Abstract
The use of entomopathogenic fungi (EPF) like Beauveria, Metarhizium, Lecanicillium, and Isaria is upsurging in recent years for the management of crop insect pests. EPF are considered better than synthetic insecticides as they are safe for humans, sustainable to the environment, and target-specific in nature. Many of these EPF are pathogenic to economically important insect pests and thus are capable of controlling them. They are cheaper in long run, show lesser residual effects, and are able to overcome the problem of resistance. EPF degrades the host cuticle and proliferates in hemolymph as hyphal bodies, secreting the toxins responsible for the death of host insects. The later saprophytic growth leads to the production of fungal spores capable of reinfecting other hosts. Different commercial formulations of EPF are available globally such as liquid formulation, wettable powder, suspensible granules, and so on. These available formulations under different trade names can be used for several crops and pests at the recommended dosage to obtain optimum results. The storage conditions should be maintained to retain the viability of EPF. Modern biotechnological interventions could be vital in enhancing the efficacy of these entomopathogens by manipulating their traits. Specialized researches are necessary to understand the interaction between EPF, host insects, crops, and their environment in order to explore the best formulation of mycoinsecticides. This review explores the overview of EPF, its mode of action, significance, commercial formulations, future prospects, and the summary of recent findings. Readers could realize the essence of EPF in sustainable agriculture through this review.
PUBLIC INTEREST STATEMENT
Entomopathogenic fungi (EPF) are capable of causing diseases and subsequently killing the insects harbouring these pathogenic fungi. This concept has been utilized in the control of insect pests damaging important crop species. They can attack only the targeted insects leaving the rest unharmed. In addition, they are admired for their lesser toxicity and lower residual effects on animal health and the environment. Farmers and researchers are interested in the utilization and exploration of EPF as an effective alternative to chemical insecticides. The regenerative capacity of EPF makes them cheap in long run. At present, global research is directed toward exploring the interaction among EPF, the environment, and the host insects. This would be monumental in finding the right EPF against a particular insect and its effective formulation for commercialization. The recent advancements in biotechnology would be a centrepiece in improving strains of EPF and increasing their virulence i.e. disease-causing ability. It demands interdisciplinary approaches and collaboration between the authoritative bodies, primarily the research organizations.
1. Introduction
Insects constitute a diverse group of animals with over a million documented species. The majority of these are beneficial for humans, but a fraction of them are damaging as they affect food production, health, and human welfare. Herbivorous insects account for 18% of the damage to the world’s agricultural production (Jankielsohn, Citation2018). A significant amount of time and effort is needed for their management. Every year, over two billion tons of pesticides are used worldwide, which include bactericides, fungicides, herbicides, and insecticides (Baron et al., Citation2019). The use of synthetic pesticides is detrimental to the environment since it contaminates water bodies and soil. Chemical pesticides pose a great threat to the ecosystem as insects could develop resistance to them in the long run. The use of broad-spectrum insecticides hampers insects such as predators of crop pests which are non-targeted and beneficial in nature (Sandhu et al., Citation2012; Sinha et al., Citation2016). Scientists are now focusing on the development of controlling techniques that are safer and more eco-friendly. Biological control of insect pests is one of the most successful strategies that include the use of predators, parasitoids, or pathogens to manage insects. Insect pathogens (entomopathogens) comprise viruses, bacteria, fungi, protists, or nematodes that infect and ultimately kill the host insect (Silva et al., Citation2020). Myco-biocontrol refers to the extensive use of fungi to lower insect density and minimize crop losses. Over 750 species of fungi have been isolated from the insect bodies which have been placed under 100 different genera (Shin et al., Citation2020; Sinha et al., Citation2016). EPF are distributed globally in almost all terrestrial ecosystems, with the greatest diversity in tropical forests and some in extreme habitats such as the Arctic tundra (Hughes et al., Citation2004).
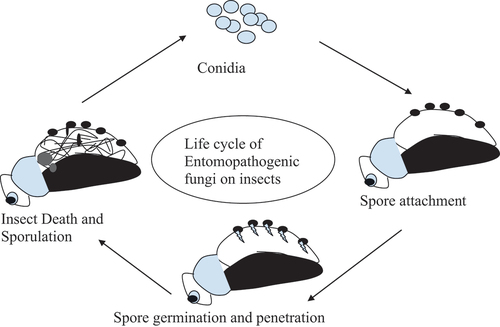
There are eight major classes of fungi, of which four classes contain EPF viz. Zygomycota, Basidiomycota, Ascomycota, and Microsporidia (Bergman et al., Citation2019). Among these, Zygomycota and Deuteromycota comprise important EPFs which have been extensively documented (Sinha et al., Citation2016). EPF can invade and propagate inside insect bodies and can be spread from one insect to another (Kaya & Vega, Citation2012). Following the contact of the spore with the host insect, EPF germinates on the cuticle of insect, penetrates the cuticle, grows further down to the inner layers, and generates the secondary conidia, which degrades the internal organs leading to the death of insect (Shin et al., Citation2020; Vega et al., Citation2012). Figure depicts the infection cycle of EPF and their rapid propagation among the host insects. Besides being pathogenic to insects, EPF can be endophytes, antagonists of plant pathogens, as well as plant growth promoters (Vega et al., Citation2008). The abundance and diversity of EPF have great prospects for sustainable insect pest management (Sinha et al., Citation2016). Regenerating capacity of EPF greatly reduces their frequency of application which results in lower cost and higher efficiency than the chemical insecticides (Gul et al., Citation2014; Mwamburi, Citation2020; Ortiz-Urquiza & Keyhani, Citation2013).
Figure 1. Infection and sporulation in host insect by entomopathogenic fungi (Modified from Sinha et al., (Citation2016).
In this review, we have explored the major entomopathogenic fungi (EPF) that are in use globally, and recent research findings related to the interaction among EPF, host insect, and the environment. This work is significant because EPF have now evolved as a sustainable alternative to chemical management. The paper comprises of an overview of EPF, its mode of action, significance, commercial formulations, future prospects, and a summary of recent research findings. It aims to enlighten a broader audience on the subject matter and spark some interest to pursue each individual topic at a deeper level.
Figure depicts the invasion of host insects by different genera of EPF. Beauveria bassiana (A), Metarhizium anisopliae (B) and Isaria fumosorosea (C) are grown on potato dextrose agar (PDA) plates. Figure D-H represent the sugarcane borer, Diatraea saccharalis colonized by B. bassiana (D and G), M. anisopliae (E and H) and I. fumosorosea (F) (Baron et al., Citation2019).
Figure 2. Effect of EPF on different host insects. (Adapted from Baron et al. (Citation2019)).

