrease of the soluble solid content and an increase of the number of volatile organic compounds. The overall quality assessment indicated that carrot kefir-like beverage was the product mostly appreciated by the judges (Corona et al., Citation2016). Cocoa pulp was also used for new cocoa beverages (Puerari, Magalhães, & Schwan, Citation2012). A microbial steady structure was detected in the analyzed kefir cocoa beverages and kefir grains. These beverages had the greater acceptance based on taste, odor, and appearance of the beverages. Based on the chemical characteristics and acceptance in the sensory analysis, these results open up perspectives for this innovative application of kefir grains for developing cocoa pulp-based beverages. Cui, Chen, Wang, and Han (Citation2013) experimented with walnut milk to produce kefir beverage. The kefir grains can be used to ferment walnut milk. The suggested optimum fermentation conditions are the following: fermentation temperature of 30°C, fermentation time of 12 h, inoculum size of 3 g of kefir grains (wet weight) and sucrose concentration of 8 g/100 mL.
Cheese whey is the liquid remaining after the precipitation and removal of milk casein during cheese-making. This by-product represents approximately 85–90% of the milk volume and retains 55% of milk nutrients (Rico, Muñoz, & Rico, Citation2015). Cheese whey represents an important environmental problem because of the high volumes produced and its high organic matter content (Magalhães et al., Citation2010). The pressure of antipollution regulations together with whey nutritional value challenges the dairy industry to face whey surplus as a resource and not only as a waste problem (Magalhães et al., Citation2010). The production of a functional beverage produced upon whey fermentation by kefir grains could be an interesting alternative for cheese whey utilisation. Cheese whey fermentation by kefir microrganisms could decrease the high lactose content in cheese whey, producing mainly lactic acid and other metabolites such as aroma compounds contributing to the flavour and texture and increasing carbohydrate solubility and sweetness of the end product (Magalhães et al., Citation2011). Manufacture of beverages through lactic fermentations can provide desirable sensory profiles and have already been considered an option to add value to cheese whey (Magalhães et al., Citation2010, Citation2011; Mazaheri Assadi, Abdolmaleki, & Mokarrame, Citation2008; Nambou et al., Citation2014). Magalhães et al. made a tentative and more comprehensive study (including morphological and microbial variations, Chemical composition and sensory analysis) of the kefir grains as a starter culture for cheese whey-based beverages production (Magalhães et al., Citation2010, Citation2011). A steady structure and dominant microbiota, including probiotic bacteria, was detected in the analyzed kefir beverages. Besides, based on the chemical characteristics and acceptance in the sensory analysis, the kefir grains showed potential to be used for developing cheese whey-based beverages. Some researchers prepared fermented dairyfruits juice beverage making use of juice and whey. The dairyfruits juice beverage provided desirable sensory profiles and uses of whey can be applied to change it from a waste to a delicious beverage (Sabokbar & Khodaiyan, Citation2015, Citation2016; Sabokbar, Moosavi-Nasab, & Khodaiyan, Citation2015).
2. Conclusion
Kefir grains are unique symbiotic associations of different microrganisms, including lactic acid bacteria, yeasts and acetic acid bacteria, cohabiting in a natural polysaccharide and a protein matrix. Kefir is a distinctive fermented dairy product due to the unique, multi-species natural kefir grains used as the starter culture. The microbiological and chemical composition of kefir provide a complex probiotic effect due to the inherent lactic acid bacteria and yeast. Kefir grains ferment the milk from most mammals and will continue to grow in such milk. Now, kefir grains have been widely used in fermented dairy products,including cheese, kefir, whey beverage, as well as other sugary liquids.
Funding
This work was supported by the quality improvement projects of Shanghai higher vocational education.
Additional information
Notes on contributors
Xin Gao
Xin Gao is an assistant in Shanghai Urban Construction Vocational College. He received his Ms in Biochemistry and Molecular Biology from Liaoning Normal University in China (2008). He works in the area of Food Technology, with an emphasis on Dairy Science. His research focuses on traditional fermented dairy products. He is currently exploring the lactic acid bacteria strains with high yield extracellular polysaccharide that can be used in the low fat yogurt.
