?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Eighteen brands; 15 domestic and 3 imported beers were collected from different market across ten sub cities of Addis Ababa. Aflatoxin B1, B2, G1 and G2 were determined by immuno-affinity column cleanup and reversed-phase liquid chromatography with fluorescence detection. Knowledge, attitude and practice (KAP) related to food quality and safety issues of the beer industries were also assessed. The result showed, out of twelve alcoholic domestic beer brands eleven were positive with a range of total aflatoxin between 1.23 and 12.47 μg/l and one brand was less than Limit of Detection. When comparing this research finding with other studies conducted in different countries, high aflatoxin level was detected in Ethiopian alcoholic beer. Conversely, aflatoxin was not detected in any of non-alcoholic domestic beer brands. Aflatoxin was detected but not quantified in all the three imported beer bands. On the other hand, knowledge about mycotoxin specifically aflatoxin in the beer factories was not satisfactory. In terms of their practice, only 35% of breweries are working under HACCP and most of breweries do not determines level of aflatoxin in incoming raw materials and final products in their laboratories. Fortunately, majority of the alcoholic domestic beer brands were less than 4 μg/l, which is the maximum allowable limit set by EU standard for total aflatoxins. However, two brands were found to be greater than 4 μg/l and unsafe for consumption and require action. Thus, government policymakers should incorporate aflatoxin legislation as mandatory standard and enforce to regulate in Ethiopian beers filling up the existing critical gap.
Keywords:
Public Interest Statement
In Ethiopia, there has been large increase in the consumption frequency and quantity of beer by referring to the brewing capacity increment and variety of beer brands in the market. However, there are no studies carried out so far to determine the level of aflatoxins in Ethiopian beers and their compliance with food safety laws.
Results from this study indicate that Ethiopian alcoholic beers have relatively higher aflatoxin content. As a result, there might be a significant risk to the health of Ethiopian beer consumers since aflatoxins are highly toxic chemical that may cause liver cancer. Hence, the government should regulate and incorporate aflatoxin legislation as mandatory standard in Ethiopian beers. Breweries also should check the quality of their raw materials (Barely) and final product (Beer) to ensure the safety of beers from the “Barely to Beer” and meet the “farm to fork” food safety management system.
Competing Interests
The authors declare no competing interest.
1. Introduction
Mycotoxins are naturally occurring secondary metabolites of fungi and can be produced on a wide range of agricultural commodities. Aflatoxins (AFs), a kind of mycotoxins, are the main toxic secondary metabolites of some Aspergillus molds such as Aspergillus flavus, Aspergillus Parasiticus and the rare Aspergillus nomius (Alcaide-Molina, Ruiz-Jiménez, Mata-Granados, & Luque de Castro, Citation2009; Ali et al., Citation2005). Such toxins can be separated into aflatoxins B1, B2, G1, and G2. Its order of toxicity is B1 > G1 > B2 > G2 (Hussein & Brasel, Citation2001; Weidenbörner, Citation2001). Aflatoxins can be both acute and chronic toxins; acute poisoning is usually rare and exceptional, while chronic toxicity is of serious international concern (Adams & Moss, Citation2000). Aflatoxin B1 is toxic for a wide range of animal species. The International Agency for Research on Cancer has concluded that there is sufficient evidence for the carcinogenicity of AFB1 in humans and hence placed this mycotoxin in group I (IARC, Citation1993).
Beer is the very old drink in history in which the making of ferment beverages was discovered by primitive humans and has been practiced as an art for thousands of years. As a result of the development of science of beer brewing become known and could be taught. In several countries where beer was initially brewed research laboratories and institutes where created and in the country of time these developed in to teaching institutes and organization (Kunze, Citation1996).
Ethiopia was just beginning to see the first sparks of technology when St. George brewery was founded in 1922. Currently Ethiopia’s beer industry is comprised of seven major breweries plants. These are BGI Ethiopia (Addis Ababa, Kombolcha and Hawassa plants), Meta Abo, Heineken (Addis Ababa, Bedele, and Harar plants), Dashen (Debre Birhan and Gonder plants), Habesha Raya and Zebider. Thus breweries brew and deliver more than fifteen commercial brand beers to the consumer (Sisay, Citation2015).
