?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In recent years, the use of artificial fruit ripening agents has become prevalent mostly due to the commercial purposes. Different ripening agents are reported to be used to initiate the ripening process in fruits during off-seasons. However, the effects of these agents on the nutritional values of fruits are yet to be fully understood. The purpose of this study is to measure, analyze and compare the nutritional value of naturally and artificially ripened fruits. Bari-1 hybrid banana (Musa Spp.; local name: Sagor Kola) was chosen to carry out the experimental study. Ripe samples were collected directly from orchards or local markets. Additionally, unripe green samples were collected and artificially ripened in the laboratory using artificial ripening agents: ethephon, calcium carbide, ethylene glycol and fume from the kerosene stove. Different nutrition parameters, such as moisture content, total titratable acidity, vitamin C and sugar content were assessed for these banana samples. The assessed parameters of naturally and artificially ripened fruits were compared and analyzed to identify the change in nutritional values and determine the health hazards associated with them. The chemical compositions of artificial ripening agents were also investigated and possible health risks related to the impurities of ripening agents were assessed. Therefore, the key objective of this study is to address the changes in nutritional values and health risks associated with the artificial ripening which will be helpful for the consumers, nutritionists, physicians and other shareholders.
PUBLIC INTEREST STATEMENT
Fruit ripening is a natural process that can also be stimulated using different artificial fruit ripening agents. In recent years, the use of artificial fruit ripening agents has become prevalent mostly due to the commercial purposes. However, artificial fruit ripening is considered as a matter of concern because of various health-related issues. There are direct and indirect health hazards associated with artificial ripening agents and their impurities. This study addresses the changes in nutritional values and health risks linked with the artificial ripening. The findings from this study demonstrated the presence of some potentially harmful impurities in commonly used ripening agents. The finding of this study will be useful for the consumers, researchers, legal authorities and other stakeholders working on food safety, food processing and preservation.
Competing Interests
The authors declare no competing interests.
1. Introduction
Fruit ripening is a natural process in which fruits go through various physical and chemical changes and gradually become sweet, colored, soft and palatable (Bouzayen et al., Citation2010; Brady, Citation1987; Prasanna et al., Citation2007). With the advancement of science and technology, various methods have been developed to artificially stimulate this ripening process, mostly to meet the high demand of consumers. However, in recent years, artificial fruit ripening is considered as a matter of concern because of various health-related issues (Fattah & Ali, Citation2010; Jayan, Citation2011; Siddiqui & Dhua, Citation2010; Vila et al., Citation2003).
The natural process of fruit ripening is a combination of physiological, biochemical and molecular processes (Brady, Citation1987; Prasanna et al., Citation2007; . Kendrick, Citation2009; Bouzayen et al., Citation2010). Fruit ripening involves coordination of different metabolism with activation and deactivation of various genes leading to change in color, increase in sugar content, decrease in acidity, softening of fruit and increase in flavor and aroma (Tucker and Grierson Citation1987; Lizada, Citation1993; . Prasanna et al., Citation2007; Kendrick, Citation2009; Bouzayen et al., Citation2010; Singal et al., Citation2011; El Hadi et al., Citation2013). This ripening process can be instigated artificially by using different chemical agents (Goonatilake, Citation2008; Hakim et al., Citation2012; Rahman et al., Citation2008; Siddiqui & Dhua, Citation2010). Different research groups have reported that ethanol, methanol, methyl jasmonate, ethylene glycol, ethephon and calcium carbide are used to ripen fruits and vegetables artificially (Chace, Citation1934; Goonatilake, Citation2008; Koros, Citation2014; Nagel, Citation1989; Rahman et al., Citation2008; Siddiqui & Dhua, Citation2010). In recent days, fume from the kerosene stove or lantern has been used to initiate the ripening process of different fruits which is the combustion product of kerosene that induces ripening (House et al., Citation1929). However, the effects of these artificial ripening agents on the nutritional values of fruits and associated health hazard caused by consuming artificially ripened fruits are yet to be fully understood (Mursalat et al., Citation2013). Different research groups have reported that possible health hazards are caused by direct exposure or direct consumption of artificial ripening agents (Goonatilake, Citation2008; Siddiqui & Dhua, Citation2010). For example, calcium carbide is alkaline in nature and irritates the mucosal tissues of the abdominal region. Several cases of stomach disorder after eating carbide-ripened mangoes have also been reported (Siddiqui & Dhua, Citation2010). A generous consumption of ethylene glycol may cause kidney failure (Goonatilake, Citation2008). Chemicals such as ethylene and methyl jasmonate are reported to be nontoxic for human consumption. They are relatively expensive and therefore are not widely used (Rahman et al., Citation2008). In many developing countries, chemicals such as calcium carbide (Rahman et al., Citation2008; Siddiqui & Dhua, Citation2010), ethylene glycol (Goonatilake, Citation2008) and ethephon (Hakim et al., Citation2012) are preferred due to their low cost (Islam, Mursalat, & Khan, Citation2016; Islam, Rahman, Mursalat, Rony, & Khan, Citation2016).
Therefore, it is important to conduct systematic scientific research to identify and quantify any change in nutritional values of artificially ripened fruits and also investigate any possible health hazard associated with the consumption of these fruits.
