Abstract
Avocado production has increased exponentially over the years worldwide. This has been necessitated by the various uses of avocado fruits other than as a source of nutrients which includes pharmaceutical, cosmetic and oil industries. These various uses of avocado have resulted to improved livelihood in terms of income generation. Increased avocado production worldwide has been at the expense of other tree and food crops. In Kenya, it is attributed to decreased acreage of key cash crops such as coffee and tea as well as staple food crops such as maize and beans. Avocado production, however, has been faced by several constraints such as poor rootstalks, diseases, pests, abiotic factors, poor harvesting technology, poor handling of harvested fruits and post-harvest diseases. All these challenges lead to poor fruit quality at the market both local and export market leading to losses economically. Of major concern is anthracnose disease caused by Colletotrichum gloeosporioides, Colletotrichum boninense, Pestalotiopsis microspora both in the field and after harvest. The disease is associated with 60% losses as a result of abortion of fruits in the field and post-harvest rots. Limited studies on the the interaction of the causal agents, the disease cycle and its epidemiology in Kenya, has rendered disease management impractical. Control of this disease has been through pruning and sorting of the diseased fruits. However, majority of the farmers do not apply any control measures. Furthermore, the use of chemical control has been limited due to a lack of available fungicides registered for use in Kenya. Fruit rots and quality issues due to black spots has affected the marketability of avocado fruits in the export market mainly EU market. Due to this, the avocado farmer in Kenya has been receiving low returns from their avocado export. Further understanding of the anthracnose disease epidemiology, virulence and genetic variation of the causal agent and the sensitivity of the causal agent to available fungicides will enhance the effectiveness of the anthracnose disease management, resulting in improved avocado productivity.
PUBLIC INTEREST STATEMENT
The researcher focus on fruit diseases specifically post-harvest diseases of avocado in Kenya. The avocado has become a major fruit and economic earner for the country as well as at the world level. Anthracnose disease being a major production constraint of avocado in Kenya. It has led to low avocado production and also affect marketability of the fruits both locally and internationally. The research therefore geared towards undertaking studies on management/control options of these diseases in order to have quality, safe fruits acceptable by the consumers both locally and internationally.
1. Avocado: origin and spread
Avocado (Persea americana Mill.), is known to have originated in Central America and Southern Mexico (Chen et al., Citation2008).It is divided into 3 sub-species, i.e. Mexican (sub-tropical), Guatemalan (semi-tropical) and West Indian (tropical) (Wasilwa et al., Citation2006). Avocado popularity has grown exponentially over the years and it is currently cultivated all over the world, including tropical and subtropical areas of Africa (Schaffer et al., Citation2013 and Zentmyer, Citation1994). It has been among export produce in countries such as South Africa, East Africa including Rwanda and Burudi. Kenya and South Africa are ranked among the 10 major avocado producer in the world (FAOSTAT, Citation2013).Avocado plants were first introduced in Kenya in the 1930s by the Portuguese (Griesbach, Citation1985, Citation2005) to be used for subsistence, whereas commercial cultivation of avocado in Kenya started in the early 1960s (Griesbach, Citation1985). Currently, avocado is produced in various agro-ecological zones of Kenya by small-scale growers (85%) and large-scale growers for subsistence, local markets and export (Cooper et al., Citation2003; Horticultural Crop Development (HCD), Citation2018; Wasilwa et al., Citation2006).
2. Avocado production world wide
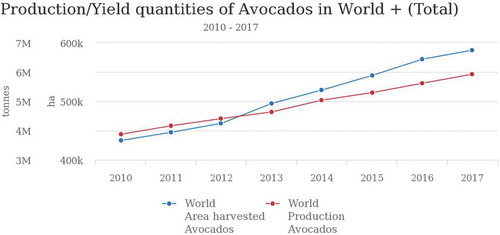
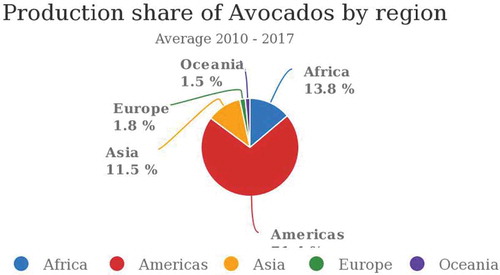
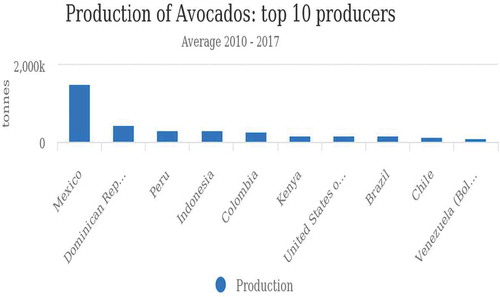
Avocado production has grown gradually over the years among the avocado-producing countries, for example, from 3,871,140 tons in 2010 to 5,924,398 tons in 2017 (FAOSTAT, Citation2013) (Figure ). Region-wise, America has the highest average avocado production with 71.4%, followed by Africa at 13.8% while Oceania has the lowest at 1.5% (FAOSTAT, Citation2013) (Figure ).Among the top-producing countries, Mexico is the leading producer, with an average production of 1,529,922.25 tonnes between 2010 and 2017. In the same period Kenya had an average production of 177,714.75 tonnes (Figure ).
Avocado plays an important role in Mexico in terms of food security, as it is a staple food for Mexican households (Hernandez, Citation2011). Furthermore, avocado contributes to the economy of the country as more than 13% of the total production is traded internationally (FAO, Citation2004). In Chile, South Africa, Israel and Spain, the avocado production is mainly intended for the overseas export market.
Figure 3. Avocado average production by the 10 top avocado-producing countries of the world between 2010 and 2017.
The major avocado markets are the European Union and the United States (FAO, Citation2004), and Kenya is ranked sixth after Mexico among avocado exporters globally (FAOSTAT, Citation2013). Avocados are produced mainly on a small scale in the tropics and sub tropics, though its large-scale production is gaining popularity. In Kenya, avocado is the most important fruit at 62%, followed by mango at 26%, in terms of foreign exchange earnings in the mid-2000s up to the year 2018 (HCD Citation2018).
In Kenya, avocado is mainly grown in highlands, where like other crops it is susceptible to various pests such as fruitflies and false codling moth and diseases such as anthracnose, cercospora and stem-end rot. However, Pests and diseases of significant economic importance to avocado production in Kenya are fruit flies, false codling moth, anthracnose, stem end rot (Horticultural Crop Development (HCD), Citation2018). Anthracnose, caused by the a multiple fungi (Kimaru et al., Citation2018a) leads to reduction in yield, shelf-life and quality of avocado fruits, due to their high susceptibility (Pernezny et al., Citation2000). Over 60% of the Kenyan avocado production cannot be marketed because of damage due to anthracnose and their low quality, emanating from poor production procedures (Kimaru et al., Citation2018b). The disease leads to significant economic losses of fruits in the tropics due to the reduced shelf life of fruits during storage and transport, and hampered market accessibility (Freeman et al., Citation1998). In Kenya, these losses represent about 40% of the avocado export revenue (Horticultural Crop Development (HCD), Citation2018).