Cereals used in beer production, particularly barley, wheat and maize, can be contaminated by different mycotoxins; the contamination can occur both in field and during storage, if conditions of high moisture are present, and during malting. Barley (Hordeumvulgare) is the cereal grain most often malted and it is the main raw material for beer production. The raw material that is capable of supporting microbial growth has the potential to produce unwanted metabolites that can persist through the brewing process and produce adverse effects. Malt and adjuncts derived from cereals present the greatest threat. Malts are made from selected cereal grain, usually barley, (but sometimes wheat, rye, oats, sorghum or millet), that has been cleaned and stored until dormancy has declined and it is needed. It is then germinated under controlled conditions. Poor control of the steps involved in the manufacture of these ingredients can result in mold growth. Species of molds from genera such as, Alternaria, Aspergillus, Cladosporium, Fusarium and Rhizopus have all been reported to produce adverse effects. Since cereals are raw materials of beer and beer-based drinks, it is necessary to consider that the risk of contamination also exists in beer and beer-based drinks, produced in this case by Aspergillus carbonarius. Aflatoxins get to beer either from contaminated input materials or adjuncts added in the course of brewing. Studies with different mycotoxins added at various stages of the brewing process show that AFB1 may be transmitted from contaminated grains into beer (Karolina, Sylvie, Renata, & Zdenek, Citation2011).
The presence of concentrations of aflatoxins has been demonstrated in commercial beer from many parts of the world. A survey on 304 samples of Canadian and imported beer, the toxin was found only in some samples. From India (highest value 0.230 μg/l), Spain, Portugal and Mexico (all lower than 0.02 μg/l) (Mably et al., Citation2005). Much higher levels of aflatoxins have been found in African beers both commercial and home-brewed (Scott, Citation1996).
In Ethiopia, Brewing production capacity rise from a level of just 1 million hectoliters in 2003/2004, to nearly 10 million hectoliters by 2015/2016 (Access Capital, Citation2010). Even if there have been large increases in the consumption frequency and quantity of beer is high in Ethiopia by referring the brewing capacity increment, according to our search there are no studies carried out related to mycotoxins specifically aflatoxin content of Ethiopian commercial beers still not determined. In most countries beer is classified as a foodstuff and so is subject to some food hygiene regulations including mycotoxins. This means regular inspection by agents of local or national government according to the standards. But the occurrence and concentration of aflatoxins in food chains of Ethiopia specifically processed foods are not well studied. Moreover, aflatoxin is not a mandatory standard and the government did not enforce and regulate it in Ethiopian beer. In this regard, it can be counted as one of the threats to food safety in Ethiopia.
2. Materials and method
2.1. Study area
The study was conducted in Addis Ababa, the capital city of Ethiopia and the experiments were carried out from December 2015 to April 2016 in Addis Ababa University Center for Food Science and Nutrition, Food toxicology Laboratory.
2.2. Sample and Sampling
Commercial beer samples were randomly collected from different retail outlets and food market across ten Sub Cities of Addis Ababa. 18 brands; 15 domestic (12 from alcoholic and 3 from non-alcoholic) and 3 imported alcoholic beers were analyzed in duplicate. Each brand samples correspond to 20 bottles into two sample container for the duplicate analysis by assigned a code for each. The beer samples were first transferred into 500 ml plastic bottles containers and were mixed by gentle shaking and sonicated for 30 min in Ultra sonic bath at room temperature. Twenty milliliters was collected from the composite sample for the analysis and remaining was stored in a freezer.
2.3. Determination of aflatoxins in beer
Analysis of aflatoxins (B1, B2,G1 and G2) in the beer samples was carried out by the method of Scott and Lawrence (Citation1997) with some modifications and method validations.
2.3.1. Chemical and reagents
Acetonitrile and methanol were HPLC grade, distilled water and phosphate buffered saline (PBS: NaCl 8 g/l, KCl 0.2 g/l, Na2HPO4 1.15 g/l, KH2PO4 0.2 g/l; and adjust pH to 7.4 using 0.1 M HCl or 0.1 M NaOH) were purchased from local market.
2.3.2. Immunoaffinity columns
LCTechAflaCLEANImmunoaffinity column used for sample clean-up for the Aflatoxin analysis with a maximum loading capacity of 100 ng aflatoxin B1 and selectivity against Aflatoxins B1, B2, G1 and G2 were purchased (imported) from, LCTech GmbH Dorfen, German.