2. Trends in the use of EPF
Old Chinese texts on medicinal herbs such as “Bencao Gangmu” (from the middle ages) have records of Cordyceps sinensis infected insects (Hepialidae family of Lepidoptera order), C. sobolifera infected cicadas and B. bassiana infected silkworm (Shin et al., Citation2020). Even earlier records from ancient China (221–220 BC) show the incidents of B. bassiana infected silkworms (Li & Sheng, Citation2007). Historically, the commercial raising of insects such as silkworms (Bombyx mori) sparked an interest in EPFs like B. bassiana and M. anisopliae, which cause muscardine diseases in silkworms (Shin et al., Citation2020). The earliest systematic studies of EPF occurred in the early 1800s while developing strategies to manage the silkworm muscardine diseases in France. Beauveria bassiana (formerly Botrytis bassiana) was established as the causative agent of white muscardine disease in silkworms by Agostino Bassi in 1835. Later, it was found that fungi like B. bassiana could infect other insects, making them an alternative means of controlling insect pests (Vega et al., Citation2008). In the late nineteenth century, Russian zoologist Elie Metchnikoff began studies on the diseases of the cockchafer beetle, of which green muscardine was found to be caused by Metarhizium anisopliae (previously Entomophthora anisopliae). This fungus was later mass-produced and used against sugar beet weevil in the field by Krassilsstchik in 1888 (Griffin, Citation2007; Ownley et al., Citation2008). In the two decades (1970–1990), following the successful use of EPF, multiple companies and cooperatives stopped the commercial production of myco-biocontrols. The prime reasons for this were the loss of farmers’ confidence in such biocontrol agents and the heavy reduction in subsidies by the government e.g., China (Li et al., Citation2010). Mycoinsecticides were replaced heavily by synthetic insecticides after the 1990s due to their easy accessibility and greater affordability (Li et al., Citation2010). The use of EPF leads to prolonged pest control since the strategy depends on successive generations of fungus. This is not possible with the use of chemicals and other pest control strategies. Hundreds of products have been developed with dozens of species of entomopathogenic fungi (Faria & Wraight, Citation2007). The presence of EPF is not restricted to diseased insects only. A study conducted by Greif and Currah (Citation2007) on the body surface of 1700 arthropods showed the presence of EPF mycelia even in non-diseased insects. Detailed studies on pathogen-insect-environment interactions are required to improve the efficacy of these biocontrol agents. The enhancement of the endophytic activity of EPF can equally boost the ability of pest control (Vega et al., Citation2009).
3. EPF mechanism of action
The process of infection by EPF is initiated when the conidia get attached to the epicuticle of the host. Following the attachment, the conidia germinate and the appressoria are formed on the insect’s body. Then the mycelium passes into the procuticle and reaches the hemolymph, forming blastospores as shown in Figure . After invading (killing) the host insect, the fungus again transforms into mycelium and penetrates the cuticle. Genes used for primary penetration and invasion are reused during the later cuticle degradation. Abiotic factors such as water and wind help in the dispersal of conidia from one host to another (Shin et al., Citation2020).
Figure 3. Infection process of EPF. (Modified from Vega et al. (Citation2012), Sandhu et al., (Citation2012)).
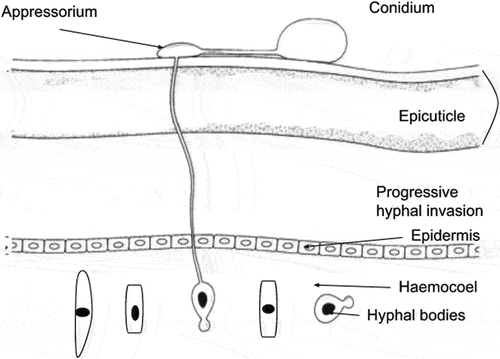
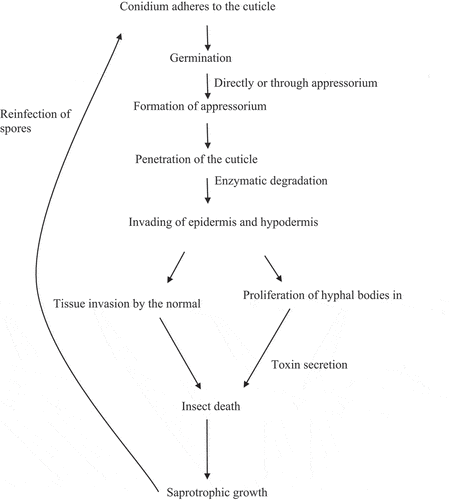
The above Figure depicts the process of infection in an insect by B. bassiana. The appressorium formed from the conidia leads to invasion of the cuticle and hyphal penetration into the haemocoel. The invasion of tissues and the proliferation of fungal hyphae lead to the eventual death of the insect (Sandhu et al., Citation2012). The following flow chart (Figure ) shows the process of infection by EPF in brief.
Figure 4. Diagrammatic representation of infection steps by EPF. (Modified from Sinha et al. (Citation2016)).
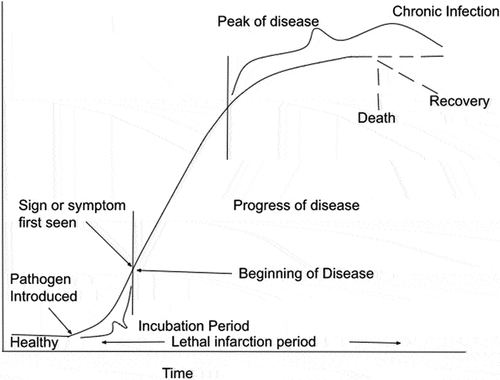
After acquiring a sufficient concentration of EPF a healthy and susceptible insect develop a sequence of events referred as the course and progress of disease as shown in figure . The intensity of the disease increases as the pathogen reproduces and produces toxins. Kaya and Vega (Citation2012) defined the period of lethal infection as the interval from the acquisition of a pathogen to the death of an insect. The effectiveness of infection depends on the lytic enzymes, secondary metabolites, and adhesion compounds produced by EPF (Litwin et al., Citation2020). Optimum level of humidity and temperature varies with the species of EPF, however for most EPF the humidity required is high i.e. 75-100% and temperature required is moderate i.e. 15-30 °C (Islam et al., Citation2021).
Figure 5. Multiplication of entomopathogens inside the body of host insect over time (Adapted from Kaya and Vega (Citation2012)).
The primary mode of use of EPF is by developing them as inundative biocontrol agents. The mechanism of insect control by EPF such as B. bassiana includes antibiosis, competition, and induced systemic resistance (Griffin, Citation2007; Ownley et al., Citation2008). EPF either kills or disables the insect pests by mycelial growth, invasion, and production of toxins. Based on the mode of infection, they may be opportunistic pathogens (which become pathogenic when host immunity is impaired) like Aspergillus flavus, potential pathogens (which have no method of invading or infecting the host but can be pathogenic once they gain entry), facultative pathogens (can infect and multiply in the host as well as outside) like B. bassiana, or obligate pathogens (which can multiply within the host body but not outside) like Microsporidia (Kaya & Vega, Citation2012). The relationships by which different fungal species obtain energy from their insect hosts (i.e., their eco-nutritional mode) include biotrophy (nutrition derived only from living cells, which ceases once the cell has died), necrotrophy (killing and utilization of dead tissues), and hemibiotrophic (initially biotrophic and then becoming necrotrophic) (Humber, Citation2008; Sinha et al., Citation2016). Beauveria (white muscardine) and Metarhizium (green muscardine) are the most extensively studied EPF genera while the greatest diversity of fungal pathogens is documented in scale insects like Coccidae and Aleyrodidae (Humber, Citation2008).
Table shows the major toxins produced by important EPF species. These toxins have a specific chemical structure, distinct site of action and a unique mode of action within the insect’s body. Some toxins like Beauvercin obtained from Beauveria bassiana are the lactone trimer with cytotoxic effects whereas Destruxins produced by Metarhizium anisopliae are cyclic depsipeptides with immunodepressant effects on host insects.
Table 1. Toxins produced by different entomopathogenic fungi (EPF)
Bojke (Citation2018) conducted a qualitative and quantitative analysis on seven different species of major EPF and identified 63 different volatile (primarily of the following groups: aldehydes, ketones, alcohols, acids, esters, and terpenes) (Bojke et al., Citation2018). Similarly, toxic compounds like beauvericin, destruxin, and bassianolide were reported from Beauveria and Metarhizium (Weng et al., Citation2019). The secondary metabolites of I. fumosorosea include the mycotoxins of non-ribosomal peptides (NRPs) (beauvericin and beauverolides), terpenes (trichocaranes and fumosorinone), lactone compounds (cepharosporolides), acids (dipicolinic acid and oxalic acid), etc. Whereas, the secondary metabolites of I. farinose include NRP mycotoxins (cycloaspeptides) and the terpene compounds (farinosones and militarinones) (Weng et al., Citation2019).