This study aims to develop a scientific understanding of the changes in nutrition values of artificially ripened fruits. In this study, Bari-1 hybrid banana sample was used as sample fruit. Banana is a highly consumed fruit; in 2016, about 17 million tons of banana were exported worldwide (Food and Agriculture Organization, Citation2017). Bananas contain easily digestible carbohydrates, vitamin C and minerals (Chadha, Citation2007; Kazi et al., Citation2015). For this study, natural and artificially ripened banana samples were collected from the banana orchards and local markets. Unripe banana samples were artificially ripened in the laboratory by ethephon, kerosene fume, calcium carbide and ethylene glycol. Moisture content, total titratable acidity (TTA), vitamin C (ascorbic acid) and sugar content of unripe and ripe (naturally and artificially) banana samples were assessed and compared. Furthermore, different ripening agents were chemically analyzed and health risks posed by the impurities in the ripening agents were assessed. This systematic approach of investigating artificially ripened bananas can also be used to analyze other fruit items.
2. Health hazards related to impurities in ripening agents
Different kinds of impurities are reported to be found in ripening agents (Robertson et al., Citation1947; Nadel et al., Citation1965, Charan et al., Citation1979; US EPA, Citation1988; Marcus, Citation1990; Segall et al., Citation1991, Kulling et al., Citation1992; Hanif et al., Citation1995; O’Brien et al., Citation1998; Pirson et al., Citation2003; Rahman et al., Citation2008; Goonatilake, Citation2008; Siddiqui & Dhua, Citation2010; Fattah & Ali, Citation2010; Hakim et al., Citation2012). Their possible health effects are briefly discussed below.
2.1. Ethylene glycol
The main impurities of ethylene glycol are diethylene glycol (3-oxapentane-1,5-diol), triethylene glycol (3,6-dioxaoctane-1,8-diol), methanol and aldehydic oxidation products (Marcus, Citation1990). Diethylene glycol is a highly toxic organic solvent that causes acute renal failure and death when ingested (Hanif et al., Citation1995; O’Brien,Selanikio et al., Citation1998). Triethylene glycol has low toxicity (Robertson et al., Citation1947).
2.2. Ethephon
Ethephon contains impurities like 1,2-ethanediylbis (phosphonic acid) and monochloroethyl ester of (2-chloroethyl)-phosphonic acid (Segall et al., Citation1991, US EPA, Citation1988). The later may degrade to monochloroacetic acid (US EPA, Citation1988). Skin exposure to monochloroacetic acid can cause burn injury and fatal systematic poisoning (Kulling et al., Citation1992; Pirson et al., Citation2003).
2.3. Kerosene
Kerosene contains impurities like sulfur (emitted as SOx), aromatic compounds and hydrocarbons (Khan, Khan,, & Beg, Citation2013). Inhalation of SO2 (4–6 ppm) for 10 min decreases airway conductance (increased airway resistance) of a healthy person (Nadel, Salem Citation1965). Acute exposure to high concentration of sulfur dioxide can cause pulmonary injuries which sometimes lead to death (Charan et al., Citation1979).
2.4. Calcium carbide
Calcium carbide reacts with water to produce acetylene, which behaves similarly to the ethylene gas, a natural ripening hormone (Fattah & Ali, Citation2010). Industrial grade calcium carbide may contain arsenic and phosphorous hydride (Siddiqui & Dhua, Citation2010). Workers may have direct contact with calcium carbide while applying it to the fruits. High exposure to arsenic and phosphorus may cause a buildup of fluids in lungs with early symptoms of vomiting, diarrhea with or without blood, burning sensation in chest and abdomen, thirst, weakness, difficulty in swallowing, irritation or burning in the eyes and skin, permanent eye damage, ulcer on the skin, sore throat, cough and shortness of breath (Siddiqui & Dhua, Citation2010). The impurities present in ripening agents and their possible health hazards are summarized in Table .
Table 1. Impurities present in ripening agents and possible health hazards [Robertson et al., Citation1947, Nadel, et al., Citation1965, Charan et al., Citation1979, Citation1988, Marcus, Citation1990, Segall et al., Citation1991, Kulling et al., Citation1992, Hanif, et al., Citation1995 O'Brien, et al., Citation1998, Pirson, et al., Citation2003, Rahman, et al., Citation2008, Goonatilake, Citation2008, Siddiqui and Dhua Citation2010, Fattah and Ali Citation2010, Hakim, et al., Citation2012]
3. Materials and methodology
The fruit sample used in this experimental study was Musa Spp. (locally known as Sagor kola), a hybrid developed by Bangladesh Agriculture Research Institute (Azam et al., Citation2010). Both green and ripe banana samples were collected from the local wholesale market (Sadarghat). Ripe banana samples were also collected from banana orchards. In this experimental study, reagent grade sodium hydroxide, vitamin C, hydrochloric acid and sulfuric acid (MERCK KGaA, Germany) were used. Calcium carbide ethephon and ethylene glycol were collected from local markets in Dhaka, Bangladesh. Spectrophotometry (HACH DR-6000 UV-Vis spectrophotometer), multi-gas analyzer (NOVA model600), XRF (Shimadzu, XRF-1800) and Scanning Electron Microscopy (acceleration voltage: 5 kV; JSM-7600F SEM) were used for elemental analysis of different ripening agents. Experiments were carried out at room temperature (26°C).
3.1. Sample treatment
Different treatment methods to artificially ripen green banana samples are discussed below.
3.1.1. Ethephon treatment
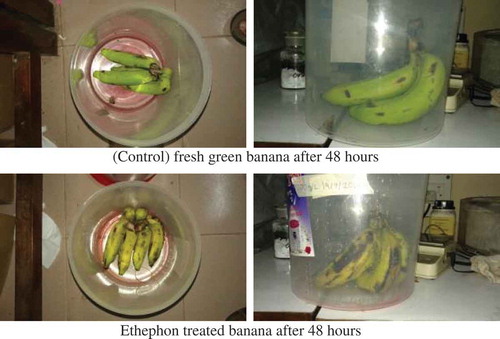
Unripe green banana samples were treated with 50 ml of ethephon solution (300 ppm). Treated banana samples were kept in a confined space for 24 h. Afterwards, they were exposed to open air as shown in Figure .