2.3.3. Standards
Aflatoxin B1, aflatoxin B2, aflatoxin G1 and aflatoxin G2 and mixed Aflatoxin standards purchased from Sigma Aldrich (St. Louis, MO, USA)
2.3.4. Mobile phase
The mobile phase was a water-methanol-acetonitrile (60:25:15, v/v/v) isocratic method applied for better resolutions of the chromatographic peak. The mobile phases were filtered by applying vacuum in a filter unit and degassed.
2.3.5. Apparatus
Immunoaffinity column, lab stand with clump, Volumetric and Graduated pipettes (1, 5, 10, 25 and 50 ml), volumetric flask (10, 25, 50, 100, 500 and 1,000 ml), Measuring cylinder (50 and 100 ml), Beaker (50, 100 and 500 ml), conical flask (250, 500 and 1000 ml), Mixer, Stirrer, Ultra bath Sonicator, Wash bottle, Micropipettes, Micropipette tips, Millipore Filter, Electronic balance, syringes(5 and 10 ml), Paraffin, Sample label, Vials with screw cap. HPLC system setup contains auto sampler, injector, oven, column, Link, Degasser, fluorescence detector and desktop computer with chromatography software.
2.3.6. Chromatographic system
Aflatoxin analysis was conducted with Shimadzu HPLC system (Shimadzu, USA). The HPLC system consisted of pump (LC-20AB), a degasser (DGU-20A 3R), an auto-injector (SIL-20A), column oven (CTO-20AC) and Florescence detector (RF-20A) and Data solution software. A Shim-pack FC-ODS column (5 μm, 150 × 4.6 mm diameter) at 25 °C temperature and 1.2 ml min−1 f low rate was used. The run time was 15 min, injection volume 20 μl, diluent methanol and Needle wash (Water: Methanol 90:10 v/v). Aflatoxins were detected at 365 nm excitation and 440 nm emission wavelengths.
2.4. Procedure for aflatoxin analysis
2.4.1. Method adaptation
To evaluate the analytical performance of the instrument and validity of the method, first identification then limit of detection, limit of quantification, precision, recoveries, linearity and asserting the working range was done.
2.4.2. Identification
Identification of Aflatoxins was determined by retention time of individual and mixed aflatoxins (AFG2, AFG1, AGB2 and AFB1) injecting at the same condition and its precision determined by percent relative standard deviation (% RSD).
2.4.3. LOD and LOQ
LOD was determined by injecting (0.01, 0.2, 0.01, and 0.2) μg/l of individual aflatoxins G2, G1, B2 and B1 respectively to obtain the lowest amount of analyte greater than three times of noise level S/N > 3. In the same way LOQ was determined by injecting (0.05, 0.8, 0.05, and 0.8) μg/l of individual aflatoxins G2, G1, B2 and B1 respectively to obtain the lowest amount of analyte which can be reproducibly quantitated above the baseline noise, that gives S/N > 10.
2.4.4. Precision
Precision of the method was evaluated through the repeatability of the method by assaying ten replicate injections of aflatoxin mixed standard at the same concentration (30 μg/l) during the same day under the same experimental conditions to obtain an acceptable % RSD.
2.4.5. Linearity
Linearity was determined by injecting a serious of (2, 5, 10, 20, 30, 50, and 100) μg/l aflatoxins standard. The concentration range (2–100) μg/land regression equation was found by plotting the peak area (Y) vs. the Aflatoxins Concentration (X) expressed in μg/l.
2.4.6. Recovery
Recoveries were determined by spiking (50 and 100) μg/l aflatoxin mixed standard in 20 ml beer sample which diluted by PBS (10 ml) and purified through Immunoaffinity column. The column washed with PBS (2 ml) and AFs was slowly eluted with methanol (2 ml) into a graduated glass vial. The extract was injected in HPLC system for recovery and accuracy check.
2.4.7. Standard preparation
Aflatoxin standards obtained from Sigma Aldrich (St. Louis, MO, USA). From the stock solution aflatoxin mixed standard which have a concentration of (2, 5, 10, 20, 30, 50, and 100) μg/l were prepared for method validation. Standards solutions prepared in 10 ml volumetric flasks using HPLC grade Methanol as a diluent. The prepared standards were transferred to vials and stored at 4 OC and protected from light to avoid deterioration of the aflatoxins in the solution.