4. Host response to fungal invasion
There are various methods of fungus resistance by insects, viz. cuticular, humoral, and cellular defenses. The primary barrier offering resistance to fungus is the cuticle which contains fungistatic fatty acids, phenol oxidases and pigments like melanin (Dubovskiy et al., Citation2013). Even if the pathogen breaks the cuticular barrier, it still has to encounter the host’s cellular and humoral defenses. The components like hemocytes help in the fungal encapsulation that is large enough for phagocytosis. The humoral elements responsible for pathogenic defense are phenoloxidase, reactive oxygen species, and other antimicrobial peptides (Yan et al., Citation2005). Dubovskiy et al. (Citation2013) and Srygley (Citation2012) reported that the darkened cuticle gives some degree of intrinsic resistance against fungal growth in some melanic insects. The damage done by the pathogens can be limited by the repair and stress pathways in insects or by their immune response. However, the link between the remedial pathways and immune response for limiting pathogens is still unclear (Chakrabarti et al., Citation2012). During infection by certain EPFs, it has been observed that the insects direct their attention to targeted responses (in cuticle and epidermis) rather than the switching-on of the systemic immune defences. However, the responses are selective and depend on the types of EPF involved (Dubovskiy et al., Citation2013).
5. Bodyguard hypothesis
In the course of evolution, plants have developed mechanisms to favour the natural enemies of their insect pests, which protect them from consequential damage. This phenomenon is explained by the bodyguard hypothesis (Elliot et al., Citation2000). Such natural enemies include insect parasitoids, predators, and entomopathogens (fungi, bacteria, and viruses). The volatiles from healthy plant suppresses conidiation of EPF like Neozygites tanajoae whereas the volatiles from herbivore-damaged plants promote the growth of N. tanajoe (Agrawal et al., Citation2015). Multiple studies have shown the greater presence of M. anisopliae in the rhizosphere region than in bulk soil, indicating the potential co-evolutionary relation (St. Leger, Citation2008). It is not clear whether plants supply nutrition to EPF, however, it seems to have an evolutionary advantage for the plants to combat insect pests (Roy et al., Citation2005). Some EPFs, like Pandora neoaphidis (affecting aphids), use herbivore-associated plant signals i.e., volatiles produced after the insect damage, to identify the host plant. The germination of P. neoaphidis conidia is enhanced in those plants damaged by the aphids (Baverstock et al., Citation2005; Roy et al., Citation2005). The production of such signalling volatiles is not restricted to the foliar region but is observed in the stem and roots as well. Herbaceous plants harbour a wide range of endophytic fungi, which can be both entomopathogenic and non-entomopathogenic. Even if most of the fungi are non-entomopathogenic, they show negative effects on the growth of herbivorous insects. They reduce the growth and performance of polyphagous and sucking insects but not those of monophagous insects. This can be attributed to the better adaptation of monophagous insects to secondary metabolites produced by fungi (Gange et al., Citation2019).
6. Major entomopathogenic fungi
6.1. Beauveria spp. (White muscardine fungus)
Beauveria bassiana is the asexual form (anamorph) of Cordyceps bassiana fungus, which is the sexual reproducing form (teleomorph). It is an important EPF which belongs to the class Deuteromycetes. It consists of several species like B. bassiana, B. amorpha, B. brongniartii, and B. calendonica, of which B. bassiana is widely recognized as an insect pathogen. It causes white muscardine disease in insects like whiteflies, aphids, thrips, grasshoppers, and beetles (Sinha et al., Citation2016). However, studies have shown that many isolates of this fungus are host-specific. Once the host is infected with the fungus, a white covering of mycelium can be observed, emerging from the exoskeleton of insects and forming synnemata (the reproductive structures). The commercial formulation of B. bassiana-based myco-insecticides is comparatively more stable and effective against lepidopteran insect pests (Sandhu et al., Citation2012). Multiple reports suggest that B. bassiana, being a generalist, has no particular host preference and can be used as a broad-spectrum fungicide (Uma Devi et al., Citation2008). It has already been used successfully in crops like tomato, potato, cabbage, bean, coffee and corn. Spores of the fungi are sprayed on the affected crops as wettable power or emulsified suspension which paralyses the insect pests (Sandhu et al., Citation2012).
Several studies have been conducted over the years using Beauveria fungus on different insect pests. B. bassiana (ZK-5 isolate) was found to reduce the feeding behaviour in first, second and third instars larva of Fall armyworm (Spodoptera frugiperda) (Idrees et al., Citation2021). Endophytic application of B. bassiana on corn showed reduced feeding of European corn borer (Ostrinia nubilalis) and African pink stem borer (Sesamia calamistis) (Cherry et al., Citation2004; Vega et al., Citation2008). It was also effective against pine caterpillars (Dendrolimu sp) and green leafhoppers (Nephotettix sp) (Sandhu et al., Citation2012). Vega et al. (Citation2009) reported the efficacy of fermented broth of B. bassiana against corn earworm (Helicoverpa zea) when used with its synthetic diets (Vega et al., Citation2009). SEM (Scanning Electron Microscopy) based studies in greasy cutworms (Agrotis ipsilon) larvae revealed that B. bassiana was capable of infecting it (Gabarty et al., Citation2014). Nearly 90% mortality of whiteflies (Bemisia tabaci) was observed with endophytic colonization of B. bassiana in wild tomatoes, while the efficacy was less with leaf surface exposure only (Qayyum et al., Citation2021). In a study by Akmal et al. (Citation2013), B. bassiana was found to be effective against adults of different aphid species: Schizaphis graminum, Brevicoryne brassicae, Rhopalosiphum padi and Lipaphis erysimi, under laboratory conditions at a concentration ranging from 106 to 108 spores/ml. According to a recent study, B. bassiana isolates were also effective against stored pests of wheat such as lesser grain borer (Rhyzopertha Dominica), granary weevil (Sitophilus granarius), red flour beetle (Tribolium castaneum) and khapra beetle (Trogoderma granarium) (Wakil et al., Citation2021). B. bassiana is also reported to be effective against damping-off pathogens like Rhizoctonia solani and Pythium myriotylum, as it has antagonistic effects associated with endophytic colonization (Ownley et al., Citation2008). Posada et al. (Citation2007) reported the capacity of B. bassiana to establish itself as an endophyte in coffee seedlings after the foliar sprays, stem injections and soil drenching of the fungal spores. They also demonstrated that the Beauveria fungus can move throughout the plant since the fungus was successfully recovered from the sites distant from the site of inoculation (Posada et al., Citation2007). Sharma et al. (Citation2021) pointed out the increased levels of two monoterpenes and three sesquiterpenes following the application of B. bassiana in tomatoes responsible for defending the plant against herbivorous insects. Solid-state fermentation is preferred for mass production of Beauveria to yield hydrophobic aerial conidia which is the primary active ingredient of the formulation (Mascarin & Jaronski, Citation2016).
Resistance of insects to entomopathogens is rare. However, micro-evolutionary adaptations and selection pressure against B. bassiana by the greater wax moth (Galleria mellonella) [25th generation larvae] led to the development of resistance. However, the resistance was restricted to B. bassiana and it was absent against M. anisopliae (Dubovskiy et al., Citation2013). The B. bassiana fungus was found to be compatible with certain synthetic insecticides and incompatible with the rest. For instance, the synthetic insecticide flufenoxuron was reported to be incompatible due to its inhibitory effect on the growth and development of fungus. In the same paper, another insecticide, imidacloprid, was reported to be compatible with the B. bassiana fungus (Alizadeh et al., Citation2007). More specialized research is required to understand the interaction of entomopathogenic fungi with chemical insecticides.