3. Uses of avocado fruit
Avocado fruit is being used for both its nutritive and medicinal values.
4. The nutritive use of avocado fruits
For nutrition, avocado fruits are eaten alone, pressed for oil, as dessert drinks, used to make guacamole and added to salads. In Kenya, the domestic consumption of avocado is about 1–2 kg/person/year and is on the increase as the population is sensitized by its nutritional aspects. The fruit is rich in palmitic, oleic, palmitoleic acids, linoleic and lipids (Lu et al., Citation2009).
This fruit contains twice as much protein compared to any other fruit, is very rich in minerals and vitamins (Dreher, Citation2013, Schafer et al., Citation2013). Its nutritive composition contributes positively to the health of those who consume it, including the maintenance of good cholesterol levels (Naveh et al., Citation2002; Griesbach, Citation2005). In cosmetics, avocado oil is considered to be superior to vegetable oils due to its higher penetration ability and its nourishment to the glands beneath the skin. Avocado oil is rich in vitamins and has excellent keeping quality of hair. Oil is associated with regenerative properties the reason why it is used to heal scars and spots on the skin (Eyres et al., Citation2001). In addition, oil is used as facial creams, hand lotions and soap. Further, the pulp residue obtained after oil extraction is used as stock feed.
5. Medicinal uses
The avocado plant is used as a source of medicine in different cultures where it is grown although not based on scientific findings. The leaf juice and roasted seeds have been used as an antibiotic to cure bacterial diseases, such as dysentery and diarrhoea. In other places like Cuba, a juice derived from shoots and seeds is used to treat cough and toothache, respectively. Studies by Idris et al. (Citation2009) reported antimicrobial activities of leaf and seed extract on Escherichia coli, Klebsiella pneumoniae, Bacillus subtilis, Streptococcus pyogenes, Pseudomonas aeruginosa, Staphylococcus aureus, Corynebacterium ulcerans, Salmonella typhi, Neisseria gonorrhoea and Candida albicans which are known to cause variuous ailment in human.
6. Avocado tree
The avocado tree grows to a height in the range of 9 m to 12 m. The shape of the leaves is either elliptic or oval and is 7 cm to 25 cm in length. The flowers have both female and male parts and are green in colour. The avocado fruit shape varies from pear-shaped, round or ovoid. The fruit exhibits various colours from greenish through purplish to blackish and while the texture of the skin is either smooth or rough. The fruit has a flesh that is greenish-yellow to yellow when ripe. Further, the flesh may appear fibrous or buttery. The avocado fruit has one seed which weight ranges between 0.1 kg to 1.3 kg. The weight of the seed accounts for between 10 and 25% of the total fruit weight (Schaffer et al. Citation2013).
Pollination of the avocado fruits occurs by insects, especially honeybees. The avocado plant exhibits two flowering types; A and B, where each flower opens twice, meaning that the functionally female opens first and the male opens second. Normally, the Type A flower opens first in the morning, closes at midday, and reopens in the afternoon of the following day, while Type B opens first in the afternoon, closes in the evening, and reopens the following morning. The presence of both types of trees is important in orchards to improve production by adequate pollination (Bruce et al. (2013).
Avocado tree propagation is achieved through grafted seedling or from non-grafted seedling. Grafting, however, is the commonest method in avocado and where grafted plants produce after three years, while those trees from sown seeds produce by the eighth year. Therefore, the trees propagated from seed are associated with low fruit yield and quality as compared to the grafted type which mature earlier (Brecht et al., Citation2009).
Avocado fruit is categorized as climacteric fruit, where it matures while on tree but ripens after harvest (Brecht et al., Citation2009; Hofman et al., Citation2013). Based on this, it is important to assess the maturity of the fruit while at the tree before harvesting is done. Fruits that are not mature however, if harvested do not ripen properly and therefore affects fruit quality. Fruit destined for export markets may require strategies to delay ripening to ensure that they reach the destination market when they are not overripe. Ripening process is delayed through lowing temperature, reduce ethylene production and avoid mechanical damage of the fruits (Yahia, Citation2011). However, ripening of avocado can be started artificially by spraying ethylene gas for example, (Gamage & Rehman, Citation1999).
7. Avocado varieties grown in Kenya
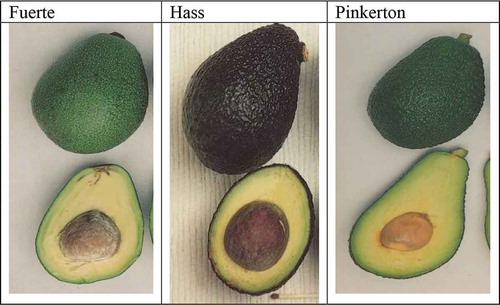
In Kenya, several avocado cultivars have been introduced (Puebla, Pinkerton, Hass and Fuerte), both for the local and the export market. However, only Fuerte, Hass and Pinkerton are in production, together with some local varieties for commercial purposes. The studies have shown that these varieties are susceptible to anthracnose disease where fuerte is comparatively the most affected (Kimaru et, al. Citation2018b). The various characteristics of different avocado varieties grown in Kenya are as described in the book “The Avocado: Botany production and uses” by Bruce et al. (2013).
8. The pathogen: Colletotrichum gloeosporioides
Filamentous fungi belonging to the Colletotrichum gloesporioides species- complex are known to cause anthracnose disease in various fruit crops such as apple (Malus domestica), citrus (Citrus sinensis), papaya (Carica papaya), passion fruit (Persiflora)mango (Mangifera indica), guava and avocado (Persea Americana), annona (Annona cherimola), coffee (Coffea arabica), apple (Malus domestica), peach (Prunus persica), mango (Mangifera indica), and, more recently, tea oil (Camellia oleifera) (Alahakoon et al., Citation1994; Cai et al., Citation2009; Crouch & Beirn, Citation2009; cai, et al., Citation2009 and Dean et al., 2012Silva et al., Citation2012; Lima et al., Citation2013; Li H, et al, Citation2016; Cristobal-Martınez et al., Citation2017; Kimaru et al, Citation2018a). There are difficulties of identifying individual Colletotrichum species as a result, few and variable cultural, morphological characteristics, an extensive host range (Latunde-Dada, Citation2001).