2.4.8. Sample preparation and Clean Up
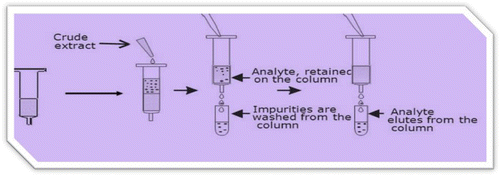
The beer samples were transferred to 500 ml volumetric flask. The beer sample were mixed by gentle shaking and degassed for 30 min in Ultra Sonic Bath at room temperature. Twenty milliliters was collected from the composite for analysis and remaining sample stored in a freezer. An aliquot (20 ml) of beer sample was diluted with PBS (10 ml) and purified through an AflaCLEANImmunoaffinity column as indicated in Figure . The column was washed with PBS (2 ml) and AFs was slowly eluted with methanol (2 ml) into a graduated glass vial. The extracts filtered with Millipore (0.45 mm) and injected (20 μl).
2.4.9. HPLC determination and calculations
The elute Aflatoxins (B1, B2, G1 and G2) methanol solution were determined at microgram per litter (μg/L) levels in beer by Immunoaffinity column cleanup and high performance liquid chromatography with fluorescence detection and calculated according to the following equation.
where:-
W = amount of aflatoxin in the test sample in μg/l
Wa = amount of aflatoxin corresponding to area of aflatoxin peak of the test extract (ng)
Vf = the final volume of re-dissolved eluate (μl)
Vi = volume of injected eluate (μl)
Vs = volume of test portion (Beer) passing through the column (ml).
2.5. Study design for KAP (knowledge attitude and practice)
The study design employed for the survey was a purposive sampling technique and Semi-structured questionnaires used to get more information. The questionnaire evaluation carried out for knowledge attitude and practice assessments related to quality and food safety issues of the breweries based on the purposive sampling. A total of 60 professional employees who are working in the side of production and quality aspects participated in the survey. The response was classified in three parts; the first part was general information about the breweries and the respondents, the second part contained quality standards and certification of the breweries and the final part was KAP related to mycotoxins in lighted on aflatoxin in the beer industries.
2.6. Statistical data analysis
The print out results was collected after HPLC quantification then all data entered in IBM SPSS version 20 and the required descriptive statistical analysis was calculated accordingly.
3. Results and discussion
3.1. Validation of the chromatographic method
3.1.1. Identification
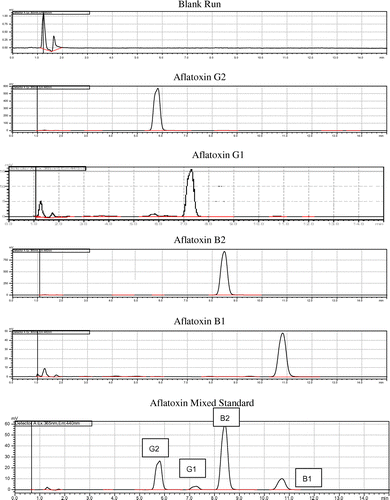
The retention time of individual and mixed aflatoxins gave a good precession having a range between (0.22 and 0.80) % RSD, which is acceptable according to FDA (Citation2002) standard which is less than 2% RSD (Table ). The elution order of individual Aflatoxins was in the order of AFG2, AFG1, AGB2 and AFB1 with 5.824, 7.312, 8.487 and 10.828 retention times respectively. In addition to the retention time chromatographic result for Blank (diluent), individual (AFG2, AFG1, AGB2 and AFB1) and mixed aflatoxins as shown in Figure demonstrate the qualitative aspect of identification test was acceptable.
Table 1. Statistics for aflatoxin retention time identification
3.1.2. Limit of detection and quantification (LOD and LOQ)
Detection performance of the HPLC was determined by the limit of detection and was found to be (0.01, 0.2, 0.01 and 0.2) μg/l for aflatoxin AFG2, AFG1, AGB2 and AFB1 respectively. LOD was determined by the amount of analyte that can be detected above baseline noise; typically, three times the noise level S/N > 3 as shown in Table below.
Table 2. Limit of detection (LOD) and limit of quantification (LOQ)
On the other hand the limits of quantification for individual aflatoxin (AFG2, AFG1, AGB2 and AFB1) were (0.05, 0.8, 0.05 and 0.8) μg/l, respectively. LOQ was determined based on the amount of analyte which can be reproducibly quantitated above the baseline noise, that gives S/N > 10. The limits of quantification were in the range of (0.05–0.8) μg/l which shows satisfactory quantification of the instrument on the desired working range.