6.2. Metarhizium spp. (Green muscardine fungus)
Metarhizium anisopliae is a well-studied soil-inhabiting fungus, having a worldwide distribution ranging from the arctic to the tropics (Senthil-Nathan, Citation2015). It is commonly referred to as the green muscardine fungus. It was originally described by Metschbikoff in 1879 as Entomophthora anisopliae and was later transferred to the Metarhizium genus (Sorokin, Citation1883). Metarhizium has a more restricted host range than Beauveria. The fungus can infect the soil-inhabiting insects [over 200 insect species in 7 orders], producing lethal effects. The susceptible insect hosts of Metarhizium include beetles [coleopteran order, primarily scarabidae family], root weevils, flies, thrips, and gnats (Azizoglu et al., Citation2020; Senthil-Nathan, Citation2015). It is probably best understood EPF at the molecular level with respect to its virulence and host specificity. It produces conidia in chains and sticks together more or less closely to conidial columns. Conidia of Metarhizium fungi adhere to the upper 20 cm of soil and can persist for up to 34 years. The toxins produced by the Metarhizium, such as destruxins, don’t affect plant and non-targeted species but only act upon the targeted insect pests (Roberts & St Leger, Citation2004). M. acridum was reported to be effective against locusts and grasshoppers. It has been used successfully in countries like Mexico, Australia, and Africa under different trade names (Greenguard and Green Muscle) (Niassy et al., Citation2011). Earlier instars of locusts were more susceptible to Metarhizium than later nymphal instars (Klass et al., Citation2007; Valizadeh et al., Citation2011). M. anisopliae is a generalist and can also control insects like termites, mosquitoes, and ticks (Aw & Hue, Citation2017). The commercial and indigenous strains of Metarhizium fungus were found to be effective against white grubs (Bohara et al., Citation2018). Also, the pod borer (Helicoverpa armigera) in chickpeas was effectively controlled using M. anisopliae in a study conducted by Rijal and Godfrey (Citation2018). It was effective against Ophiostoma ulmi (Ceratocystis ulmi), which causes Dutch elm disease. A better understanding of ecological interactions is required to develop effective strains against root-damaging insects (Bruck, Citation2009). Unlike B. bassiana, M. anisopliae has shown pathogenicity towards natural enemies of insect pests such as green lacewing (Chrysoperla carnea) and Mirid bug (Dicyphus tamaninii) (Thungrabeab & Tongma, Citation2007). The efficacy of the fungus depends on the application time of conidia, the temperature during application, the strain of the fungus, the culture medium, and the method of application (Guerrero-Guerra et al., Citation2013; Niassy et al., Citation2011).
Multiple factors (physical, chemical, and biological) influence the growth and development of Metarhizium fungus, like temperature, light, pH, the toxicity of metals, nutrient availability, and reactive oxygen species (ROS). The virulence of the fungus was found to be reduced when subjected to UV light and temperature extremities (Wang et al., Citation2019). The fungus was effective against some phytopathogenic fungi as well, while it was suppressed by the other fungi. The South Asian region has a greater distribution of different strains of Metarhizium, showing greater potential for exploitation (Bidochka & Small, Citation2005). Precautions are necessary to avoid direct conidial contact with skin and its inhalation when applied, as it may lead to allergies in animals, including humans (Zimmermann, Citation2008). Newer studies are concentrated on speeding up the mode of action of Metarhizium fungus through genetic engineering (Aw & Hue, Citation2017).
6.3. Verticillium lecanii/ Lecanicillium spp.
The Lecanicillium genus comprises important insect pathogenic fungi which were formerly classified under a single species, viz. Verticillium lecanii (Faria et al., Citation2010). They fall under the fungal order Hypocreales and are described as the anamorphic (asexual) form of Cordyceps group in Clavicipitaceae. The earlier genera (Verticillium) had several hosts such as insect pests, nematodes and fungi whereas the new genera Lecanicillium exclusively comprises insect pathogenic fungi (Zare & Gams, Citation2001). The fungus Verticillium lecanii is one of the members of Deuteromycetes which can be used for crop protection. V. lecanii lacks a sexual phase (perfect stage) and reproduces utilizing non-motile, asexual spores called conidia. Germination of these conidia produces hyphae. After subsequent growth, these hyphae produce conidiophores which finally produce conidia (AlAVO, Citation2015). The major species of Lecanicillium fungi include L. lecanii, L. attenuatum, L. longisporum, L. nodulosum and L. muscarium. The fungus is effective against several insect pests (especially the sucking type) like aphids, whiteflies, mealybugs and scales. Lecanicillium follows a similar mode of infection as Beauveria and Metarhizium fungi (Faria et al., Citation2010).
Lecanicillium fungi are found in different environments such as soil, animal bodies and fungal materials (Chen et al., Citation2017). The efficacy of Lecanicillium spp as EPF depends on the optimum temperature and relative humidity under the field condition (Reddy, Citation2020). Goettel et al. (Citation2008) reported the potential of Lecanicillium spp. to develop as a single microbial control agent for multiple insect pests (aphids, mealy bugs, scale insects and whiteflies), plant pathogenic nematodes and plant pathogenic fungi. L. attenuatum was found to be effective against the Asian Tiger mosquito (Aedes albopictus) and Diamondback moth (Plutella xylostella) (Woo et al., Citation2020). Whitefly of tomato and cucumber was found to be controlled by Lecanicillium spp. hybrid strain 2aF43 (Aiuchi et al., Citation2011). Some strains of the fungi L. psalliotae were reported to associate with the cardamom plant for the pathogenicity against insect pests such as thrips. L. psalliotae was found to respond to the volatile compounds produced by cardamom following the insect feeding and damage (Nicoletti & Becchimanzi, Citation2020). According to Vandermeer et al. (Citation2009), L. lecanii has an ability to hyperparasitize the pathogen of coffee leaf rust i.e. Hemileia vastatrix fungus. Lecanicillium hybrids, produced more recently by protoplast fusion, have shown greater virulence and an increased host range, viz., aphids to whiteflies (Su et al., Citation2019; Xie et al., Citation2019). Different Lecanicillium isolates were found to be less virulent against whitefly of tomato plants at greater temperature. Temperature greater than 32 °C was detrimental suggesting appropriate selection of fungal isolates against environmental stresses prior to the commercial formulations (Rivas et al., Citation2014). Advanced biotechnological studies are underway to explore the further interactions and utility of Lecanicillium as an effective entomopathogen.
6.4. Isaria spp.
The Isaria genus comprises the important EPFs which were earlier known as Paecilomyces. The major species of Isaria include Isaria farinosa and Isaria fumosorosea (Zimmermann, Citation2008). These fungi have a wider host range and primarily infect the lepidopteran insects. The fungus invades the reproductive tissues of host insects during infection (Gao et al., Citation2017). I. fumosorosea is referred to as a species complex. Its different strains have shown efficacy against whiteflies, thrips, aphids, termites and some dipterans. There is limited information on the toxins and metabolites produced by Isaria species, unlike Beauveria and Metarhizium. The first metabolite to be isolated from I. fumosorosea was Beauvericin (Zimmermann, Citation2008).Over 80% mortality of Greenish silk-moth (Ocinara varians) was reported when the larva was dipped in the fungal spore suspension (107 spores/ml) (Hussain et al., Citation2009). The metabolites of I. fumosorosea were effective against Cutworms (Spodoptera litura) (Vinayaga Moorthi et al., Citation2015). The enzymes isolated from the I. fumosorosea viz. chitosanase, chitinases, and lipase were reported to reduce the feeding rate and weight of Diamondback moth (Plutella xylostella) larva (Ali et al., Citation2010). The pupation rate and the rate of adult emergence were also controlled with the cultured filtrates of the fungus (Ali et al., Citation2010). Xu et al. (Citation2017) reported the potential of I. fumosorosea to suppress the immune response of Diamondback moth thereby controlling the infestation of insect pests (Xu et al., Citation2017). The Texas isolates of the fungus caused upto 95% mortality of Asian citrus psyllid (Diaphorina citri). It also checked the spread of huanglongbing disease (citrus greening) since citrus psyllid is the vector of the disease (Avery et al., Citation2011; Moran et al., Citation2011). Bugti et al. (Citation2018) reported I. fumosorosea strain (Ifu13a) as an effective biocontrol agent for sap-sucking insect pests like tea jassid (Jacobiasca formosana), Cotton aphid (Aphis gossypii), tobacco whitefly (Bemisia tabaci) and pear lace bug (Stephanitis nashi). The mortality rate ranged between 81 to 100% when used at the concentration of 108 conidia/ml (Bugti et al., Citation2018).