References
- Ahmed, Z., Wang, Y., Anjum, N., Ahmad, A., & Khan, S. T. (2013). Characterization of exopolysaccharide produced by Lactobacillus kefiranofaciens ZW3 isolated from Tibet kefir-Part II. Food Hydrocolloids, 30, 343–350.10.1016/j.foodhyd.2012.06.009
- Leite, A. M. O., Miguel, M. A. L, Peixoto, R. S., Rosado, A. S., Silva, J. T., & Paschoalin, V. M. F. (2013). Microbiological, technological and therapeutic properties of kefir: A natural probiotic beverage. Brazilian Journal of Microbiology, 44, 341–349. doi:10.1590/S1517-83822013000200001
- Arslan, S. (2015). A review: Chemical, microbiological and nutritional characteristics of kefir. CyTA-Journal of Food, 13, 340–345.10.1080/19476337.2014.981588
- Beshkova, D. M., Simova, E. D., Frengova, G. I., Simov, Z. I., & Dimitrov, Z. P. (2003). Production of volatile aroma compounds by kefir starter cultures. International Dairy Journal, 13, 529–535.
- Chen, H. C., Wang, S. Y., & Chen, M. J. (2008). Microbiological study of lactic acid bacteria in kefir grains by culture-dependent and culture-independent methods. Food Microbiology, 25, 492–501.10.1016/j.fm.2008.01.003
- Chen, M. J., Liu, J. R., Lin, C. W., & Yeh, Y. T. (2005). Study of the microbial and chemical properties of goat milk kefir produced by inoculation with taiwanese kefir grains. Asian-Australasian Journal of Animal Sciences, 18, 711–715.10.5713/ajas.2005.711
- Corona, O., Randazzo, W., Miceli, A., Guarcello, R., Francesca, N., Erten, H., ... Settanni, L. (2016). Characterization of kefir-like beverages produced from vegetable juices. LWT-Food Science and Technology, 66, 572–581.10.1016/j.lwt.2015.11.014
- Cui, X. H., Chen, S. J., Wang, Y., & Han, J. R. (2013). Fermentation conditions of walnut milk beverage inoculated with kefir grains. LWT-Food Science and Technology, 50, 349–352.10.1016/j.lwt.2012.07.043
- Dimitrellou, D., Kourkoutas, Y., Koutinas, A. A., & Kanellaki, M. (2009). Thermally-dried immobilized kefir on casein as starter culture in dried whey cheese production. Food Microbiology, 26, 809–820.10.1016/j.fm.2009.05.006
- Dimitrellou, D., Kandylis, P., Mallouchos, A., Komaitis, M., Koutinas, A. A., & Kourkoutas, Y. (2010). Effect of freeze-dried kefir culture on proteolysis in feta-type and whey-cheeses. Food Chemistry, 119, 795–800.10.1016/j.foodchem.2009.06.052
- Ertekin, B., & Guzel-Seydim, Z. B. (2010). Effect of fat replacers on kefir quality. Journal of the Science of Food and Agriculture, 90, 543–548.
- Fujisawa, T., Adachl, S., Toba, T., Arihara, K., & Mitsuoka, T. (1988). Lactobacillus kefiranofaciens sp. nov. isolated from kefir grains. International Journal of Systematic and Evolutionary Microbiology, 38, 12–14.
- Garbers, I. M., Britz, T. J., & Witthuhn, R. C. (2004). PCR-based denaturing gradient gel electrophoretictypification and identification of the microbial consortium present in kefir grains. World Journal of Microbiology and Biotechnology, 20, 687–693.10.1007/s11274-004-2624-3
- Garofalo, C., Osimani, A., Milanović, V., Aquilanti, L., De Filippis, F. D., Stellato, G., ... Clementi, F. (2015). Bacteria and yeast microbiota in milk kefir grains from different Italian regions. Food Microbiology, 49, 123–133.10.1016/j.fm.2015.01.017
- Garrote, G. L., Abraham, A. G., & De Antoni, G. L. (2001). Chemical and microbiological characterisation of kefir grains. Journal of Dairy Research, 68, 639–652.
- Gaware, V., Kotade, K., Dolas, R., Dhamak, K., Somwnshis, S., Nikam, V., ... Kashid, V. (2011). The magic of kefir: A review. Pharmacology Online, 1, 376–386.