3.1.2. Kerosene treatment
Banana samples were placed in an airtight container (50 L). An open-lid kerosene lantern was placed at the center of the container and was allowed to burn until the oxygen inside was exhausted. Banana samples were kept in the container for 24 h and then exposed to open air.
3.1.3. Calcium carbide treatment
Fresh unripe banana samples were placed in a container (25 L). Then 10 g of calcium carbide powder was placed at the middle of the container. As shown in Figure , it took around 30 h for the samples to start ripening. After 48 h, the samples were fully ripened.
3.1.4. Ethylene glycol treatment
Ethylene glycol solution (20 weight percent, wt%) was applied on the outer surface of the unripe green banana samples using a brush. Then the samples were kept in an open-lid container (25 L). It took around 48 h for the samples to be completely ripened.
3.2. Experimental techniques
Experimental techniques to determine ripening condition, moisture content, TTA, vitamin C, sugar contents of banana samples, and toxicity and diffusivity of ripening agents are discussed in the following sections.
3.2.1. Determination of ripening condition
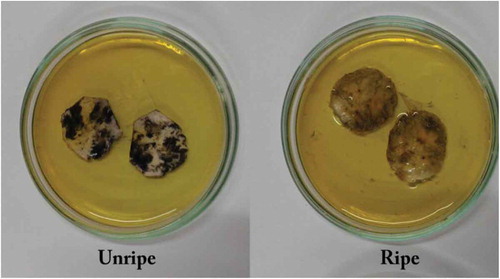
The degree of ripening was determined using starch-iodine test (Figure ) (Blanpied & Silsby, Citation1992; Ministry of Justice, Canada, Citation2009, UC Davis, 2010; Dwivany et al., Citation2012; Hollo and Szejtli Citation1958). Starch-iodine test is widely used to determine the maturity of fruits in which amylase present as starch in the unripe fruits produces violet color in the presence of iodine (Hollo & Szejtli, Citation1958). After dipping the banana samples into iodine solution; the starch content of the unripe samples gave violet color, whereas the ripe samples showed no color change. This is because starch is converted to sugar with the progression of ripening process (Bouzayen et al., Citation2010). So a ripe fruit will show no color when strained with iodine but an unripe fruit will (Figure ).
3.2.2. Determination of moisture content
The moisture content was determined by oven-dry method (Abano and Amoah Citation2011; Canet., Citation1988; Nielsen, Citation2010; Senthilkumar et al., Citation2005). Freshly cut banana samples were dried in an oven (National, NB-7500E) at 110°C until a constant weight was reached. Weight readings of the samples were taken before and after drying; the weight difference denoted the amount of moisture in the samples. The moisture content is expressed in gram of moisture available per 100 g of fruit sample (g of moisture/100 g of fruit stem) (Shahnawaz, Sheikh, & Nizamani, Citation1980).
3.2.3. Determination of TTA
TTA was measured using the method (method 22.060 of 13th edition) described by Association of Official Analytical Chemists (AOAC), USA (AOAC Citation1980). Juice from banana samples was titrated with standardized 0.1M NaOH solution using phenolphthalein indicator. The resultant acid content from the titration is multiplied by the citric acid factor and the result is expressed in gram citric acid per 100 g of fruit stem (John Bean Technologies Corporation Citation2011).
3.2.4. Determination of vitamin C
Vitamin C (ascorbic acid) concentration was determined by redox titration using standardized iodine solution (Majidi and Y-AlQubury Citation2016; Munir et al., Citation2013; Mussa & Sharaa, Citation2014; Patel, Citation2017; Said et al., Citation2016). In this method, ascorbic acid in fruit reacts with iodine to produce dehydroascorbic acid and iodide ions (Equation (1)) (Mussa & Sharaa, Citation2014; Patel, Citation2017). Excess iodine then reacts with the starch indicator to produce violet color and indicate the end point of titration (Mussa & Sharaa, Citation2014; Patel, Citation2017). The results obtained are expressed in ppm (mg/L).
3.2.5. Determination of sugar content
The sugar content of the fruit samples was determined by measuring the refractive index of the samples (Cejpek, Citation2012). A KRUSS (Germany) refractometer was used to measure the refractive index of the banana solutions (10 wt%). Afterwards, sugar content (wt%) was determined from this refractive index.
3.2.6. Analysis of ripening agents
X-ray fluorescence spectrometer (Shimadzu, XRF-1800) was used for the elemental analysis of calcium carbide. During elemental analysis, high amount of sulfur was found in calcium carbide samples. Energy-dispersive X-ray spectroscopy (EDS, JSM-7600F SEM) was used to cross-check the value of elemental sulfur in calcium carbide samples. EDS was done using a field emission secondary electron microscope (FESEM; JSM-7600F SEM). Kerosene fume was analyzed using a NOVA model 600–8 gas analyzer. Kerosene was burned in a confined space (25 L), fitted with a small stack. The fume was collected from the stack and analyzed using the gas analyzer. Sulfur content in ethephon and ethylene glycol was determined using a HACH DR-4000 UV-Vis spectrophotometer (Company 2018) (USEPA).
3.2.7. Analysis of diffusion of sulfur from ripening agent to fruit
To understand the diffusion of sulfur from calcium carbide to the flesh and peel of fruits, sulfate and sulfide content of fresh and carbide-treated banana samples (flesh and peel) were measured using HACH DR-6000 UV-Vis spectrophotometer (Company 2018, Company 2018) (USEPA).