I. fumosorosea was found to be effective against subterranean termites like Coptotermes curvignathus and Coptotermes gestroi of the Rhinotermitidae family which damage living trees, woods and other cellulosic materials (Jessica et al., Citation2018). Another strain of the fungus Ifu TR-78-3 controlled the bark beetle (Anisandrus dispar) and Ambrosia beetle (Xylosandrus germanus) (Kushiyev et al., Citation2018). The pupal emergence of apple maggot (Rhagoletis pomonella) was checked (3%) with the combination of I. javanica and entomopathogenic nematode (Steinernema riobrave) (Usman et al., Citation2020). Whitefly (Bemisia tabaci) was controlled with the combination of I. fumosorosea (IfB01 strain; 2.5 × 106 conidia/mL) and Imidacloprid (12.5 mg/L) (Zou et al., Citation2014). D’Alessandro et al. (Citation2011) reported the use of I. fumosorosea with propamocarb and Trichoderma harzianum as appropriate combination for the IPM programs against Greenhouse whitefly (Trialeurodes vaporariorum) (D’Alessandro et al., Citation2011).
The demerit of using Isaria as a pest control measure is that it affects non-targeted insects. I. farinose was infective to silkworm (Bombyx mori), peteromaids (Dibrachys affinis), honeybee (Apis mellifera) and ground beetle (Broscus cephalotes) (Zimmermann, Citation2008). The occurrence of resistance was negligible with the application of Isaria fungi. For instance, five generations of whiteflies were unable to show resistance to fungus even upon the continuous application (Gao et al., Citation2017). The recent studies of EPFs are focused on improving the efficacy of EPFs through induced mutations, viz. ion beam and gamma rays (Shinohara et al., Citation2013).
6.5. Miscellaneous
The Entomophthorales fungal order consists of some important EPFs such as Entomophthora (attacking aphids), Conidiobolus (attacking aphids), Erynia (infecting aphids), Zoophthora (effective against aphids, beetles, and caterpillars) and Entomophaga (effective against caterpillars and grasshoppers). They are found primarily in temperate climates and less in the subtropics and tropics. Application of Zoophthora radicans was effective against exotic aphids, whereas that of Entomophaga maimaiga was found effective against gipsy moths (Georgiev et al., Citation2013; Nielsen & Wraight, Citation2009). These fungi flourish more on biotrophs since they can obtain nutrients from them and later sporulate from the bodies of dead insects. Due to slow biotrophic growth, special growth media are required for their culture (Gryganskyi et al., Citation2017). E. muscae is pathogenic to flies, killing them in 5–7 days. A strange behaviour (extended phenotype) was observed in the flies infected with E. muscase in which flies crawled to the highest point and spread their wings and legs, ensuring effective dispersal of fungal spores. Adult Mediterranean fruit flies (Ceratitis capitata) were found to be infected by E. muscae and E. schizophorae (Uziel et al., Citation2003). In a recent study conducted by Becher et al. (Citation2018), E. muscae was reported to be infective against spot-winged Drosophila (Drosophila suzukii) (Becher et al., Citation2018).
Hirsutella is another EPF with three important species, viz., H. gigantea, H. thompsonii, and H. citriformis (Reddy et al., Citation2020). It forms a greyish-yellow mycelium with a lesser number of conidia (Quesada-Sojo & Rivera-Méndez, Citation2016). Due to the huge number of species, greater variability, and shorter shelf-life, the fungi of these genera are difficult to identify. This fungus is effective against adults and nymphs of the red spider mite, the varroa mite, and the coconut eriophyid mite. They enter the host through the legs of insects. Hirsutella sp. is reported to be harmless to non-target organisms (Reddy et al., Citation2020). Another EPF, Neozygites sp belongs to the Neozygitaceae family of Entomopthorales fungal order. Neozygites floridanais is a mite pathogenic fungus capable of controlling cassava spider mites (Mononychellus tanajoaI) (Elliot et al., Citation2002). Mite infection by Neozygites follows a captivated biological mechanism. The male green spider mite prefers the Neozygites floridana-infected female than the healthy living female. The inability of males to avoid N. floridana leads to the transmission of fungal infection to mite colonies due to their fatal attraction. Also, the male doesn’t guard the body of a dead female (with conidial infection), which opens the space for other males to get infected. This is an example of parasitic manipulation of the host (Trandem et al., Citation2015). Nomuraea rileyi is an effective EPF, tested successfully against insect pests like tobacco cutworm, cotton bollworm, cabbage loopers, velvet bean caterpillars, and soybean loopers. The fungus is infective against all the life stages of the tobacco cutworm. However, the efficacy decreases as the development of insects progress (Sahayaraj & Namasivayam, Citation2008). Another fungal genus, Tolypocladium (T. extinguens), has been reported to be pathogenic against mosquito larvae and glowworms, while T. guangdongense possesses some medicinal properties and is edible (Zhang et al., Citation2020).
In an experiment conducted in China, L. lecanii and M. anisopliae were found to kill over 80% of Citrus red mite (Panonychus citri) after 9 days of fungal exposure while B. bassiana and L. lecanii were effective at minimum dosage after 9 days of inoculation (Qasim et al., Citation2021). B. bassiana (ZK-5 isolate), Penicillium citrinum (CTD-24 isolate) and Cladosporium tenuissimum (SE-10 isolate) were found to be effective against fall armyworm (S. frugiperda) from early egg stage to the third instar larva (Idrees et al., Citation2021). Qasim et al. (Citation2020) isolated 17 different toxin compounds (alkaloids, peptides and polyketides) from Cordyceps (Isaria) fumosorosea (an important EPF) of which Bassianolide killed over 70% of citrus psyllids (Diaphorina citri) within 2 days of application. B. bassiana (IBCB-240 strain) and M. anisopliae (IBCB-364 strain) were reported to be lethal against Eucalyptus snout beetle (Gonipterus platensis) which has been a major problem in Brazil (Jordan et al., Citation2021). New studies are focused on the management of insect-pests threatening plants with high commercial values.
7. Commercial production of EPF
According to a recent report, out of 171 commercial EPF products, majority of them were based on B. bassiana (33.9 %), M. anisopliae (33.9 %), I. fumosorosea (5.8%) and B. brongniartii (4.1%) (Islam et al., Citation2021). The EPFs such as Beauveria, Metarhizium, Lecanicillium, and Isaria, are abundant on a commercial scale due to the ease of mass-production. The major factors to be considered for commercial production include ecological interactions, economic viability, greater stability and better efficiency (Humber, Citation2008). The major constituents of EPF based biopesticides are active ingredients (conidia/ dry spores), surfactants, carriers, UV protectants, and stickers. The quality of the product depends primarily on the viability of conidia (spores) (Islam et al., Citation2021). The commercial production of EPF begins with isolate selection, followed by production and formulation. In addition to these, confirmation of the best application method, assurance of safety and product registration is required. Isolate selection is based on laboratory bioassays using cultured insects. Selection is done for various conditions that include soil chemistry, light, and pH of the medium. It is followed by in-vivo cultivation. The major production systems of EPF include submerged (liquid) fermentation, surface cultivation and biphasic fermentation. The method of application influences the degree of effectiveness of EPF. For instance, the endophytic conidial application of B. bassiana has greater efficacy against Tuta absoluta (leaf miner) than the direct foliar spray (El-Deeb et al., Citation2012). Russo et al. (Citation2019) reported the lesser efficacy of seed treatment in terms of endophytic colonization, with a conidial suspension of B. bassiana in tobacco, corn, soybean, and wheat. Specialized research is needed to find the best method of applying the newly developed EPF formulation. The timing of application is equally important for maintaining the efficacy of EPF, which includes optimum humidity, light and temperature, in addition to the insect’s susceptible stage (McKinnon et al., Citation2018; Vega, Citation2018). The EPF formulations should be host specific and safe for non-targeted species. For instance, the Beauveria-based mycoinsecticides shouldn’t threaten silkworm production (Vega et al., Citation2009). The registration of the mycoinsecticide requires studies on efficacy, toxicity and harm to non-targeted studies. The major problems with this process are elevated cost, procedural complexity and inconsistency of registration between the countries (Thomas, Citation2000).