Molecular approaches are being used to discriminate among Colletotrichum gloeosporioides species complex, since morpho-taxonomic criteria are not accurate. An A1 T-rich DNA have been used to group C. gloeosporioides isolates obtained from strawberries (Freeman, et al., Citation1996). Further, arbitrarily primed-PCR (ap-PCR) analysis used to categorize C. gloeosporioides isolates from infected strawberries, mangoes, avocado and papayas (Freeman & Rodriguez, Citation1995; Mills et al., Citation1992). Similarly, polymorphisms in mitochondrial DNA (mtDNA) and DNA encoding ribosomal RNA (rDNA) have been used to determine variability within populations of C. gloeosporioides infecting different fruits (Hodson et al., Citation1993; Alahakoon et al., Citation1994) while other studies have followed single locus based on the internal transcribed spacer (ITS) region of rDNA (Abang et al., Citation2002; Chung et al., Citation2006; Shivas & Tan, Citation2009).
Further studies involving multilocus phylogenetic analysis based on sequencing data from the ITS region and from the βtubulin-2 and glyceraldehyde-3-phosphate dehydrogenase genes has identified Colletotrichum asianum, Colletotrichum fructicola, and Colletotrichum musae as members of the Colletotrichum gloeosporioides species complex, known to cause anthracnose in various fruit (Gañán et al., Citation2015). More so, Bayesian multilocus phylogenetic analyses performed using amplified sequences of the internal transcribed spacer region of the nuclear ribosomal DNA; actin, chitin synthase, glyceraldehyde-3-phosphate dehydrogenase partial genes; and APn2-Mat1-2 intergenic spacer and mating type Mat1-2 partial gene reported Colletotichum fruticola as one of the species that forms Colletotrichum gloeosporioides. (Fuentes-Aragón et al., Citation2018). These findings clearly demonstrate that a lot more research need to be done to understand the complexity of Colletotrichum gloeosporioides which cause anthracnose disease in various crops and in different regions. Similarly, multi-locus phylogenetic analyses involving combinations of ITS, act, ApMat, cal, chs1, gapdh, gs, his3, tub2 gene/markers identified (C. aenigma, C. alienum, C. fructicola, C. gloeosporioides sensu stricto, C. karstii, C. nupharicola, C. siamense, C. theobromicola) and a novel species (C. perseae) as avocado anthracnose pathogens in Israel (G. Sharma et al., Citation2017).
Colletotrichum gloeosporioides (Penz.) Penz. & Sacc. is an asexual stage (anamorph imperfect) parasite, which belongs to the family of Phyllachoraceae of the division Ascomycota is the most distributed worldwide (Cannon et al., Citation2012). Glomerella cingulata is the sexual (perfect) teleomorph state. The fungus thrives best in warm humid environment where it is associated with the spread of anthracnose (Farr et al., Citation2006; Ajay, Citation2014). The fungus/host relationship is broad and imprecise (Sanders & Korsten, Citation2003a).
The fungus produces abundant conidia, acervuli, setae and appressoria on infected plant organs such as fruits and leaves. Fungal propagules are dispersed by splashing rain or overhead irrigation and cause new infections on leaves, blossoms and fruits (Agrios, Citation2005; Farr et al., Citation2006). The fungus may cause infection, forming pepper spots (Giblin & Coates, Citation2010) or remain dormant after infecting fruits in the field until ripening starts, after it becomes active and causes lesion formation. The fungus produces acervuli in the infected hosts' tissues of the fruits (Ajay, Citation2014). The fungus produces hyaline, one-celled, oblong, slightly curved conidia with obtuse ends (Freeman et al., Citation1998). The fungus thrives best at temperatures around 28°C and humid conditions (Agrios, Citation2005).
In culture media, the fungus generally produces circular, woolly or cottony colonies with a characteristic colour that is pale brown or grayish white (Prabakar et al., Citation2005; Vidyalakshni & Divya, Citation2013 and Kimaru et al., Citation2018b).
9. Management of anthracnose disease
To ensure quality of the avocado fruits in the market, anthracnose disease needs to be controlled by avocado growers worldwide. The postharvest decay of the infected fruits during the avocado supply chain contributes immensely to postharvest loss and subsequent economic loss (Prusky & Keen, Citation2011). The disease occurs at different stages of fruit formation and during postharvest chain (Prusky & Keen, Citation2011).
10. Cultural control
The main cultural method used by farmers has been pruning of the avocado trees to enhance air circulation and reduce moisture in the leaf canopy which favours the fungal growth that causes anthracnose. In addition, farmers have been collecting the diseased fruits and leaves from the orchard, dispose them through burning or burying.
11. Chemical control
Chemical control is used to reduce the incidence anthracnose disease in avocado. Application of fungicides, such as prochloraz at a commercial level has been used in Australia, New Zealand and South Africa (Everett et al., Citation2005; Scheepers et al., Citation2007; Smith et al., Citation2011). Prochloraz, affects the mycelial growth of the pathogen through inhibition of the ergosterol synthesis, a component of the fungal cell membrane (Bill et al., Citation2014).
Other fungicides used to control the disease with good results include copper oxychloride, Mancozeb, Metiram, Propineb and Thiabendazole (Agrios, Citation2005).
However, the use of fungicides is not sustainable due to the development of strains of the fungi that are resistant to fungicide (Ippolito & Nigro, Citation2000). For example, isolates of C. gloeosporioides from avocado and mango fruits showed varied sensitivity towards prochloraz (Arauz, Citation2000) and benzimidazole, respectively (Sanders et al., Citation2000). Benzimidazole fungicides function by inhibiting β-tubulin biosynthesis (Davidse, Citation1973). Furthermore, the fungus has a wide host range, which ensures re-infection after application of chemicals on fruits of a particular host (Sideney & Dirlane, Citation2014). In addition, fungicides are a source of environmental contamination of soil and water resources due to the disposal of large volumes of fungicide solutions.
Due to the risks associated with the use of postharvest fungicides, avocado farmers need to find seek for alternatives to fungicide applications. Such methods include; controlled and modified atmosphere storage (Kader, Citation1994), biocontrol agents (Janisiewicz et al., Citation2001), heat treatments (Fallik & Luire, Citation2007), microwave treatments (Karabulut and Baykal, Citation2002) and the application of chitosan and natural products such as essential oils (Burt, Citation2004; Kalemba & Kunicka, Citation2003).
Sodium bicarbonate and C. oleophila have been used to control anthracnose in papaya as an environmental friendly compared to chemicals. In addition, essential oils are known to have fungicidal properties and are therefore being used as a safer alternative to the environment than synthetic chemicals (Pitarokili et al., Citation1999).
As a strategy of controlling diseases, chemical use has raised concerns worldwide due to the harmful effect of pesticide and their residues on humans, animals and the environment. For example, the set maximum residue level (MRL) of prochloraz is 2 mg/kg for the European Union countries and Republic of Japan (Njombolwana et al., Citation2013).