4. Results
4.1. Nutritional value analysis
The experiments were carried out to analyze the changes in nutritional values in the artificially ripened banana samples with respect to those of naturally ripened samples. The moisture content, TTA, vitamin C and sugar content of naturally ripened, artificially ripened (treated with ethephon, kerosene fume, calcium carbide and ethylene glycol) and commercially available banana samples are enlisted in Table . Each experiment was repeated five times (n = 5) and the average results were used for further analysis.
Table 2. Nutrition value of naturally and artificially ripened banana (Bari-1 hybrid banana; Musa Spp.) (Standard deviation for n = 5 samples)
4.1.1. Moisture content
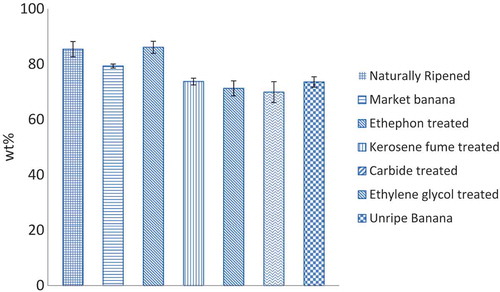
Figure presents moisture contents of different banana samples. Moisture content values of the fruit samples ranged from 70 to 86wt%. The experimental results are in accordance with the results reported by the Dutch Food Composition Database and Abano and Amoah Citation2011.
4.1.2. Total titratable acidity
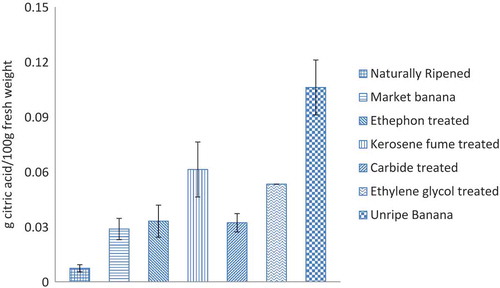
Figure represents TTA of different banana samples. TTA content was found comparatively higher in artificially ripened banana samples than in the naturally ripened samples. The unripe banana sample exhibited a significantly higher TTA with respect to naturally and artificially ripened samples. The experimental value of fresh sample was compatible with the reported literature (Ho et al., Citation2012; Uma et al., Citation2005).
4.1.3. Vitamin C
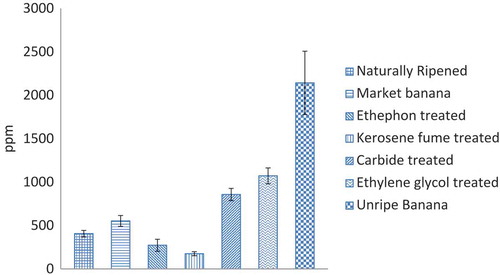
Vitamin C content of ripe banana samples during the experiment ranged between 175 and 1,072 ppm (Figure ). In addition, vitamin C content of the unripe banana sample was higher than the ripened samples, which is supported by the available literature (Lizada, Citation1993; Prasanna et al., Citation2007; Tucker and Grierson Citation1987).
4.1.4. Sugar content
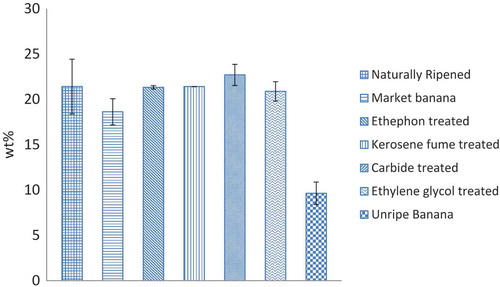
Figure presents sugar content of different banana samples. Initially, the unripe banana sample had low sugar content (9.64 wt%); as the samples got ripened, their sugar content increased. The sugar content of the ripened samples varied from 18.63% to 22.7% which is in accordance with the values reported by the Netherlands Nutrition Centre and US Food and Drug Administration (FDA, Citation2008; NNC, Citation1996).
4.2. Characterization of ripening agents
4.2.1. Calcium carbide
Figure shows SEM images of calcium carbide. The images indicate a large surface area in calcium carbide microstructure. When calcium carbide comes into contact with environmental moisture, this large surface area facilitates the formation of acetylene gas (Equation (2)). This acetylene gas initiates the ripening process in fruits (Rahman et al., Citation2008).
Figure 8. SEM images of calcium carbide used as an artificial fruit ripening agent. (a) 600 times magnification, (b) 1,000 times magnification, (c) 10,000 times magnification, (d) 50,000 times magnification. The high surface area of calcium carbide microstructure ameliorates the formation of acetylene gas when it comes into contact with environmental moisture.
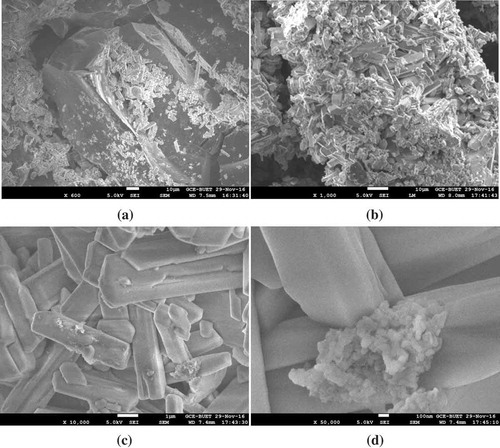
Table presents the elemental analysis of calcium carbide samples. The results show a high amount of sulfur content in calcium carbide sample. EDS using a FESEM was also performed on the carbide sample (Figure ). EDS is a qualitative detection method for the elemental analysis of a sample. The EDS analysis reconfirms high S-content in calcium carbide sample.