3.1.3. Precision
As verified in Table the precision was evaluated through the repeatability of the method by assaying ten replicate injections of aflatoxin mixed standard at the same concentration (30 μg/l), during the same day, under the same experimental conditions. It shows an acceptable % RSD which had a values of <0.05 and <3.0% for the retention time and peak area respectively. A precision criterion the instrument precision (repeatability) and is normally expressed as the percent relative standard deviation for a statistically significant number of samples should be ≤5% RSD in FDA (Citation2002) standard.
Table 3. Descriptive statistics for precision check
3.1.4. Linearity and working range
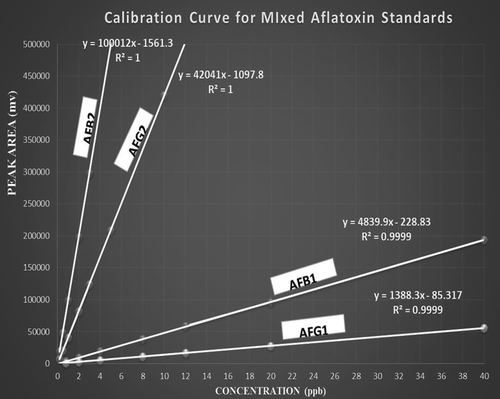
Linearity was studied by selecting seven concentrations (2, 5, 10, 20, 30, 50 and 100) μg/lin order to demonstrate a proportional relationship of peak area vs. analyte concentration over the working range. The International Conference on Harmonization (ICH) guidelines specified a minimum of five concentration levels, along with certain minimum specified ranges. Regression equation was found by plotting the peak area (y) vs. the aflatoxins concentration (x) expressed in μg/l as presented in Figure .
Acceptability of linearity data is often judged by examining the correlation coefficient and y-intercept of the linear regression line for the peak area versus concentration plot. As revealed in Figure the demonstration coefficient (r2) obtained for the regression line demonstrates the excellent relationship between peak area and concentration of aflatoxin and its coefficient of correlation (R2) sited on 0.9999 and 1.000. The regression coefficient (r2) is >0.998 is generally considered as evidence of acceptable fit of the data to the regression line on the FDA (Citation2002) standard.
3.1.5. Recovery check
The accuracy of this analytical method was obtained by standard additions, which can also be used to determine recovery of spiked analyte. This approach was used due to the difficulty to obtain a blank sample matrix without the presence of the analyte. As illustrated in Table , the concentration of 50 and 100 μg/l aflatoxin standards spiked in beer sample and injected in duplicate. A percent recovery of response factor (area/concentration) was calculated. Accuracy criteria for an assay method FDA (Citation2002) is that the mean recovery will be 100 ± 20% at each concentration over the range of (80–120) % of the target concentration. The results of recovery and accuracy studies shown in the range between (84.0 and 97) % and it is evident that the method is accurate within the desired recovery range.
Table 4. Statistics for aflatoxin recovery and accuracy check
3.2 The level of aflatoxin in beer
The 18 branded beers which are listed in Table below coded as (A to L) for domestic alcoholic beer, (M to O) for domestic nonalcoholic beer and (P to R) for imported alcoholic beers. Each samples run in duplicate according to the procedure and specification.
Table 5. The Level of aflatoxin in different brands of beer
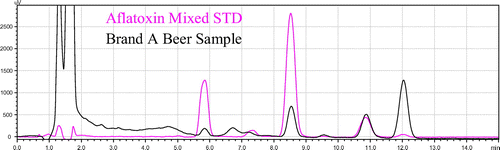
Figure shows that the standard and sample peaks supper imposed perfectly; this is evidence for the beer sample contaminated by aflatoxin. All the samples were determined by supper imposing with the standard peak and the level of aflatoxin calculated in μg/l according to the formula.