- Goncu, A., & Alpkent, Z. (2005). Sensory and chemical properties of white pickled cheese produced using kefir, yoghurt or a commercial cheese culture as a starter. International Dairy Journal, 15, 771–776.10.1016/j.idairyj.2004.10.008
- Gulitz, A., Stadie, J., Wenning, M., Ehrmann, M. A., & Vogel, R. F. (2011). The microbial diversity of water kefir. International Journal of Food Microbiology, 151, 284–288.10.1016/j.ijfoodmicro.2011.09.016
- Guzel-Seydim, Z., Seydim, A. C., & Greene, A. K. (2000). Organic acids and volatile flavor components evolved during refrigerated storage of kefir. Journal of Dairy Science, 83, 275–277.10.3168/jds.S0022-0302(00)74874-0
- Guzel-Seydim, Z., Wyffels, J. T., Seydim, A. C., & Greene, A. K. (2005). Turkish kefir and kefir grains: Microbial enumeration and electron microscobic observation. International Journal of Dairy Technology, 58, 25–29.10.1111/idt.2005.58.issue-1
- Guzel-Seydim, Z. B., Kok-Tas, T., Greene, A. K., & Seydim, A. C. (2011). Review: Functional properties of kefir. Critical Reviews in Food Science and Nutrition, 51, 261–268.10.1080/10408390903579029
- Hamet, M. F., Londero, A., Medrano, M., Vercammen, E., Van Hoorde, K. V., Garrote, G. L., ... Abraham, A. G. (2013). Application of culture-dependent and culture-independent methods for the identification of Lactobacillus kefiranofaciens in microbial consortia present in kefir grains. Food Microbiology, 36, 327–334.10.1016/j.fm.2013.06.022
- Hamet, M. F., Piermaria, J. A., & Abraham, A. G. (2015). Selection of EPS-producing Lactobacillus strains isolated from kefir grains and rheological characterization of the fermented milks. LWT-Food Science and Technology, 63, 129–135.10.1016/j.lwt.2015.03.097
- Hsieh, H. H., Wang, S. Y., Chen, T. L., Huang, Y. L., & Chen, M. J. (2012). Effects of cow’s and goat’s milk as fermentation media on the microbial ecology of sugary kefir grains. International Journal of Food Microbiology, 157, 73–81.10.1016/j.ijfoodmicro.2012.04.014
- Huang, Y., Wu, F., Wang, X., Sui, Y., Yang, L., & Wang, J. (2013). Characterization of Lactobacillus plantarum Lp27 isolated from Tibetan kefir grains: A potential probiotic bacterium with cholesterol-lowering effects. Journal of Dairy Science, 96, 2816–2825.10.3168/jds.2012-6371
- Kalamaki, M. S., & Angelidis, A. S. (2016). Isolation and molecular identification of yeasts in Greek kefir. International Journal of Dairy Technology. doi:10.1111/1471-0307.12329
- Katechaki, E., Panas, P., Rapti, K., Kandilogiannakis, L., & Koutinas, A. A. (2008). Production of hard-type cheese using free or immobilized freeze-dried kefir cells as a starter culture. Journal of Agricultural and Food Chemistry, 56, 5316–5323.10.1021/jf703585y
- Katechaki, E., Panas, P., Kourkoutas, Y., Koliopoulos, D., & Koutinas, A. A. (2009). Thermally-dried free and immobilized kefir cells as starter culture in hard-type cheese production. Bioresource Technology, 100, 3618–3624.10.1016/j.biortech.2009.02.061
- Koroleva, N. S., & Robinson, R. K. (1991). Products prepared with lactic acid bacteria and yeasts. In R. K. Robinson (Ed.), Therapeutic properties of fermented milks (pp. 159–179). London: Elsevier Applied Science.
- Latorre-García, L., del Castillo-Agudo, L. D., & Polaina, J. (2007). Taxonomical classification of yeasts isolated from kefir based on the sequence of their ribosomal RNA genes. World Journal of Microbiology and Biotechnology, 23, 785–791.10.1007/s11274-006-9298-y
- Leite, A. M. O., Mayo, B., Rachid, C. T. C. C., Peixoto, R. S., Silva, J. T., Paschoalin, V. M. F., & Delgado, S. (2012). Assessment of the microbial diversity of Brazilian kefir grains by PCR-DGGE and pyrosequencing analysis. Food Microbiology, 31, 215–221.10.1016/j.fm.2012.03.011
- Leite, A. M. O., Miguel, M. A. L., Peixoto, R. S., Ruas-Madiedo, P., Paschoalin, V. M. F., Mayo, B., & Delgado, S. (2015). Probiotic potential of selected lactic acid bacteria strains isolated from Brazilian kefir grains. Journal of Dairy Science, 98, 3622–3632.10.3168/jds.2014-9265
- Macuamule, C. L. S., Wiid, I. J., Helden, P. D. V., Tanner, M., & Witthuhn, R. C. (2015). Effect of milk fermentation by kefir grains and selected single strains of lactic acid bacteria on the survival of Mycobacterium bovis BCG. International Journal of Food Microbiology, 217, 170–176.