Table 3. X-ray fluorescence spectroscopy analysis of calcium carbide sample
Figure 9. Energy-dispersive X-ray spectroscopy of calcium carbide sample used in artificial fruit ripening.

Elemental analysis of calcium carbide also indicates the presence of arsenic (As) and phosphorous (P). When applied as a ripening agent, sulfur, arsenic and phosphorous from calcium carbide may diffuse into the peel and flesh of fruits and may pose serious health risk.
4.2.2. Kerosene fume
Table represents the results of kerosene fume analysis. According to Table , kerosene fume contains SO2, NO, NO3 and CO.
Table 4. Analysis of kerosene fume using a gas analyzer (NOVA model 600–8)
4.2.3. Ethephon and ethylene glycol
In addition to calcium carbide and kerosene, the sulfur content of ethephon and ethylene glycol was also assessed. As shown in Table , ethephon and ethylene glycol samples contained a trace amount of S-content.
Table 5. S-content in different artificial ripening agents (standard deviation for n = 2 samples)
4.3. Sulfate and sulfide content in banana samples
To understand the effect of high sulfur content in calcium carbide, the sulfate (SO42−) and sulfide (S2−) contents of naturally and carbide-ripened banana samples were determined.
Results in Table indicate that sulfate contents in the peel and flesh of carbide-ripened samples were higher than that of naturally ripened samples. In addition, experimentally quantified sulfide content in the peel of carbide-treated sample showed an augmented level compared to the naturally ripened sample. However, the sulfide content in banana flesh remained below detection limit.
Table 6. Sulfate and sulfide content of naturally and carbide-ripened banana (standard deviation for n = 3 samples)
5. Discussion
The changes in moisture content, TTA, vitamin C and sugar content of different artificially ripened banana samples were investigated in this study. It was found that, with respect to the green and naturally ripened samples, the changes in moisture contents and sugar contents of artificially ripened banana samples did not show any substantial change (Figures and ). The unripe banana samples showed significantly higher TTA content and lower sugar content. The ripening process increases gluconeogenesis (metabolic pathway that generates glucose), hydrolyzes polysaccharides, decreases acidity and accumulates sugars and organic acids (Lizada, Citation1993; Prasanna et al., Citation2007; Tucker and Grierson Citation1987) which may cause lower TTA and higher sugar content in ripened banana samples. The TTA value was significantly lower in the naturally ripened samples in comparison with the artificially ripened ones, which could be used as the marker to identify artificially ripened bananas. Furthermore, researchers have reported that high TTA might cause dental erosion, especially among children (Featherstone & Lussi, Citation2006; Fatima Enam et al., Citation2016). Hence, regular consumption of artificially ripened banana could be harmful to dental health (Fatima Enam et al., Citation2016).
Among artificially ripened banana samples, the kerosene fume-treated ones contained the lowest concentration of vitamin C (175 ppm). According to the ripening chemistry, vitamin C decreases with the increase in temperature (Njoku et al., Citation2011). Thus, the heat evolved from the kerosene lamp could be a cause of temperature increase which ultimately led to the decrease in vitamin C content of fume-treated samples.
From the elemental analysis of the locally available artificial ripening agents, sulfur, arsenic and phosphorous were found in calcium carbide samples. The sulfur contents found in calcium carbide samples were higher than the toxic dose set by the National Academy of Sciences, USA (600 ppm) (Florin et al., Citation1993, IMFNB, Citation2005). In carbide samples, arsenic content was found as high as 160 ppm which is much higher than the permissible limit set by US FDA (0.5–2 ppm) (ATSDR, Citation2009). Centers for Disease Control and Prevention (CDC) has reported the lower lethal dose (LDLo) of phosphorous for dog and cat are 10 and 4 ppm, respectively (NIOSH, Citation1994). Phosphorous present in the analyzed samples were higher (80 and 120 ppm) than LDLo of these animals. The possible reason for high sulfur content in calcium carbide is the carbide samples used in fruit ripening are often obtained from steelmaking plants where it is used as a desulfurizing agent (LLC, Citation2009; Svyazhin et al., Citation2004). Use of cheaply sourced industrial grade carbide sample can also be a reason for the presence of arsenic and phosphorous, which researchers have reported may contain both of these as impurities (Fattah & Ali, Citation2010; Siddiqui & Dhua, Citation2010).
Analysis of the kerosene fume indicated the presence of SO2, NO, NO3 and CO in fume. Formation of sulfur dioxide indicates the presence of sulfur as an impurity (Nicholas et al., Citation2013). The presence of high carbon monoxide (CO) content could be due to operating the kerosene lamp in an inadequately ventilated area where reduction of air oxygen ultimately led to incomplete combustion of kerosene and produced CO. (Lionel et al., Citation1986).
Experimentations also indicated a significantly higher sulfur content in calcium carbide-treated banana samples than the naturally ripened samples. This could be due to the diffusion of sulfur from calcium carbide to fruit body during the ripening process. Exposure to higher sulfur concentration may cause diarrhea (IMFNB, Citation2005) and exposure to arsenic and phosphorous may cause complications in different organs of human body (Fattah & Ali, Citation2010; Siddiqui & Dhua, Citation2010).