Table presents a brief summary of major EPF genera used globally, their host insects, commercial names and formulation of EPF, and the crops on which they are primarily used.
Table 2. Major EPF (Entomopathogenic Fungi) formulations at commercial scale globally
Oliveira et al. (Citation2018) reported the enhancement in temperature tolerance of fungus with emulsifiable oil-based formulations. Similarly, the addition of vegetable oils to the conidia of EPF showed increased protection against harmful UV rays (Kaiser et al., Citation2019). Significant improvements in self-life, spore desiccation and stress-bearing ability are observed with cryopreservation (low-temperature storage) and oil formulations. However, spore germination and spore adhesion are reduced in the process, thereby decreasing the overall efficacy of EPF (Vega et al., Citation2009). The virulence of EPF is affected by several biotic and abiotic factors. For instance, exposure to high levels of UV radiation, lesser relative humidity and unfavorable temperature (< 20 °C and > 35 °C) reduce the virulence of EPFs. Conidia or mycelial forms of EPF are used for the application which later sporulates. The insecticide resistance to synthetic chemicals can be managed by combining them with the right EPF (Usta, Citation2013).
8. Mode of application of commercial formulation
Inoculation methods of EPF in plants include leaf spraying, injection through the stem, seed treatment and soil irrigation (Quesada-Moraga et al., Citation2006). The spray of spore suspensions is an effective mode of application of EPF for controlling insect pests in the phylloplane region (leaf surface) (Vega et al., Citation2009). Endophytic application of EPF controls the insects that perforate and feed inside the leaves, roots, stems, seeds, and rhizomes (Resquín-Romero et al., Citation2016). Foliar spray of EPF like Metarhizium brunneum and Beauveria bassiana has been found to temporarily colonize plants like alfalfa, tomato, sweet pepper, and melons, endophytically (Jaber & Araj, Citation2018).The endophytic presence of EPF in tomato plants through artificial inoculation showed effective control of Tuta absoluta (Leaf miner) (Klieber & Reineke, Citation2016; Resquín-Romero et al., Citation2016). The shelf life of most of the EPF formulations currently available on the market ranges from three to six months. The concentration of fungal spores in these formulations is in the order of 109 to 1010 spores per gm. The dose of application varies according to the formulation, severity of the infestation, type of insect and environmental conditions. Detailed instructions regarding the usage can be found on the label of the mycoinsecticide.
9. Advantages and constraints of EPF
The EPFs have several advantages over conventional methods of insect pest control. These fungi have high insecticidal effectiveness, so are being increasingly used in organic farming (Litwin et al., Citation2020). Most of the EPF have limited growth outside the body of the insect (host), so they do not disturb the content of soil like carbon and nitrogen (Ownley et al., Citation2008). EPF products are safe for humans, harmless to non-target organisms and environmentally sustainable. So, these could be effective alternatives to chemical insecticides for the IPM programme. EPFs are environmentally sustainable as they pose minimal negative effects on the environment (Sandhu et al., Citation2012). Some of the EPF could be combined with synthetic insecticides and are able to produce synergistic effects (Islam et al., Citation2021). The use of EPF is monumental in the IPM programs since it is host-specific (maybe a single target or multi-target specific), effective in small quantities and degrades quickly. Endophytic EPF has no negative effects on the growth and development of plants; rather, they are stimulative in nature (Vega, Citation2018). Since EPF are able to self-perpetuate under optimum environment conditions, they are cheaper in long-run. Due to their unique mode of action, EPF are reported to have lesser problem of insect resistance development (Islam et al., Citation2021). In addition they reduce the reliance of farmers on the chemical insecticides.
Sometimes, the narrow host range of EPF could be a problem since the use of EPF could lead to the outbreak of secondary pests. The fungal inoculum has a short life span and needs a longer period (2–3 weeks) to control insect pests (Islam et al., Citation2021). The application of EPF would be ineffective when relative humidity is low and temperature is non-optimum. In addition to it, the cost of commercial formulations is high. There is some chance of environmental contamination with mycotoxins (such as aflatoxins, fumonisins, and citrinins) produced by entomopathogenic fungi (Sinha et al., Citation2016). In few occasions EPF can affect the non-targeted species such as predators, parasitoids, earthworms or honeybees. Some of the farmers have greater expectations with EPF as they tend to compare their efficacy with synthetic insecticides. However the unsystematic handling and lack of expertise has further decreased their efficacy, leading to unfulfilled expectations among farmers (Jaber & Ownley, Citation2018).
10. Further prospects of EPF
The use of chemicals for insect pest control was accelerated during the 1940s. However, the negative impacts of synthetic chemicals were realized, and the developed countries shifted their attention toward alternative practices such as the adoption of transgenic plants. The use of transgenic plants for resistance or the use of chemicals is not sustainable since insects are in a constant state of evolution (Wheelis, Citation2002). The global market for biopesticides is around US$ 7.7 billion with an annual growth rate of 14.1%, which is 3% of the global pesticide market (Sharma et al., Citation2021). These figures indicate the prospect of EPF as an alternative method of insect pest control. The production of EPF is based on simple techniques but is labour-intensive. Hence, it is suitable for developing countries, where cheap labour is abundant and the problem of chemical insecticides is grave. Countries like Brazil and China have successfully demonstrated the biological control of insect pests using EPF (Li et al., Citation2010). Further studies should be carried out to understand the ecology of fungi concerning their roles as endophytes, rhizosphere colonizers (the region around the roots), plant growth promoters, and plant disease suppressors. These studies could help in understanding the concrete niche of EPF within the given ecosystem. In addition, it helps in enhancing the ability of pest control by improving the production and efficacy of formulation (Vega et al., Citation2009). There is a growing body of molecular research on improving the effectiveness of insect pathogenic fungi. Future work on EPF should be directed toward the improvement of fungal strains to enhance their resistance and broaden their host range. Also, the focus should be shifted to the development of effective metabolites and the effective combination of EPF with insecticides for IPM programs (Sinha et al., Citation2016).
11. Conclusion and recommendation
The use of EPF could lead to sustainable agriculture since it poses a minimal threat to human and animal health, helps in managing pesticide resistance and is environmentally friendly. The use of EPF is cheaper than the use of chemicals as it is based on the repeated infection cycle due to its persistence in the soil. It could be an important tool for the Integrated Pest Management (IPM) programmes. Farmers should be encouraged to use mycoinsecticides for controlling the insect pests in their fields. For this, the concerned authorities (governmental and non-governmental agencies, research bodies) should educate farmers regarding the necessity and benefits of EPF and provide them with financial subsidies and technological knowledge related to handling (storage to field application). The protocols necessary for maintaining food safety and avoiding environmental hazards must be followed promptly before the application of EPF. This biological method of pest control holds immense potential with improved pathogenesis through biotechnological interventions in the upcoming years. Considering the recent developments, new EPF formulations would be of longer shelf life, non-allergic to humans and safer to non-target species. Specialized researches related to host-environment-pathogen interaction are necessary to explore the newer formulations of mycoinsecticides to combat greater numbers of insect pests and ensure optimum yield and quality of food crops. This could pave a path to sustainable agriculture and food security.