In Kenya, the use of fungicides to control anthracnose disease in avocado is limited due to lack of registered fungicides that are allowed for use in avocado (PCPB, Citation2019). This has resulted to use of fungicides by some farmers registered for the control of diseases in other crops other than avocado. Such fungicides include Bayleton 25WP (Triadimefon), milraz 76WP (Propineb), Cymoxaxil and Green cop 500WP (copper oxychloride) (Kimaru et al., Citation2018b). The use of fungicides that have not been registered for control of anthracnose has also been reported in Europe, where the fungicide guazatine is used as a pre-storage drench treatment to control sour rot caused by Geotrichum candidum in citrus fruit during the wet season. (European Food Safety Authority EFSA, Citation2013).
12. Biological control
Biological control of the anthracnose disease can be achieved through use of Bacillus subtilis, which inhibits the growth of C. gloeosporioides in vitro (Piteira and Rodrigues, Citation1999). B. subtilis, have been found to maintain dormancy of C. gloeosporioides through removal of nutrients surrounding its appresoria when applied as a wax formulation. (Korsten & Jeffries, Citation2000). Bacillus spp have proofed to be effective in control of anthracnose disease of avocado on their own or in combination with fungicide (Korsten & Jeffries, Citation2000; Korsten et al., Citation1995).
The Bacillus spp. isolated from avocado leaves were found to be more effective in controlling anthracnose as compared to prochloraz when applied as a postharvest dip (El Ghaouth et al., Citation2002; Korsten et al., Citation1995). The use of biocontrol agents aids in reducing the use of synthetic fungicides, which are known to be an environmental hazard (Janisiewicz & Cornway, Citation2010). To control the disease, in South Africa, Bacillus subtilis B246; Avogreen® (Korsten et al., Citation1991) has been registered for commercial use by avocado growers and is popular with organic avocado growers. Though biocontrol is an appropriate alternative to chemical fungicides, it has the characteristics of not having an immediate knock-down effect when applied and also little is known on how to properly handle biocontrol agents.
The use of botanical extracts as fungicides, for example, extract derived from Ocimum basilicum L. and Allium sativum L for the control of the pathogen may be safer to human and the ecosystem and may serve as an alternative to application of fungicides (Ogbebor et al., Citation2007). Among the botanical fungicides, the use of essential oils (EOs) which are economical and show antimicrobial properties, low mammalian toxicity, biodegradability and eco-friendliness (Burt, Citation2004; Isman, Citation2000; Kalemba & Kunicka, Citation2003) is the most accepted alternative method by the consumer.
13. Resistant avocado cultivars
The use of resistant cultivars to anthracnose could be the most appropriate method to manage the disease. However, the varieties that are popular to the consumers are susceptible. In this regard, breeding avocado for resistance to anthracnose is paramount while maintaining the desirable character by the consumers.
14. Molecular characterization of Colletotrichum spp
Molecular techniques have been employed to identify and characterize Colletotrichum spp. (Crouch & Beirn, Citation2009 and Garrido et al., Citation2008). For example, arbitrarily primed polymerase chain reactions (PCRs), which use primers whose nucleotide sequence is arbitrarily chosen (similar to random-amplified polymorphic DNAs (RAPDs)) and PCR-amplified ribosomal RNA (rRNA) were employed to categorize C. acutatum and C. gloeosporioides isolates from different hosts (Freeman, Citation2000; Tapia-Tussell et al., Citation2008). Further, primers that are specific to a species designed from ITS region of rRNA gene of Colletotrichum, has been used to distinguish between C. acutatum from C. gloeosporioides (Harp et al., Citation2008; Sreenivasaprasad et al., Citation1992, Citation1994). Further, a combination of molecular and morphological methods have enhanced the determination of the two species of Colletotrichum (Damm et al., Citation2012; Poulivong et al., Citation2010). However, in recent time, a copy an intron of the glutamine synthetase (GS) gene (885 to 915 bases) has been used to classify and differentiate various species of Colletotrichum (Guerber et al., Citation2003; Liu & Correll, Citation2007; Crouch & Beirn, Citation2009; Than et al., Citation2008a).
Freeman et al. (Citation1996), demonstrated pathogenic and genotypic diversity among C. gloeosporioides isolates from avocado. However, they did not observe phenotypic correlations between isolates from Israel and USA. In their study, the avocado isolates were identified as C. gloeosporioides by morpho-taxonomic criteria, but there was a possibility of other species being present, such as C. acutatum being involved in the infection (Hartill & Everett, Citation2002).
The fungal isolates from avocado from Israel and the United States showed diversity, characterized by many arbitrarily primed-PCR phenotypes. Further, southern hybridization of the nuclear-DNA element GcpR1 to PstI-digested genomic DNA of avocado isolates expressed fragments which were polymorphic among the isolates (Freeman, 1996). This clearly shows there exist some variations within C. gloeosporioides isolates from different localities.
15. Current research on the management of anthracnose disease in avocado
Field surveys to determine the incidence and severity of the anthracnose are paramount. This information should indicate the extent of the spread and damage associated with the disease in avocadoes. The morphological and molecular characteristics of the pathogen causing anthracnose in avocadoes should lead to the exact identification of the pathogen up to the species/strain level. This will then provide clear information on the exact causal agent of the disease, which has up till now been associated with various species of Colletotrichum, namely C. gloeosporioides, C. acutatum, C. boninense and C. karstii. This information is essential, as anthracnose is identified as a serious post-harvest disease of avocado worldwide (Silva-Rojas & Avila-Quezada, Citation2011, Damm et al., Citation2012; Velázquez-del Valle et al., Citation2016). Furthermore, the wide host range associated with the causal agent makes the disease management a challenge (Abang et al., Citation2002). For example, Lopez & Lucas et al. (Citation2010) verified that C. gloeosporioides isolated from different plant hosts (avocado, mangabeira, passion fruit and acerola) are also pathogenic to cashew tree. This served as a clear indication that cultures of C. gloeosporioides from different hosts are a source of inoculum for avocado, therefore pose a challenge when it comes to control the disease in avocado where the alternate hosts are within the vicinity (Sideney & Dirlane, Citation2014).
The need to identify the particular Colletotrichum species affecting avocado is of great concern, due to the current innovations where C. gloeosporioides strains are being developed as bio-control agents for weed management (Legar et al., Citation2001). Furthermore, reduced pathogenicity mutants of C. gloeosporioides are being explored as control agents of the virulent types (Yakoby et al., Citation2002). In addition, some formae speciales of the fungus have been formulated for commercial use as biocontrol agents of weeds in various countries. Such agents include C. gloeosporioides f. sp cuscutae, C. gloeosporioides f. sp malvae and C. gloeosporioides f. sp aeschynomere registered in China, Canada and USA, respectively (Evan et al., Citation2001 and Kaewchai, et al., Citation2009).