As summarized in Table , eleven domestic alcoholic beer samples were positive for aflatoxin greater than the limit of quantitation (LOQ) which is in the range between (1.23 and 12.47)μg/l for aflatoxin sum and one brand is less than LOQ. On the other hand aflatoxin is not detected in non-alcoholic domestic beer which had a result less than the limit of detection (LOD) and aflatoxin is detected but not quantified in all the three imported alcoholic beer which has the result between the limit of detection and quantification. Aflatoxin was determined at microgram per litter (μg/l) levels in beer by immunoaffinity column cleanup and reversed-phase liquid chromatography with fluorescence detection.
Table 6. Review results for aflatoxins sum in alcoholic and non-alcoholic beer
When comparing this research finding with other studies conducted in different countries, high aflatoxin level detected in Ethiopian commercial alcoholic beer. A study conducted in domestic (Canadian) and imported beers from 36 countries were picked up for the determination of aflatoxins B1, B2, G1 and G2. Twelve samples were positive with aflatoxins greater than the limit of quantitation (LOQ) (aflatoxin B1, 4.4 ng/l; aflatoxin B2, 3.4 ng/l aflatoxin G1, 11.2 ng/l; and aflatoxin G2, 6.2 ng/l). Five samples from Mexico, two samples from Spain and one from Portugal contained aflatoxin B1. From India (highest value 0.230 μg/l), Spain, Portugal and Mexico (all lower than 0.02 μg/l). The remaining samples contained less than the LOQ for aflatoxins B1, B2, G1 and G2 which is very low as compare with this research finding (Mably et al., Citation2005).
In contrary with this study, aflatoxin was not detected in another review paper on mycotoxin occurrence in beer produced in several European countries by Bertuzzi, Rastelli, Mulazzi, and Pietri (Citation2011). The results from a survey investigating the presence of some mycotoxins in beer samples produced in several European Countries, AFs were not detected in any samples whereas DON, OTA and FBs were found in a relatively high number of samples.
Additionally, study conducted by Scott and Lawrence (Citation1997) analysis of 24 beer samples, the majority from the United States and Mexico. The result showed natural contamination of one sample of Mexican beer at 49 ng B1/L when determined at 360 nm excitation, but reanalysis of 23 of the samples using 340 nm excitation indicated that an additional 4 Mexican samples and one Brazilian sample contained aflatoxin B1 at low levels (<10 ng/l).
Moreover, a survey of aflatoxins in industrially brewed South African sorghum beer and beer straining by Trinder (Citation1988). The aflatoxin content of samples of sorghum beer was less than 0.1 μg/l in 97% of the samples examined. Three percent of the beer samples analyzed had an average aflatoxin content of 0.1 μg/l and no sample exceeded 0.2 μg/l. From the results of analysis of 211 samples of beer straining it was deduced that most of the corresponding beers contained less than 0.1 μg/l aflatoxin and only in a few instances did the aflatoxin level reach 0.5 μg/l.
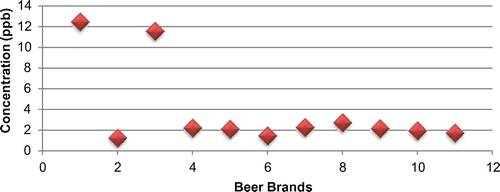
When comparing by brand difference of Ethiopian alcoholic beer as shown in the diagram below (Figure ). Majority of alcoholic beers had less than 4 μg/l aflatoxin concentration which is tolerable level in EU standard setting for the sum (B1, B2, G1 and G2). Nonetheless two brands (Brand A and C) are high relative to the others and beyond the tolerable level in EU standard for total aflatoxin, this inferred that unsafe for consumption and require an action.
For the toxicological significance of regular beer drinker in Ethiopia the exposure of aflatoxins should be identified. It is expressed in micro gram of the chemical per kilogram of body weight. Beer lovers in Ethiopia have now the choice to drink a 330 milliliters bottle. Aflatoxins exposure if a person consumes 6 bottles of beer per dayand adult population is 60 kg, according to Table the average aflatoxin content in alcoholic beer is 3.52 μg/l. The amount of aflatoxin in one bottle will be 1.173 μg while in 6 bottles it becomes 7.04 μg.
Even if the European legislation, (European Food Safety Authority, [EFSA], Citation2010) setting the maximum allowable limit for total content of all aflatoxins (Sum B1, B2, G1 and G2), 4.0 μg/kg, the chronic exposure provided the individual’s intake averaged over longer periods of time exceed the level set. As a result the European and international bodies have not set an acceptable daily dose (ADI) for aflatoxins. These substances have genotoxic carcinogenic effects, with no threshold, and the only realistic approach is to reduce exposure to as low a level as possible following the ALARA (As Low As Reasonably Achievable) principle (French Food Safety Agency, Citation2006).