6. Conclusion
The analysis of nutritional value and health effects of chemically adulterated foods and artificially ripened fruits is a dynamic process. In recent times, the use of artificial ripening agents has become widespread globally mainly due to the economical reasons. It is, therefore, important to conduct an elemental analysis of fruit ripening agents, point out the changes in nutritional values caused by artificial ripening and associated health effects. In this study, the nutritional values of naturally and artificially ripened banana samples were measured and compared. Moisture and sugar content of naturally and artificially ripened banana samples did not show any significant difference. However, the Vitamin C content of kerosene fume-treated sample was drastically lower than all other samples and the TTA contents of artificially ripened samples were higher than the naturally ripened sample. In addition, elemental analysis of the four artificial ripening agents (calcium carbide, ethephon, ethylene glycol and kerosene fume) revealed that, the carbide sample contained a high amount of sulfur and a trace amount of arsenic and phosphorous. The other three agents also contained trace amount of sulfur. Presence of impurities may cause health-related issues to the consumers eating fruits ripened with these agents. Since artificial fruit ripening process involves health and socioeconomic aspects, it is a complex issue. Therefore, the understanding of this study will be useful for the consumers, researchers, legal authorities and other stakeholders working on food safety.
Acknowledgements
This research was supported by BCEF Academic Research Fund. The authors would like to acknowledge, Renata Pharmaceutical Ltd, Department of Glass and Ceramic Engineering, BUET, Mr A. H. M. S. Rahman, Mr A. F. Pasha and Ms M. Mursalat for technical assistance and discussion.
Additional information
Funding
Notes on contributors
Mohidus Samad Khan
Authors of this article are researchers of the “Bio and Environmental Technology (BET) Research Group”, Department of Chemical Engineering, Bangladesh University of Engineering and Technology (BUET). Research interest of BET Research Group includes chemical, biotechnological and environmental engineering. BET Research Group aims to address different scientific, engineering and socioeconomic issues related to Health, Food, and Environment. Artificial fruit ripening process is a complex issue since it involves health and socioeconomic aspects. Therefore, the understanding of this study will be useful for the consumers, researchers, legal authorities and other stakeholders working on food safety.
References
- Abano, E. E., & Amoah, L. K. S. (2011). Effect of different parameters on drying characteristics of banana slice. ARPN Journal of Engineering and Applied Sciences, 6(3), 121-129.
- AOAC (Association of Official Analytical Chemists). (1980). In Official methods of analyses (13th Ed.). Washington, DC.
- ATSDR. (2009). Agency for toxic substances and disease registry. USA: Arsenic Toxicity.
- Azam, F. M. S., Islam, S., Rahmatullah, M., & Zaman, A. (2010). Clonal propagation of banana (MUSA SPP.) Cultivar ‘BARI-1ʹ (AAA genome, Sapientum Subgroup), in International Conference on Banana and Plantain in Africa: Harnessing International Partnerships to Increase Research Impact. Mombasa, Kenya, 5-9 October, 2008.
- Blanpied, G. D., & Silsby, J. K. (1992). Predicting harvest date windows for apples. Cornell Cooperative Extension.
- Bouzayen, M., Latche, A., Nath, P., & Pech, J. C. (2010). Mechanism of fruit ripening. In E. C. Pua & M. R. Davey (Eds.), Plant developmental biology - Biotechnological perspectives. New York, NY: Springer-verlag berlin heidelberg.
- Brady, C. J. (1987). Fruit ripening. Annual Review of Plant Physiology and Plant Molecular Biology, 38, 155–178. doi:10.1146/annurev.pp.38.060187.001103
- Canet., W. (1988). Determination of the moisture content of some fruits and vegetables by microwave heating. Journal of Microwave Power and Electromagnetic Energy, 23(4), 231–235. doi:10.1080/08327823.1988.11688062
- Cejpek, K. (2012). Determination of carbohydrates in foodstuff: Analysis of food and natural products. Prague: Institute of Chemical Technology.
- Chace, E. M. (1934). Health problems connected with the ethylene treatment of fruits. American Journal of Public Health and the Nation’s Health, 24(11), 1152–1156. doi:10.2105/AJPH.24.11.1152
- Chadha, K. L. (2007). Handbook of horticulture (1st Ed.). New Delhi: Indian Council Of Agricultural Research.
- Charan, N. B., Myers, C. G., Lakshminarayan, S., & Spencer, T. M. (1979). Pulmonary injuries associated with acute sulfur dioxide inhalation. American Review of Respiratory Disease, 119(4), 555–560.
- Dwivany, F., Esyanti, R. R., Robertlee, J., Paramaputra, I. C., Permatadewi, R. K., Tambun, D. H., ... Zaskia, H. (2012). Environment effect on fruit ripening related gene to develop a new post harvest technology, in 4th International Conference on Mathematics and Natural Sciences. 8-9 November, Bandung, Indonesia.
- El Hadi, M. A. M., Zhang, F. J., Wu, F. F., Zhou, C. H., & Tao, J. (2013). Advances in fruit aroma volatile research. Molecules, 18.
- Enam, F., Mursalat, M., Guha, U., Aich, N., Anik, M. I., Nisha, N. S., ... Khan, M. S. (2016). Dental erosion potential of beverages and bottled drinking water in Bangladesh. International Journal of Food Properties, 20(11).
- EPA, U. S. (1988). Guidance for the reregistration of pesticide products containing ethephon as the active ingredient. Washington, DC.
- FAO. (2017). Banana market review 2015-2016. Rome: Author.
- Fattah, S. A., & Ali, M. Y. (2010). Carbide ripened fruits - A recent health hazard. Faridpur Medical College Journal, 5(2), 37.
- FDA. (2008). Nutrition facts for raw fruits. USA: Author.
- Featherstone, J. D. B., & Lussi, A. (2006). Understanding the chemistry of dental erosion. In A. Lussi (Eds), Dental erosion. Basel: Karger Publishers.