The ability of the fungus to overwinter in different media, infested fruits, plant debris and soil makes the disease to thrive from one season to the other (Agrios, Citation2005).
In Kenya, molecular studies have been done to identify the causal agent of anthracnose and also to compare with other findings which have reported Colletotrichum spp as the causal agent in avocado different countries/regions (Boesewinkle, Citation1982; Peres et al., Citation2002; Silva-Rojas & Avila-Quezada, Citation2011; Velázquez-del Valle et al., Citation2016). Studies have identified C. gloeosporioides, C. boninense and Pestalotiopsis microspora as the causal agents of anthracnose of avocado in Kenya (Kimaru et al., Citation2018b). The management of this disease is a still a challenge in Kenya due to lack of registered fungicides (PCPB, 2019). Further, there is need to consider other alternative control measures other than synthetic fungicides to be used to control the disease in Kenya. More studies also, are required to establish the interactions of the causal agents and their effective control strategies.
Competing interests
The authors declares no competing interests.
Acknowledgements
Thanks to the management and technical team of KARLO, Kandara, where this work was done in the Mycology lab.
Disclosure statement
The author(s) declare(s) that there is no conflict of interest regarding the publication of this article.
Additional information
Funding
Notes on contributors
K. S Kimaru
The author, Dr. Stanley Kimaru, a Plant Pathologist (Post-harvest diseases) at the Department of Plant Sciences, Kenyatta University is the principal researcher in control and management of Post –harvest diseases of avocado in Kenya. Currently, he is conducting a research aimed at characterization of the anthracnose disease-causing agents and its control in Kenya. Mr. Peterson Muchemi, a PhD students, and Ms. Jedidah Mwangi an Msc students attached to the project.
References
- Abang, M. M., Winter, S., & Green, K. R. (2002). Molecular identification of Colletotrichum gloeosporioides causing yam anthracnose in Nigeria. Plant Pathology, 51, 63–14. https://doi.org/10.1046/j.0032-0862.2001.00655.x
- Agrios, G. N. (2005). Plant Pathology (5th ed.). Elsevier Academic Press.
- Ajay, K. G. (2014). Colletotrichum gloeosporioides: Biology, pathogenicity and management in India. Journal of Plant Physiology and Pathology, 2, 2-11.
- Alahakoon, P. W., Brown, A. E., & Sreenivasaprasad, S. (1994). Cross infection potential of genetic groups of Colletotrichum gloeosporioides on tropical fruits. Physiological and Molecular Plant Pathology, 44(2), 93–103. https://doi.org/10.1016/S0885-5765(05)80104-3
- Arauz, L. (2000). Mango anthracnose: Economic impact and current options for integrated management. Plant Disease, 84, 600–611. https://doi.org/10.1094/PDIS.2000.84.6.600
- Bill, M., Dharini Sivakumar, A., Thompson, K., & Korsten, L. (2014). Avocado Fruit Quality Management during the Postharvest Supply Chain. Food Reviews International, 30(3), 169–202. https://doi.org/10.1080/87559129.2014.907304
- Boesewinkle, H. J. (1982). A list of 142 new plant disease recordings from New Zealand and short notes on three diseases. Australian Plant Pathology, 11(4), 40–43. https://doi.org/10.1071/APP9820040
- Brecht, J. K., Yahia, E. M., & Litz, R. E. (2009). Postharvest physiology in Litz, R. E. (eds). The mango: Botany, production and uses (2nd ed., 484–528). CABI.
- Burt, S. (2004). Essential oils: Their antibacterial properties and potential applications in foods-a review. Internaytional Journal Food Microbiology, 94(3), 223–253. https://doi.org/10.1016/j.ijfoodmicro.2004.03.022
- Cai, L., Hyde, K. D., Taylor, P. W. J., Weir, B., Waller, J., Abang, M. M., Zhang, J. Z., Yang, Y. L., Phoulivong, S., Liu, Z. Y., Prihastuti, H., Shivas, R. G., McKenzie, E. H. C., & Johnston, P. R. (2009). A polyphasic approach for studying Colletotrichum. Fungal Diversity, 39(1), 183–204.
- Cannon, P. F., Damm, U., Johnston, P. R., & Weir, B. S. (2012). Colletotrichum – Current status and future directions. Study Mycology, 73, September 2012, 181–213. https://doi.org/10.3114/sim0014
- Chen, H., Morrell, P. L., Cruz, M. D. L., & Clegg, M. T. (2008). Nucleotide diversity and linkage disequilibrium in wild avocado (Persea americana Mill.). Journal of Heredity, 99(4),382-389.
- Chung, W. H., Ishii, H., & Nishimura, K. (2006). Fungicide sensitivity and phylogenetic relationship of anthracnose fungi isolated from various fruit crops in Japan. Plant Disease, 90(4), 506–512. https://doi.org/10.1094/PD-90-0506
- Cooper, J., Dobson, H., & Orchard, J. (2003). Avocado production protocol- a document in consultation with avocado growers and exporters in Kenya. Natural Resources Institute. University of Greenwich, UK. 63.
- Cristobal-Martınez, A. L., Ya~nez-Morales, M. J., & Solano- Vidal, R. (2017). Diversity of Colletotrichum species in coffee (Coffea arabica) plantantions in Mexico. European Journal of Plant Pathology, 147(3), 605–614. https://doi.org/10.1007/s10658-016-1029-0
- Crouch, J. A., & Beirn, L. A. (2009). Anthracnose of cereals and grasses. Fungal Diversity, 39, 19–44. http://www.fungaldiversity.org/fdp/sfdp/FD39-2.pdf
- Damm, U., Cannon, P. F., Woudenberg, J. H. C., & Crous, P. W. (2012). The Colletotrichum acutatum species complex. Studies in mycology, 73, 37-113.
- Davidse, L. C. (1973). Antimitotic activity of methyl benzimidazol-2-yl carbamate (MBC) in Aspergillus nidulans. Pesticide Biochemistry and Physiology, 3(3), 317–325. https://doi.org/10.1016/0048-3575(73)90030-8
- Dean, R., Van Kan, J. A., Pretorius, Z. A., Hammond-Kosack, K. E., & Di Pietro, A. (2012). The Top 10 fungal pathogens in molecular plant pathology. Molecular Plant Pathology, 13(4), 414–430. https://doi.org/10.1111/j.1364-3703.2011.00783.x
- Dreher, M. L., & Davenport, A. J. (2013). Hass avocado composition and potential health effects. Critical reviews in food science and nutrition, 53(7), 738-750.