3.3. Aflatoxin survey result
A semi-structured questionnaire result shows KAP (Knowledge, attitude and practice) assessments related to quality and food safety issues of the breweries based on a purposive sampling. On this survey 60 professional employees working in breweries are participated and the response summarized in three parts.
There is a noticeable gap on production system related to food toxins. Only 35% of breweries are working under ISO specifically HACCP based systems and 42% of breweries inspect and test the incoming raw material. According to (Karolina et al., Citation2011) aflatoxins get to beer either from contaminated input materials or adjuncts (barley, malt, hop, brewery yeasts) added in the course of brewing. It is necessary to consider that the risk of contamination exists in beer and beer-based drinks since the raw materials of beer have a chance to contaminate by molds. The fact that not inspect and testing of incoming raw materials, it is doubtful for the quality of the final product. On the other hand only 48% of professional employees got training on food safety issue and the remains still seeking the chance.
With regard to KAP in related to mycotoxins most breweries had serious gap as. The level of mycotoxin is not determined on their laboratories in any desired methods and 35% of respondents expect mycotoxin eliminate by pasteurization. Thirty-three percent of respondents had no knowhow about food toxin like mycotoxin. It is recommended for a possible inclusion of mycotoxin in the curriculum is the department of agronomy, veterinary sciences, food technology, medicine, chemistry and pharmacy. Bhat (Citation1987) stated that the information on mycotoxins has to be incorporated in the syllabus at various levels for certificate, diploma and the courses offered in higher education.
Eventually on the side of aflatoxin, 30% of respondents have no idea about the cause and effect of aflatoxin and only 17% knew its toxicity level. 45% of respondents not expect aflatoxin in bottled beer and the remains expect insignificant level this implies the quality and food safety issue still questionable.
4. Conclusion
In relation to the objectives of the study, 91.6% of alcoholic beer were positive to aflatoxin with the range between (1.23 and 12.47)μg/l. Conversely none of domestic non-alcoholic beers were positive for aflatoxin. Moreover, aflatoxin was detected but not quantified in imported alcoholic beer cross over in this study. From KAP assessment knowledge about mycotoxin specifically aflatoxin in the beer industry is not satisfactory. Attitudinally, most don’t expect and seem to worry about the presence of aflatoxin in beer. In terms of their practice, few are applied HACCP system and almost all breweries do not determine the level of aflatoxin in the incoming raw materials and final products on their laboratories. The government also does not regulate and incorporate aflatoxin legislation as mandatory standard in Ethiopian beers. Since aflatoxins get to beer either from contaminated input materials or adjuncts in the course of brewing, particularly the malt (the main raw material for beer) is the greatest threat for it requires unremitting follow up. Poor control of the steps involved in the manufacturing process and unable to use HACCP based system may provide unwanted metabolites that can persist through the brewing process and produce adverse effects. As a result, there is a significant risk to the health of Ethiopian people due to the chronic exposure to aflatoxin in beer and it may contribute to an increase in the incidence of hepatocellular carcinoma.
Funding
The authors received no direct funding for this research.
Acknowledgments
Authors would like to thank Center for Food Science and Nutrition, Addis Ababa University, Ethiopian Conformity Assessment Enterprise, Food, Medicine, Health care Administration and Control Authority (FMHACA) and Bless Agri-Food Laboratory PLC for their contribution in the research.
Additional information
Notes on contributors
Ashagrie Zewdu Woldegiorgis
Dr Ashagrie Zewdu research group (DARG) is a research group comprised of graduate students (Msc and PhD) under the supervision of the senior faculty staff member of Dr Ashagrie Zewdu Woldegiorgis. Dr Ashagrie is a full time Assistant Professor of Food Science and Nutrition at the Centre for Food Science and Nutrition, Addis Ababa University since September 2009. He has a Bachelor degree (Bsc) in chemistry 2003 and a Master of Science (Msc) in Food Science and Nutrition in 2009 and Doctorate of philosophy (PhD) in Food Science and Nutrition in 2013. DARG research interest areas are:
| • | Industrialization of traditional foods, | ||||
| • | Antioxidant properties, | ||||
| • | Food safety and quality (especially aflatoxins in foods), | ||||
| • | Product development and as well as nutrition related areas such as omega-3 fatty acids, fortification etc. | ||||
| • | Food and nutrition analysis | ||||
Under the research group so far four PhD and more than 30 Msc students has completed their research thesis/dissertation with good results and this will continue in the future also. The first author, Afework Nigussie was the member of the DARG and this is his Msc thesis research work. He had a Bsc in Chemistry in 2010 and completed his Msc in Food Science and Nutrition in 2016 from the Center.