Acknowledgements
We would like to thank our parents, teachers and colleagues for their support in completion of this review article.
Disclosure statement
The authors have no known competing interest.
Additional information
Funding
Notes on contributors

Amrit Sharma
Amrit Sharma is an agriculture research scholar at Agriculture and Forestry University (AFU), Chitwan, Nepal. He had been an associate student member of NAPA (Association of Nepalese Agricultural Professionals of Americas) and has published research and review articles in international journals. He has received some grants and funding for his scientific writings and research works. He has conducted research on plant pathology and has been working with fungi like Rhizoctonia solani and Trichiderma viride. He is passionate with the studies concerning biological control of agricultural pests (pathogen and insects). Mr. Sharma has devoted his time and resources on the study of fungal entomopathogens, biological antagonism, botanical pesticides, and integrated management of agricultural pests. He likes to explore the field of biotechnology, genetics, plant pathology and entomology and wishes to further continue interdisciplinary researches. Besides the academics he is a part-time educator.
References
- Agrawal, Y., Khatri, I., Subramanian, S., & Shenoy, B. D. (2015). Genome sequence, comparative analysis, and evolutionary insights into chitinases of entomopathogenic fungus Hirsutella thompsonii. Genome Biology and Evolution, 7(3), 916–21. https://doi.org/10.1093/gbe/evv037
- Aiuchi, D., Horie, S., Watanabe, T., Yamanaka, S., & Koike, M. (2011). Biological Control of Greenhouse Whitefly, Trialeurodes Vaporariorum by Entomopathogenic Fungus Lecanicillium Spp. Hybrid Strain in Greenhouse. Biological Control of Greenhouse Whitefly, Trialeurodes Vaporariorum by Entomopathogenic Fungus Lecanicillium Spp. Hybrid Strain in Greenhouse, 66, 255–258. https://www.cabdirect.org/cabdirect/abstract/20113280327
- Akmal, M., Freed, S., Malik, M. N., & Gul, H. T. (2013). Efficacy of Beauveria bassiana (Deuteromycotina: Hypomycetes) against different aphid species under laboratory conditions. Pakistan Journal of Zoology, 45(1), 71–78. https://www.academia.edu/download/32275050/71-78__10__PJZ-963-12_22-11-12_Efficacy_of_Beauveria_bassiana__Deuteromyco_.pdf
- AlAVO, T. B. (2015). The insect pathogenic fungus Verticillium lecanii (Zimm.) Viegas and its use for pests control: A review. Journal of Experimental Biology and Agricultural Sciences, 3(4), 337–345. https://doi.org/10.18006/2015.3(4).337.345
- Ali, S., Huang, Z., & Ren, S. (2010). Production of cuticle degrading enzymes by Isaria fumosorosea and their evaluation as a biocontrol agent against diamondback moth. Journal of Pest Science, 83(4), 361–370. https://doi.org/10.1007/S10340-010-0305-6
- Alizadeh, A., Khezri, M., & Saberi Riseh, R. (2007). Compatibility of Beauveria bassiana (Bals.) Vuill. With several pesticides. International Journal of Agriculture and Biology, 9(1), 31–34. http://www.fspublishers.org/published_papers/19773_.pdf
- Altinok, H. H., Altinok, M. A., & Koca, A. S. (2019). Modes of action of entomopathogenic fungi. Current Trends in Natural Sciences, 8(16), 117–124. https://www.researchgate.net/profile/Abdurrahman-Koca-2/publication/338390298_Modes_of_Action_of_Entomopathogenic_Fungi/links/5e10f8a792851c8364b0c41f/Modes-of-Action-of-Entomopathogenic-Fungi.pdf
- Avery, P. B., Wekesa, V. W., Hunter, W. B., Hall, D. G., McKenzie, C. L., Osborne, L. S., Powell, C. A., & Rogers, M. E. (2011). Effects of the fungus Isaria fumosorosea (Hypocreales: Cordycipitaceae) on reduced feeding and mortality of the Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae). Biocontrol Science and Technology, 21(9), 1065–1078. https://doi.org/10.1080/09583157.2011.596927
- Aw, K. M. S., & Hue, S. M. (2017). Mode of infection of Metarhizium spp. Fungus and their potential as biological control agents. Journal of Fungi, 3(2), 30. Article 2. https://doi.org/10.3390/jof3020030
- Azizoglu, U., Jouzani, G. S., Yilmaz, N., Baz, E., & Ozkok, D. (2020). Genetically modified entomopathogenic bacteria, recent developments, benefits and impacts: A review. Science of the Total Environment, 734, 139169. https://doi.org/10.1016/j.scitotenv.2020.139169
- Baron, N. C., Rigobelo, E. C., & Zied, D. C. (2019). Filamentous fungi in biological control: Current status and future perspectives. Chilean Journal of Agricultural Research, 79(2), 307–315. https://doi.org/10.4067/S0718-58392019000200307
- Baverstock, J., Elliot, S. L., Alderson, P. G., & Pell, J. K. (2005). Response of the entomopathogenic fungus Pandora neoaphidis to aphid-induced plant volatiles. Journal of Invertebrate Pathology, 89(2), 157–164. https://doi.org/10.1016/j.jip.2005.05.006
- Becher, P. G., Jensen, R. E., Natsopoulou, M. E., Verschut, V., & De Fine Licht, H. H. (2018). Infection of Drosophila suzukii with the obligate insect-pathogenic fungus Entomophthora muscae. Journal of Pest Science, 91(2), 781–787. https://doi.org/10.1007/s10340-017-0915-3
- Bergman, M. E., Davis, B., & Phillips, M. A. (2019). Medically useful plant terpenoids: Biosynthesis, occurrence, and mechanism of action. Molecules, 24(21), 3961. https://doi.org/10.3390/molecules24213961
- Bidochka, M. J., & Small, C. L. (2005). Phylogeography of Metarhizium, an insect pathogenic fungus. In Vega, Fernando E., & Blackwell, M. (Eds.), Insect-Fungal Associations (pp. 28–49). Oxford University Press. https://www.cabdirect.org/cabdirect/abstract/20053125550
- Bohara, J. R., Maharjan, S., Poudel, A., Karki, K., Bist, V., Regmi, R., Marahatta, S., & Kafle, L. (2018). Efficacy of different concentration of Metarhizium anisopliae (Metsch.) Sorokin against white grub at lab condition in Chitwan, Nepal. Journal of Pharmacognosy and Phytochemistry, 7(1S), 149–153. https://www.phytojournal.com/archives/2018/vol7issue1S/PartD/SP-7-1-291.pdf
- Bojke, A., Tkaczuk, C., Stepnowski, P., & Golcebiowski, M. (2018). Comparison of volatile compounds released by entomopathogenic fungi. Microbiological Research, 214, 129–136. https://doi.org/10.1016/j.micres.2018.06.011
- Bruck, D. J. (2009). Impact of fungicides on Metarhizium anisopliae in the rhizosphere, bulk soil and in vitro. BioControl, 54(4), 597–606. https://doi.org/10.1007/s10526-009-9213-1
- Bugti, G. A., Na, C., Bin, W., & Feng, L. H. (2018). Control of plant sap-sucking insects using entomopathogenic fungi Isaria fumosorosea strain (Ifu13a). Plant Protection Science, 54(4), 258–264. https://doi.org/10.17221/118/2017-PPS