- El Ghaouth, A., Wilson, C. L., Wisniewski, M., Droby, S., Smilanick, J. L., & Korsten, L. (2002). Biological control of postharvest diseases of citrus fruit. Biological Control of Crop Diseases.Mercel Dekker, Newyork, 289-312
- European Food Safety Authority EFSA. (2013). Reasoned opinion on the review of the existing maximum residue levels (MRLs) for guazatine according to Article 12 of Regulation (EC) No 396/20051. EFSA Journal, 11(5), 3239. https://doi.org/10.2903/j.efsa.2013.3239
- Evans, H. C., Greaves, M. P., & Watson, A. K. (2001). Fungal biocontrol agents of weeds. Fungi as Biocontrol Agents, 169–192. CABI Publishing, Wallingford, UK
- Everett, K. R., Owen, S. G., & Cutting, J. G. M. (2005). Testing efficacy of fungicides against postharvest pathogens of avocado (Persea americana cv. Hass). N. Z. Plant Protection, 58, 89–95. https://doi.org/10.30843/nzpp.2005.58.4260
- Eyres, L., Sherpa, N., & Hendriks, G. (2001). Avocado oil: New edible oil from Australasia. Lipid Technology, 13(4), 84–88.
- Fallik, E. L. A. Z. A. R., & Lurie, S. U. S. A. N. (2007). Thermal control of fungi in the reduction of postharvest decay. Heat Treatment for Postharvest Pest Control: Theory and Practice
- FAO (2004). Worldwide Regulations for Mycotoxins for 2003. A Compendium of Food and Nutrition Paper No. 81.
- FAOSTAT,(2013). The Statistical Division (FAOSTAT) of the Food and Agriculture Organization of the United Nations (FAO).
- Farr, D. F., Aime, M. C., Rossman, A. Y., & Palm, M. E. (2006). Species of Colletotrichum on agavaceae. Mycology Resource, 110(12), 1395–1408. https://doi.org/10.1016/j.mycres.2006.09.001
- FDA (2014). US food and drug administration. http://www.accessdata.fda.gov/scripts
- Freeman, S. (2000). Genetic Diversity and host specificity of Colletotrichum species on various fruits. In D. Prusky, S. Freeman, & M. B. Dickman (Eds.), Colletotrichum: Host specificity, pathology, and host- pathogen interaction (pp. 133–134). American Phytopathological Society.
- Freeman, S., Katan, T., & Shabi, E. (1996). Characterization of Colletotrichum gloeosporioides isolates from avocado and almond fruits with molecular and pathogenicity tests. Applied and environmental microbiology, 62(3), 1014-1020.
- Freeman, S., Katan, T., & Shabi, E. (1998). Characterization of Colletotrichum species responsible for anthracnose diseases of various fruits. Plant Disease, 82(6), 596–605. https://doi.org/10.1094/PDIS.1998.82.6.596
- Freeman, S., & Rodriguez, R. J. (1995). Differentiation of Colletotrichum species responsible for anthracnose of strawberry by arbitrarily primed PCR. Mycological Research, 99(4), 501–504. https://doi.org/10.1016/S0953-7562(09)80653-9
- Fuentes-Aragón, D., Juárez-Vázquez, S. B., Vargas-Hernández, M., & Silva-Rojas, H. V. (2018). Colletotrichumfructicola, a member of colletotrichum gloeosporioides sensu lato, is the causal agent of anthracnose and soft rot in avocado fruits cv. “Hass”. Mycobiology, 46(2), 92–100. https://doi.org/10.1080/12298093.2018.1454010
- Gamage, T. V., & Rehman, M. S. (1999). Post- harvest handling of foods of plant origin. In M. S. Rehman (Ed.), Handbook of food preservation (pp. 11–46). Marcel Dekker Inc.
- Gañán, L., Álvarez, E., & Castaño Zapata, J. (2015). Genetic identification of Colletotrichum spp. anthracnose causing in avocado, banana, mango and tree tomato fruits. Rev. Acad. Colomb. Science. Ex Fis. Nat., 39(152), 339–347. https://doi.org/10.18257/raccefyn.192
- Garrido, C., Carbú, M., Fernández-Acero, F. J., Budge, G., Vallejo, I., Colyer, A., & Cantoral, J. M. (2008). Isolation and pathogenicity of Colletotrichum spp. causing anthracnose of strawberry in south west Spain. European Journal of Plant Pathology, 120(4), 409–415.
- Giblin, F. R., Coates, L. M., & Irwin, J. A. G. (2010). Pathogenic diversity of avocado and mango isolates of Colletotrichum gloeosporioides causing anthracnose and pepper spot in Australia. Australasian Plant Pathology, 39(1), 50-62.
- Griesbach, J. (1985). The avocado industry in Kenya. In X African Symposium on horticultural crops 158 (pp 87-92). Doi: 10.17660/ActaHortic.1985.158.9
- Griesbach, J. (2005). Avocado growing in Kenya. World Agroforestry Centre (ICRAF). Kul Graphics Ltd. Nairobi.
- Guerber, J. C., Liu, B., & Correll, J. C. (2003). Characterization of diversity in Colletotrichum acutatum sensu lato by sequence analysis of two gene introns, mtDNA and intron RFLPs, and mating compatibility. Mycologia, 95(5), 872–895. https://doi.org/10.1080/15572536.2004.11833047
- Harp, T. L., Pernezny, K., Ivey, M. L. L., Miller, S. A., & Kuhn, P. J. (2008). The etiology of recent pepper anthracnose outbreak in Florida. Crop Protection, 27(10), 1380–1384. https://doi.org/10.1016/j.cropro.2008.05.006
- Hartill, W. F. T., & Everett, K. R. (2002). Inoculum sources and infection pathways of pathogens causing stem‐end rots of ‘Hass’ avocado(Persea Americana). New Zealand Journal of Crop and Horticultural Science, 30(4), 249-260.
- Hernandez, G. 2011. Production and trade forecast to grow GAIN Report. Foreign Agricultural Service.
- Hodson, A., Mills, P. R., & Brown, A. E. (1993). Ribosomal and mitochondrial DNA polymorphisms in Colletotrichum gloeosporioides isolated from tropical fruits. Mycological Research, 97(3), 329–335. https://doi.org/10.1016/S0953-7562(09)81130-1
- Hofman, P. J., Bower, J., & Woolf, A. (2013). Harvesting, packing, postharvest technology, transport and processing. In B. Schaffer, B. N. Wolstenholme, & A. W. Whiley (Eds.), The avocado: Botany, production and uses (2nd ed., pp. 489–540). CABI.
- Horticultural Crop Development (HCD), (2018). National Horticultural Validated Report.