References
- Access Capital. (2010). Retrieved April 27, 2016, from Access capital Web Site: http://www.acess_capital.sc.com/Research
- Adams, M. R., & Moss, M. O. (2000). Food Microbiology. Cambridge: RSC Publishing.
- Agency, French Food Safety (2006). Risk assessment for mycotoxins in human and animal food Chains ( Summary Report, pp. 12–20).
- Alcaide-Molina, M., Ruiz-Jiménez, J., Mata-Granados, J. M., & Luque de Castro, M. D. (2009). High through-put aflatoxin determination in plant material by automated solid-phase extraction on-line coupled to laser-induced fluorescence screening and determination by liquid chromatography-triple quadrupole mass spectrometry. Journal of Chromatography A, 1216(7), 1115–1125.10.1016/j.chroma.2008.12.049
- Ali, N., Hashim, N. H., Saad, B., Safan, K., Nakajima, M., & Yoshizawa, T. (2005). Evaluation of a method to determine the natural occurrence of aflatoxins in commercial traditional herbal medicines from Malaysia and Indonesia. Food and Chemical Toxicology, 43(12), 1763–1772.10.1016/j.fct.2005.05.019
- Bertuzzi, T., Rastelli, S., Mulazzi, A., & Pietri, A. (2011). Mycotoxin occurrence in beer produced in several European countries. Food Control, 22(12), 2059–2064.10.1016/j.foodcont.2011.06.002
- Bhat, R. V. (1987). Review of activities in mycotoxin prevention and control: Strategies for improvement based on experience in Asia and East Africa. Rome: Food and Agriculture Organization (FAO).
- EFSA (European Food Safety Authority). (2010). Scientific report of EFSA: Management of left censored data in dietary exposure assessment of chemical substances. EFSA Journal, 8(3), 1557.
- FDA. (2002). Investigative operations manual. Retrieved 2016, from Author: http://www.fda.gov/ora/inspect_ref/iom/Contents/ch4_TOC.html
- Hussein, H. S., & Brasel, J. M. (2001). Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology, 167(2), 101–134.10.1016/S0300-483X(01)00471-1
- IARC. (1993). IARC monographs on the evaluation of carcinogenic risks to humans. Some naturally occurring substances: Food items and constituents, heterocyclic aromatic amines and mycotoxins (vol. 56). Lyon, France: Author.
- Karolina, B., Sylvie, B., Renata, M., & Zdenek, S. (2011). Monitoring of selected aflatoxins in brewing materials and beer by liquid chromatography/mass spectrometry. Food Control, 25(2), 626–630.
- Kunze, W. (1996). Technology Brewing and Malting. Berlin: VLB Berlin.
- Mably, M., Mankotia, M., Cavlovic, P., Tam, J., Wong, L., & Pantazopoulos, P. (2005). Survey of aflatoxins in beer sold in Canada. Food Additives and Contaminants, 22(12), 1252–1257.10.1080/02652030500241884
- Scott, P. M. (1996). Mycotoxins transmitted into beer from contaminated grains during brewing. Journal of AOAC International, 79(4), 875–882.
- Scott, P. M., & Lawrence, G. A. (1997). Determination of aflatoxins in beer. Journal of AOAC International, 80(6), 1229–1234.
- Sisay, A. (2015, December). Tough competition pushes Ethiopian breweries to aggressive marketing. Retrieved 2016, from New Business Ethiopia: http://newbusinessEthiopia.com
- Trinder, D. W. (1988). A survey of aflatoxins in industrially brewed South African sorghum beer and beer strainings. Journal of the Institute of Brewing, 94, 307–309.10.1002/jib.1988.94.issue-5
- Weidenbörner, M. (2001). Encyclopedia of food mycotoxins. Berlin Heidelberg: Springer.10.1007/978-3-662-04464-3