- Magalhães, K. T., de Melo Pereira, G. V., Dias, D. R., & Schwan, R. F. (2010). Microbial communities and chemical changes during fermentation of sugary Brazilian kefir. World Journal of Microbiology and Biotechnology, 26, 1241–1250.10.1007/s11274-009-0294-x
- Magalhães, K. T., Dias, D. R., de Melo Pereira, G. V., Oliveira, J. M., Domingues, L., Teixeira, J. A., ... Schwan, R. F. (2011). Chemical composition and sensory analysis of cheese whey-based beverages using kefir grains as starter culture. International Journal of Food Science & Technology, 46, 871–878.10.1111/ifs.2011.46.issue-4
- Mainville, I., Robert, N., Lee, B., & Farnworth, E. R. (2006). Polyphasic characterization of the lactic acid bacteria in kefir. Systematic and Applied Microbiology, 29, 59–68.10.1016/j.syapm.2005.07.001
- Mazaheri Assadi, M., Abdolmaleki, F., & Mokarrame, R. R. (2008). Application of whey in fermented beverage production using kefir starter culture. Nutrition & Food Science, 38, 121–127.
- Mei, J., Guo, Q., Wu, Y., & Li, Y. (2014). Microbial diversity of a camembert-type cheese using freeze-dried tibetan kefir coculture as starter culture by culture-dependent and culture-independent methods. PLoS ONE, 9, e111648.10.1371/journal.pone.0111648
- Mei, J., Guo, Q., Wu, Y., Li, Y., & Yu, H. (2015). Study of proteolysis, lipolysis, and volatile compounds of a Camembert-type cheese manufactured using a freeze-dried Tibetan kefir co-culture during ripening. Food Science and Biotechnology, 24, 393–402.10.1007/s10068-015-0052-9
- Miguel, M. G. D. C. P., Cardoso, P. G., Lago, L. D. A., & Schwan, R. F. (2010). Diversity of bacteria present in milk kefir grains using culture-dependent and culture-independent methods. Food Research International, 43, 1523–1528.10.1016/j.foodres.2010.04.031
- Miguel, M. G. D. C. P., Cardoso, P. G., Magalhães, K. T., & Schwan, R. F. (2011). Profile of microbial communities present in tibico (sugary kefir) grains from different Brazilian States. World Journal of Microbiology and Biotechnology, 27, 1875–1884.10.1007/s11274-010-0646-6
- Morgan, C. A., Herman, N., White, P. A., & Vesey, G. (2006). Preservation of micro-organisms by drying; A review. Journal of Microbiological Methods, 66, 183–193.10.1016/j.mimet.2006.02.017
- Nambou, K., Gao, C., Zhou, F., Guo, B., Ai, L., & Wu, Z. J. (2014). A novel approach of direct formulation of defined starter cultures for different kefir-like beverage production. International Dairy Journal, 34, 237–246.10.1016/j.idairyj.2013.03.012
- Nielsen, B., Gürakan, G. C., & Ünlü, G. (2014). Kefir: A multifaceted fermented dairy product. Probiotics and Antimicrobial Proteins, 6, 123–135.10.1007/s12602-014-9168-0
- O’Brien, K. V. (2012). The effect of frozen storage on the survival of probiotic microorganisms found in traditional and commercial kefir. Retrieved from http://etd.lsu.edu/docs/available/etd-04252012-155457/
- Otles, S., & Cagindi, O. (2003). Kefir: A probiotic dairy-composition, nutritional and therapeutic aspects. Pakistan Journal of Nutrition, 2, 54–59.