- Idris, S., Ndukwe, G., & Gimba, C. (2009). Preliminary phytochemical screening and antimicrobial activity of seed extracts of Persea americana (avocado pear). Bayero Journal of Pure and Applied Sciences, 2(1), 173–176. Doi: 10.4314/bajopas.v2i1.58538
- Ippolito, A., & Nigro, F. (2000). Impact of preharvest application of biological agents on postharvest diseases of fresh fruits and vegetables. Crop Protection, 19(8–10), 715–723. https://doi.org/10.1016/S0261-2194(00)00095-8
- Isman, B. M. (2000). Plant essential oils for pest and disease management. Crop Protection, 19(8–10), 603–608. https://doi.org/10.1016/S0261-2194(00)00079-X
- Janisiewicz, W. J., & Cornway, W. S. (2010). Combining biological control with physical and chemical treatments to control fruit decay after harvest. Stewart Postharvest Reveiw, 10, 1–3.
- Janisiewicz, W. J., Tworkowski, T. J., & Kurtzman, C. P. (2001). Biocontrol potential of Metchnikowia pulcherrima strains against blue mold of apple. Phytopathology, 91(11), 1098–1108. https://doi.org/10.1094/PHYTO.2001.91.11.1098
- Kader, A. A. (1994). Modified and controlled atmosphere storage of tropical fruits. In B. R. Champ, E. Highley, & G. I. Johnson (Eds.), Postharvest Handling of Tropical Fruits (pp. 239–249). Proc. ACIAR conference.
- Kaewchai, S., K. Soytong, and K. D. Hyde. (2009). Reviews, Critiques and New Ideas.
- Kalemba, D., & Kunicka, A. (2003). Antibacterial and antifungal properties of essential oils. Current Medical Chemistry, 10(10), 813–829. https://doi.org/10.2174/0929867033457719
- Karabulut, O. A., & Baykal, N. (2002). Evaluation of the use of microwave power for the control of post harvest diseases of peaches. Post harvest Biology and Technology, 26(2),237-240.
- Kimaru, S. K., Monda, E., Cheruiyot, R. C., Mbaka, J., & Alakonya, A. (2018a). Sensitivity of Colletotrichum gloeosporioides isolates from diseased avocado fruits to selected fungicides in Kenya. Advances in Agriculture, 2018, 1–6. https://doi.org/10.1155/2018/3567161
- Kimaru, S. K., Monda, E., Cheruiyot, R. C., Mbaka, J., & Alakonya, A. (2018b). Morphological and molecular identification of the causal agent of anthracnose disease of avocado in Kenya. International Journal of Microbiology, 2018, 10. Article ID 4568520. https://doi.org/10.1155/2018/4568520
- Korsten, L., & Jeffries, P. (2000). Potential for biological control of diseases caused by Colletotichum. In D. Prusky, S. Freeman, & M. B. Dickman (Eds.), Colletotrichum host specificity, pathology and host-pathogen interaction (pp. 266–295). APS Press.
- Korsten, L., Van Harmelen, M. W., Heitman, A., & De Jager, E. (1991). Biological control of post-harvest mango diseases. South African Mango Growers Association Year Book, 11, 65–67.
- Korsten, L., Wehner, F. C., De Villiers, E. E., Kotze, J. M., & De Jagar, E. S. (1995). Evaluation of epiphytes isolated from avocado leaf and fruit surfaces for biocontrol of avocado post- harvest diseases. Plant Disease, 79(11), 1149–1156. https://doi.org/10.1094/PD-79-1149
- Latunde‐Dada, A. O. (2001). Colletotrichum: tales of forcible entry, stealth, transient confinement and breakout. Molecular plant pathology, 2(4),187-198.
- Li, H., Zhou, G. Y., Liu, J. A., & Xu, J. (2016). Population genetic analyses of the fungal pathogen Colletotrichum fructicola on tea-oil trees in China. PloS one, 11(6), e0156841.
- Lima, N. B., Batista, M. V. D. A., & De Morais, M. A. (2013). Five Colletotrichum species are responsible for mango anthracnose in north-eastern Brazil. Fungal Diversity, 61(1), 75–88. https://doi.org/10.1007/s13225-013-0237-6
- Liu, B., & Correll, J. C. (2007). Molecular tools for taxonomy of Colletotrichum species. Brazilian Phytopathology, 32S21–S24.
- Lopez, A. M. Q., & Lucas, J. A. (2010). Colletotrichum isolates related to Anthracnose of cashew trees in Brazil: morphological and molecular description using LSU rDNA sequences. Brazilian Archives of Biology and Technology, 53(4), 741-752.
- Lu, Q., Zhang, Y., Wang, Y., Wang, D., Lee, R., & GAO, K. (2009). California “Hass” avocado: Profiling of carotenoids, tocopherol, fatty acid, and fat content during maturation and from different growing areas. Journal of Agricultural and Food Chemistry, 57(21), 408–413. https://doi.org/10.1021/jf901839h
- Mills, P. R., Hodson, A., & Brown, A. E. (1992). Molecular differentiation of Colletotrichum gloeosporioides isolates infecting tropical crops. In J. A. Bailey & M. J. Jeger (Eds.), Colletotrichum: Biology, pathology and control (pp. 269–288). CAB International.
- Naveh, E., Werman, M. J., Sabo, E., & Neeman, I. (2002). Research Communication: Defatted Avocado Pulp Reduces Body Weight and Total Hepatic Fat But Increases Plasma Cholesterol in Male Rats Fed Diets with Cholesterol. The Journal of nutrition, 132(7), 2015-2018.
- Njombolwana, N. S., Erasmus, A., & Fourie, P. H. (2013). Evaluation of curative and protective control of Penicillium digitatum following imazalil application in wax coating. Postharvest Biology and Technology, 77(2013), 102–110. https://doi.org/10.1016/j.postharvbio.2012.11.009
- Ogbebor, N. O., Adekunle, A. T., & Enobakhare, D. A. (2007). Inhibition of Colletotrichum gloeosporioides (Penz) Sac. causal organism of rubber (HeveabrasiliensisMuell. Arg.) leaf spot using plant extracts. African Journal of Biotechnology, 6(3), 213–218. https://www.ajol.info/index.php/ajb/article/view/56139
- Peres, N. A. R., Souza, N. L., Zitko, S. E., & Timmer, L. W. (2002). Activity of benomyl for control of postbloom fruit drop of citrus caused by Colletotrichum acutatum. Plant disease, 86(6), 620–624.
- Pernezny, K., Belle, G., & Marlatt, R. B. (2000). Diseases of Avocado in Florida. Plant Pathology Fact Sheet pp-21.
- Pest Control Products Board. (2019). List of registered pest control products in Kenya. (9th Ed).