- Florin, T. H. J., Neale, G., Goretski, S., & Cummings, J. H. (1993). The sulfate content of foods and beverages. Journal of Food Composition and Analysis, 6(2), 140–151. doi:10.1006/jfca.1993.1016
- Goonatilake, R. (2008). Effects of diluted ethylene glycol as a fruit-ripening agent. Global Journal of Biotechnology & Biochemistry, 3(1), 8–13.
- Hakim, M. A.,Huq, A. K. O., Alam, M. A., Khatib, A., Saha, B.K., Haque, K. M. F., & Zaidul, I. S. M. (2012). Role of health hazardous ethephone in nutritive values of selected pineapple, banana and tomato. Journal of Food, Agriculture & Environment, 10(2), 247–251.
- Hanif, M., Mobarak, M. R., Ronan, A., Rahman, D., Donovan Jr, J. J., & Bennish, M. L. (1995). Fatal renal failure caused by diethylene glycol in paracetamol elixir: The Bangladesh epidemic. British Medical Journal, 311, 88–91. doi:10.1136/bmj.311.6997.88
- Ho, L. H., Aziah, A. A. N., & Bhat, R. (2012). Mineral composition and pasting properties of banana pseudo-stem flour from Musa acuminata X balbisiana cv. Awak grown locally in Perak, Malaysia. International Food Research Journal, 9(4), 1479–1485.
- Hollo, J., & Szejtli, J. (1958). The mechanism of starch-iodine reaction. Periodica Polytechnica: Chemical Engineering, 2(1), 25–37.
- House, M. C., Nelson, P. M., & Haber, E. S. (1929). The vitamin A, B, and C content of artificially versus naturally ripened tomatoes. Journal of Biological Chemistry, 81(8).
- IMFNB (Institute of Medicine, Food and Nutrition Board). (2005). Dietary reference intakes for water, potassium, sodium, chloride, and sulfate.
- Islam, M. N., Mursalat, M., & Khan, M. S. (2016). A review on the legislative aspect of artificial fruit ripening. Agriculture & Food Security, 5(1), 8. doi:10.1186/s40066-016-0057-5
- Islam, M. N., Rahman, A. H. M. S., Mursalat, M., Rony, A. H., & Khan, M. S. (2016). A legislative aspect of artificial fruit ripening in a developing country like Bangladesh. Chemical Engineering Research Bulletin, 18(1), 30–37. doi:10.3329/cerb.v18i1.26219
- Jayan, T. V. (January 3, 2011) Beware of these fruits. The Telegraph, Calcutta, Retrieved from https://www.telegraphindia.com/1110103/jsp/knowhow/story_13381969.jsp.
- John Bean Technologies Corporation. (2011). In Procedures for analysis of citrus products. Lakeland, FL.
- Kazi, N. A., Yadav, J. P., & Agale, M. G. (2015). Nutritional values of fruits. Scholarly Research Journal for Interdisciplinary Studies,3(16), 2937–2943.
- Kendrick, M. (2009). The origin of fruit ripening, in scientific American. New York: Nature America.
- Khan, M. Y., Khan, F. A., & Beg, M. S. (2013). Ethanol-kerosene blends: Fuel option for kerosene wick stove. International Journal of Engineering Research and Applications, 3(1), 464–466.
- Koros, K. (14 March, 2014). Kenya: Sweet poison - Illegal ripening of fruits exposes millions of Kenyans to cancer. In AllAfrica. The Star.
- Kulling, P., Andersson, H., Boström, K., Johansson, L. Å., Lindström, B., & Nyström, B. (1992). Fatal systemic poisoning after skin exposure to monochloroacetic acid. Journal of Toxicology: Clinical Toxicology, 30(4), 643–652.
- Lionel, T., Martin, R. J., & Brown, N. J. (1986). A comparative study of combustion in kerosine heaters. Environmental Science & Technology, 20, 78–85. doi:10.1021/es00143a010
- Lizada, C. (1993). Mango. In G. B. Seymour, J. E. Taylor, & G. A. Tucker (Eds.), Biochemistry of fruit ripening (pp. 255–271). United Kingdom: Springer Science & Business Media.
- LLC. (2009). Limited Liability Company. Calcium carbide steel making/slag conditioning grades, in steel making/slag conditioning grades. USA: Chemtrec.
- Majidi, M. I. H. A., & AlQubury, H. Y. (2016). Determination of vitamin C (ascorbic acid) contents in various fruit and vegetable by UV-spectrophotometry and titration methods. Journal of Chemical and Pharmaceutical Sciences, 9(4). 2972-2974.
- Marcus, Y. (1990). Recommended methods for the purification of solvents and tests for impurities: 1, 2-ethanediol and 2, 2, 2-trifluoroethanol. Pure and Applied Chemistry, 62(1), 139–147. doi:10.1351/pac199062010139
- Ministry of Justice, Government of Canada. (2009). Fresh fruit and vegetable regulations. Canada.
- Munir, M., Baloch, A. K., Khan, W. A., Ahmad, F., & Jamil, M. (2013). Development of iodimetric redox method for routine estimation of ascorbic acid from fresh fruit and vegetables. Journal of Chemical Society of Pakistan, 35(3), 783–792.
- Mursalat, M., Rony, A. H., Rahman, A. H. S., Islam, M. N., & Khan, M. S. (2013). A critical analysis of artificial fruit ripening: Scientific, legislative and socio-economic aspects. Chethoughts, 4(1).6-12.