- Pintado, M. E., Da Silva, J. A. L., Fernandes, P. B., Malcata, F. X., & Hogg, T. A. (1996). Microbiological and rheological studies on Portuguese kefir grains. International Journal of Food Science & Technology, 31, 15–26.10.1111/ifs.1996.31.issue-1
- Puerari, C., Magalhães, K. T., & Schwan, R. F. (2012). New cocoa pulp-based kefir beverages: Microbiological, chemical composition and sensory analysis. Food Research International, 48, 634–640.10.1016/j.foodres.2012.06.005
- Rico, C., Muñoz, N., & Rico, J. L. (2015). Anaerobic co-digestion of cheese whey and the screened liquid fraction of dairy manure in a single continuously stirred tank reactor process: Limits in co-substrate ratios and organic loading rate. Bioresource Technology, 189, 327–333.10.1016/j.biortech.2015.04.032
- Sabokbar, N., & Khodaiyan, F. (2015). Characterization of pomegranate juice and whey based novel beverage fermented by kefir grains. Journal of Food Science and Technology, 52, 3711–3718.
- Sabokbar, N., & Khodaiyan, F. (2016). Total phenolic content and antioxidant activities of pomegranate juice and whey based novel beverage fermented by kefir grains. Journal of Food Science and Technology, 53, 739–747.10.1007/s13197-015-2029-3
- Sabokbar, N., Moosavi-Nasab, M., & Khodaiyan, F. (2015). Preparation and characterization of an apple juice and whey based novel beverage fermented using kefir grains. Food Science and Biotechnology, 24, 2095–2104.10.1007/s10068-015-0278-6
- Santos, A., San Mauro, M., Sanchez, A., Torres, J. M., & Marquina, D. (2003). The antimicrobial properties of different strains of lactobacillus spp. isolated from kefir. Systematic and Applied Microbiology, 26, 434–437.10.1078/072320203322497464
- Simova, E., Beshkova, D., Angelov, A., Hristozova, T., Frengova, G., & Spasov, Z. (2002). Lactic acid bacteria and yeasts in kefir grains and kefir made from them. Journal of Industrial Microbiology and Biotechnology, 28, 1–6.10.1038/sj/jim/7000186
- Taş, T. K., Ekinci, F. Y., & Guzel-Seydim, Z. B. (2012). Identification of microbial flora in kefir grains produced in Turkey using PCR. International Journal of Dairy Technology, 65, 126–131.10.1111/idt.2011.65.issue-1
- Tratnik, L., Božanić, R., Herceg, Z., & Drgalić, I. D. A. (2006). The quality of plain and supplemented kefir from goat’s and cow’s milk. International Journal of Dairy Technology, 59, 40–46. doi:10.1111/j.1471-0307.2006.00236.x
- Vardjan, T., Mohar Lorbeg, P., Rogelj, I., & Čanžek Majhenič, A. (2013). Characterization and stability of lactobacilli and yeast microbiota in kefir grains. Journal of Dairy Science, 96, 2729–2736.10.3168/jds.2012-5829
- Vinderola, C. G., Duarte, J., Thangavel, D., Perdigón, G., Farnworth, E., & Matar, C. (2005). Immunomodulating capacity of kefir. Journal of Dairy Research, 72, 195–202.10.1017/S0022029905000828
- Wang, J., Xiao, Z., Zheng, T., Yang, Y., & Yang, Z. (2015). Characterization of an exopolysaccharide produced by Lactobacillus plantarum YW11 isolated from Tibet kefir. Carbohydrate Polymers, 125, 16–25.
- Wang, S. Y., Chen, H. C., Liu, J. R., Lin, Y. C., & Chen, M. J. (2008). Identification of yeasts and evaluation of their distribution in Taiwanese kefir and viili starters. Journal of Dairy Science, 91, 3798–3805.10.3168/jds.2007-0468
- Witthuhn, R. C., Schoeman, T., & Britz, T. J. (2004). Isolation and characterization of the microbial population of different South African kefir grains. International Journal of Dairy Technology, 57, 33–37.10.1111/idt.2004.57.issue-1
- Wszolek, M., Tamime, A. Y., Muir, D. D., & Barclay, M. N. I. (2001). Properties of kefir made in Scotland and Poland using bovine, caprine and ovine milk with different starter cultures. LWT-Food Science and Technology, 34, 251–261.10.1006/fstl.2001.0773
- Zhou, J., Liu, X., Jiang, H., & Dong, M. (2009). Analysis of the microflora in Tibetan kefir grains using denaturing gradient gel electrophoresis. Food Microbiology, 26, 770–775.10.1016/j.fm.2009.04.009