- Pitarokili, D. O., Couladis, T. M., & Verykokidou, E. (1999). Composition and antifungal activity of essential oil of Salvia pomiferas fsp. calycina growing wild in Greece. Journal Essential Oil Research, 11(5), 655–659. https://doi.org/10.1080/10412905.1999.9701233
- Piteira, M. C. C., & Rodrigues Jr, C. J. (1999). Colletotrichum gloeosporioides Penz. of cashew (Anacardium occidentale L.). Morphocultural studies and pathogenicity tests using cross inoculations on other tropical fruits. In [New Directions in Plant Protection], Oeiras (Portugal), 24–25 Sep 1998. EAN.
- Poulivong, S., Cai, L., Chen, H., McKenzie, E. H. C., Abdelsalam, K., Chukeatirote, E., & Hyde, K. D. (2010). Colletotrichum gloeosporioides is not a common pathogen on tropical fruits. Fungal Diversity, 44(1), 33–43. https://doi.org/10.1007/s13225-010-0046-0
- Prabakar, K., Raguchander, T., Parthiban, V. K., Muthulakshmi, P., & Prakasam, V. (2005). Post -harvest fungal spoilage in mango at different levels marketing. Madras Agriculural Journal, 92(1-3), 42–48.
- Prusky, D., & Keen, N. T. (2011). Involvement of preformed antifungal compounds in the resistance of subtropical fruits to fungal decay. Plant Disease, 77(2), 114–119. https://https://www.apsnet.org/publications/plantdisease/backissues/Documents/1993Articles/PlantDisease77n02_114.PDF
- Sanders, G. M., & Korsten, L. (2003a). Comparison of cross inoculation potential of South African avocado and mango isolates of Colletotrichum gloeosporioides. Microbiological Research, 128(2), 143–150. https://doi.org/10.1078/0944-5013-00186
- Sanders, G. M., Korsten, L., & Wehner, F. C. (2000). Survey of fungicide sensitivity in Colletotrichum gloeosporioides from different avocado and mango production areas in South Africa. European Journal of plant pathology, 106(8),745-752.
- Schaffer, B. A., Wolstenholme, B. N., & Whiley, A. W. (Eds.). (2013). The avocado: botany, production and uses. CABI.
- Scheepers, S., Jooste, A., & Alemu, Z. G. (2007). Quantifying the impact of phytosanitry standards with specific reference to MRLs on the trade flow of South African avocados to the EU. Agrekon, 46(2), 260–273. https://doi.org/10.1080/03031853.2007.9523771
- Sharma, G., Maymon, M., & Freeman, S. (2017). Epidemiology, pathology and identification of Colletotrichum including a novel species associated with avocado (Persea americana) anthracnose in Israel. Scientific Reports, 7(1), 1–16. https://doi.org/10.1038/s41598-017-15946-w
- Shivas, R. G., & Tan, Y. P. (2009). A taxonomic re-assessment of Colletotrichum acutatum, introducing C. fioriniae comb. et stat. nov. and C. simmondsii sp. nov. Fungal Divers, 39(111), e122.
- Sideney, B. O., & Dirlane, A. (2014). Behavior of the fungus Colletotrichum gloeosporioides (Penz & Sacc.), which causes bitter rot in apples after harvesting. Advances in Microbiology, 4(4), 202–206. https://doi.org/10.4236/aim.2014.44026
- Silva-Rojas, H., & Avila-Quezada, G. (2011). Phylogenetic and morphological identification of Colletotrichum boninense: A novel causal agent of anthracnose in avocado. Plant Pathology, 60(5), 899–908. https://doi.org/10.1111/j.1365-3059.2011.02452.x
- Smith, L. A., Dann, B. E. K., Leonardi, J., Dean, J. R., & Cooke, A. W. (2011). Exploring on traditional products for management of postharvest anthracnose and stem end rot in avocado. V11 World Avocado Congress Home page.
- Sreenivasaprasad, S., Brown, A. E., & Mills, P. R. (1992). DNA sequence variation and interrelationships among Colletotrichum species causing strawberry anthracnose. Physiology Molecular Plant Pathology, 41(4), 265–281. https://doi.org/10.1016/0885-5765(92)90026-R
- Sreenivasaprasad, S., Mills, P. R., & Brown, A. E. (1994). Nucleotide sequence of the rDNA spacer 1 enables identification of isolates of Colletotrichum as C. acutatum. Mycological Research, 98(2), 186–188. https://doi.org/10.1016/S0953-7562(09)80184-6
- St Leger, R., Screen, S., Butt, T., Jackson, C., & Magan, N. (2001). Prospects for strain improvement of fungal pathogens of insects and weeds. Fungi as biocontrol agents: Progress, problems and potential, 219-237.
- Tapia-Tussell, R., Quijano-Ramayo, A., Cortes-Velazquez, A., Lappe, P., Larque-Saavedra, A., & Perez-Brito, D. (2008). PCR-Based Detection and Characterization of the Fungal Pathogens Colletotrichum gloeosporioides and Colletotrichum capsici Causing Anthracnose in Papaya (Carica papaya L.) in the Yucatan Peninsula. Molecular Biotechnology, 40(3), 293–298. https://doi.org/10.1007/s12033-008-9093-0
- Than, P. P., Shivas, R. G., Jeewon, R., Pongsupasamit, S., Marney, T. S., Taylor, P. W. J., & Hyde, K. D. (2008a). Epitypification and phylogeny of Colletotrichum acutatum J. H. Simmonds. Fungal Diversity, 28, 97–108.
- Velázquez-del Valle, M. G., Campos-Martínez, A., & Flores-Moctezuma, H. E. (2016). First report of avocado anthracnose caused by Colletotrichum karstii in Mexico. American Phytopathological Society, 100(2), 534. https://doi.org/10.1094/PDIS-03-15-0249-PDN
- Vidyalakshni, A., & Divya, C. V. (2013). New report of Colletotrichum gloeosporioides causing anthracnose of Pisonia alba in India. Archieve Phytopathology Plant Protection, 46(2), 201–204. https://doi.org/10.1080/03235408.2012.736281
- Wasilwa, L. A., Njuguna, J. K., Okoko, E. N., & Watani, G. W. (2006). Status of avocado production in Kenya.
- Yahia, E. M. (2011). Mango (Mangifera indica L.). In E. M. Yahia (Ed.), Postharvest biology and technology of tropical and subtropical fruits cocona to mango (Vol. 3, pp. 492–550). Amsterdam.
- Yakoby, N., Beno-Moualem, D., Kobiler, I., & Prusky, D. (2002). The analysis of fruit protection mechanisms provided by reduced-pathogenicity mutants of Colletotrichum gloeosporioides obtained by restriction enzyme mediated integration. Phytopathology®, 92(11), 1196–1201 doi:10.1094/PHYTO.2002.92.11.1196
- Zentmyer, G. A. (1994). Avocado. In Compendium of tropical fruit diseases (pp. 71–84). APS Press.