- Mussa, S. B., & Sharaa, I. S. (2014). Analysis of vitamin C (ascorbic acid) contents packed fruit juice by UV-spectrophotometry and redox titration methods. IOSR Journal of Applied Physics, 6(5), 46–52. doi:10.9790/4861
- Nadel, J. A., Salem, H., Tamplin, B., & Tokiwa, Y. (1965). Mechanism of bronchoconstriction during inhalation of sulfur dioxide. Journal of Applied Physiology, 20(1), 164–167. doi:10.1152/jappl.1965.20.1.164
- Nagel, M. C. (1989). The fruits of ethylene. ChemMatters, 7(2), 11–13.
- Nicholas, L. L., Gauthier, K. R. S. A., & Bates, M. N. (2013). Kerosene: A review of household uses and their hazards in low- and middle-income countries. Journal of Toxicology and Environmental Health Part B Critical Review, 15(6), 396–432.
- Nielsen, S. S. (2010). Food analysis (4th ed.). Springer International Publishing.
- NIOSH. (1994). The National Institute for Occupational Safety and Health: Centers for Disease Control and Prevention. Phosphorus (yellow), Immediately Dangerous to Life or Health Concentrations (IDLH). Washington, DC: Centers for Disease Control and Prevention (CDC).
- Njoku, P. C., Ayuk, A. A., & Okoye, C. V. (2011). Temperature effects on vitamin C content in citrus fruits. Pakistan Journal of Nutrition, 10(12), 1168–1169. doi:10.3923/pjn.2011.1168.1169
- NNC Netherlands Nutrition Centre. (1996). Dutch Food Composition Database. Netherlands: National Institute for Public Health and the Environment, Ministry of Health, Welfare and Sport.
- O’Brien, K. L., Selanikio, J. D., Hecdivert, C., Placide, M. F., Louis, M., Barr, D. B., … Philen, R. M. (1998). Epidemic of pediatric deaths from acute renal failure caused by diethylene glycol poisoning. Journal of American Medical Association, 279(15), 1175–1180. doi:10.1001/jama.279.15.1175
- Patel, P. M. (2017). Determine the ascorbic acid content in selected fruits by using iodine solution in redox titration method and application of ascorbic acid. Der Pharma Chemica, 9(14), 61–63.
- Pirson, J., Toussaint, P., & Segers, N. (2003). An unusual cause of burn injury: Skin exposure to monochloroacetic acid. Journal of Burn Care & Rehabilitation, 24(6), 407–409. doi:10.1097/01.BCR.0000095515.03087.E0
- Prasanna, V., Prabha, T. N., & Tharanathan, R. N. (2007). Fruit ripening phenomena–An overview. Critical Reviews in Food Science and Nutrition, 47(1), 1–19. doi:10.1080/10408390600976841
- Rahman, A. U., Chowdhury, F. R., & Alam, M. B. (2008). Artificial ripening: What we are eating. Journal of Medicine, 9(1), 42–44.
- Robertson, O. H., Loosli, C. G., Puck, T. T., Wise, H., Lemon, H. M., & Lester, W. (1947). Tests for chronic toxicity of propylexe glycol and triethylene glycol on monkeys and rats by vapor inhalation and oral administration. Journal of Pharmacology and Experimental Therapeutics, 91(1), 52–76.
- Said, K. A. M., Radzi, Z., Yakub, I., & Amin, M. A. M. (2016). Extraction and quantitative determination of ascorbic acid from banana peel Musa acuminata ‘Kepok’. IIUM Engineering Journal, 17(1). 103-111.
- Segall, Y., Grendell, R. L., Toia, R. F., & Casida, J. E. (1991). Composition of technical ethephon [(2-chloroethyl) phosphonic acid] and some analogs relative to their reactivity and biological activity. Journal of Agricultural and Food Chemistry, 39(2), 380–385. doi:10.1021/jf00002a031
- Senthilkumar, T., Sivakumar, K., Sudha, R., & Alagusundaram, K. (2005). A quick method of determining the moisture content of fruits and vegetables, in Post Production Systems and Strategies to the Issues and Challenges of Food Safety and Security. Coimbatore.
- Shahnawaz, M., Sheikh, S. A., & Nizamani, S. M. (1980). Determination of nutritive values of jamun fruit (Eugenia jambolana) products. Pakistan Journal of Nutrition, 8(8), 1275–1280.
- Siddiqui, M. W., & Dhua, R. S. (2010). Eating artificial ripened fruits is harmful. Current Science, 99(12), 1664–1668.
- Singal, S., Kumud, M., & Thakral, S. (2011). Application of apple as ripening agent for banana. Indian Journal of Natural Products and Resources, 3(1), 61–64.
- Svyazhin, A. A., Krushke, E., & Svyazhin, A. G. (2004). The use of calcium carbide in the production of low-carbon steel. Metallurgist, 48, 557–561. doi:10.1007/s11015-005-0027-9
- Tucker, G. A., & Grierson, D. (1987). Fruit ripening. In D. D. Davies (Eds), The biochemistry of plants (pp. 265–319). New York: Academic Press.
- Postharvest horticulture series no. 9. (pp. 51), UC Davis Postharvest Technology. (2010)..
- Uma, S., Kalpana, S., Sathiamoorthy, S., & Kumar, V. (2005). Evaluation of commercial cultivars of banana (Musa spp.) for their suitability for the fibre industry. Plant Genetic Resources Newsletter, 112, 29–35.
- Vila, R., Granados, M. V., Hellin, P., Kauppinen, S., Laencina, J., Rumpunen, K., & Ros, J. M (2003). Biochemical changes in chaenomeles fruits and fruit juice during ripening and storage. In K. Rumpunen, et al. (Eds.), Japanese quince - Potential fruit crop for Northern Europe (pp. 159–168). Sweden: Swedish University of Agricultural Sciences Publications.