- Chakrabarti, S., Liehl, P., Buchon, N., & Lemaitre, B. (2012). Infection-induced host translational blockage inhibits immune responses and epithelial renewal in the Drosophila Gut. Cell Host & Microbe, 12(1), 60–70. https://doi.org/10.1016/j.chom.2012.06.001
- Chen, W., Han, Y., Liang, Z., & Jin, D. (2017). Lecanicillium araneogenum sp. Nov., a new araneogenous fungus. Phytotaxa, 305(1), 29–34. https://doi.org/10.11646/phytotaxa.305.1.4
- Cherry, A. J., Banito, A., Djegui, D., & Lomer, C. (2004). Suppression of the stem-borer Sesamia calamistis (Lepidoptera; Noctuidae) in maize following seed dressing, topical application and stem injection with African isolates of Beauveria bassiana. International Journal of Pest Management, 50(1), 67–73. https://doi.org/10.1080/09670870310001637426
- D’Alessandro, C. P., Padin, S., Urrutia, M. I., & López Lastra, C. C. (2011). Interaction of fungicides with the entomopathogenic fungus Isaria fumosorosea. Biocontrol Science and Technology, 21(2), 189–197. https://doi.org/10.1080/09583157.2010.536200
- Dubovskiy, I. M., Whitten, M. M. A., Yaroslavtseva, O. N., Greig, C., Kryukov, V. Y., Grizanova, E. V., Mukherjee, K., Vilcinskas, A., Glupov, V. V., & Butt, T. M. (2013). Can insects develop resistance to insect pathogenic fungi? PLoS ONE, 8(4), e60248. https://doi.org/10.1371/journal.pone.0060248
- El-Deeb, H. M., Lashin, S. M., & Arab, Y. A.-S. (2012). Reaction of some tomato cultivars to tomato leaf curl virus and evaluation of the endophytic colonisation with Beauveria bassiana on the disease incidence and its vector, Bemisia tabaci. Archives of Phytopathology and Plant Protection, 45(13), 1538–1545. https://doi.org/10.1080/03235408.2012.681246
- Elliot, S. L., Blanford, S., & Thomas, M. B. (2002). Host–pathogen interactions in a varying environment: Temperature, behavioural fever and fitness. Proceedings of the Royal Society of London. Series B: Biological Sciences, 269(1500), 1599–1607. https://doi.org/10.1098/rspb.2002.2067
- Elliot, S. L., Sabelis, M. W., Janssen, A., van der Geest, L. P. S., Beerling, E. A. M., & Fransen, J. J. (2000). Can plants use entomopathogens as bodyguards? Ecology Letters, 3(3), 228–235. https://doi.org/10.1046/j.1461-0248.2000.00137.x
- Faria, M., Hotchkiss, J. H., Hajek, A. E., & Wraight, S. P. (2010). Debilitation in conidia of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae and implication with respect to viability determinations and mycopesticide quality assessments. Journal of Invertebrate Pathology, 105(1), 74–83. https://doi.org/10.1016/j.jip.2010.05.011
- Faria, M. R. D., & Wraight, S. P. (2007). Mycoinsecticides and Mycoacaricides: A comprehensive list with worldwide coverage and international classification of formulation types. Biological Control, 43(3), 237–256. https://doi.org/10.1016/j.biocontrol.2007.08.001
- Gabarty, A., Salem, H. M., Fouda, M. A., Abas, A. A., & Ibrahim, A. A. (2014). Pathogencity induced by the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae in Agrotis ipsilon (Hufn.). Journal of Radiation Research and Applied Sciences, 7(1), 95–100. https://doi.org/10.1016/j.jrras.2013.12.004
- Gange, A. C., Koricheva, J., Currie, A. F., Jaber, L. R., & Vidal, S. (2019). Meta-analysis of the role of entomopathogenic and unspecialized fungal endophytes as plant bodyguards. New Phytologist, 223(4), 2002–2010. https://doi.org/10.1111/NPH.15859
- Gao, T., Li, Y., Ding, M., Chai, Y., & Wang, Q. (2017). The phosphotransferase system gene ptsI in Bacillus cereus regulates expression of sodA2 and contributes to colonization of wheat roots. Research in Microbiology, 168(6), 524–535. https://doi.org/10.1016/j.resmic.2017.04.003
- Georgiev, G., Mirchev, P., Rossnev, B., Petkov, P., Georgieva, M., Pilarska, D., Golemansky, V., Pilarski, P., & Hubenov, Z. (2013). Potential of Entomophaga maimaiga Humber, Shimazu and Soper (Entomophthorales) for suppressing Lymantria dispar (Linnaeus) outbreaks in Bulgaria. Comptes Rendus de L’Academie Bulgare Des Sciences, 66(7), 1025–1032. https://doi.org/10.7546/CR-2013-66-7-13101331-14
- Goettel, M. S., Koike, M., Kim, J. J., Aiuchi, D., Shinya, R., & Brodeur, J. (2008). Potential of Lecanicillium spp. For management of insects, nematodes and plant diseases. Journal of Invertebrate Pathology, 98(3), 256–261. https://doi.org/10.1016/j.jip.2008.01.009
- Greif, M. D., & Currah, R. S. (2007). Development and dehiscence of the cephalothecoid peridium in Aporothielavia leptoderma shows it belongs in Chaetomidium. Mycological Research, 111(Pt 1), 70–77. https://doi.org/10.1016/j.mycres.2006.09.016
- Griffin, M. R. (2007). Beauveria bassiana, a cotton endophyte with biocontrol activity against seedling disease. University of Tennessee. https://trace.tennessee.edu/utk_graddiss/180
- Gryganskyi, A. P., Mullens, B. A., Gajdeczka, M. T., Rehner, S. A., Vilgalys, R., & Hajek, A. E. (2017). Hijacked: Co-option of host behavior by entomophthoralean fungi. PLoS Pathogens, 13(5), e1006274. https://doi.org/10.1371/journal.ppat.1006274
- Guerrero-Guerra, C., Reyes-Montes, M., del, R., Toriello, C., Hernández-Velázquez, V., Santiago-López, I., Mora-Palomino, L., Calderón-Segura, M. E., Fernández, S. D., & Calderón-Ezquerro, C. (2013). Study of the persistence and viability of Metarhizium acridum in Mexico’s agricultural area. Aerobiologia, 29(2), 249–261. https://doi.org/10.1007/s10453-012-9277-8
- Gul, H. T., Saeed, S., & Khan, F. A. (2014). Entomopathogenic fungi as effective insect pest management tactic: A review. Applied Sciences and Business Economics, 1(1), 10–18. http://www.asbejournal.org/10-18.pdf
- Hughes, W. O., Thomsen, L., Eilenberg, J., & Boomsma, J. J. (2004). Diversity of entomopathogenic fungi near leaf-cutting ant nests in a neotropical forest, with particular reference to Metarhizium anisopliae var. Anisopliae. Journal of Invertebrate Pathology, 85(1), 46–53. https://doi.org/10.1016/j.jip.2003.12.005
- Humber, R. A. (2008). Evolution of entomopathogenicity in fungi. Journal of Invertebrate Pathology, 98(3), 262–266. https://doi.org/10.1016/j.jip.2008.02.017
- Hussain, A., Tian, M.-Y., He, Y.-R., & Ahmed, S. (2009). Entomopathogenic fungi disturbed the larval growth and feeding performance of Ocinara varians (Lepidoptera: Bombycidae) larvae. Insect Science, 16(6), 511–517. https://doi.org/10.1111/j.1744-7917.2009.01272.x
- Idrees, A., Qadir, Z. A., Akutse, K. S., Afzal, A., Hussain, M., Islam, W., Waqas, M. S., Bamisile, B. S., & Li, J. (2021). Effectiveness of entomopathogenic fungi on immature stages and feeding performance of Fall Armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) Larvae. Insects, 12(11), 1044. https://doi.org/10.3390/insects12111044
- Islam, W., Adnan, M., Shabbir, A., Naveed, H., Abubakar, Y. S., Qasim, M., Tayyab, M., Noman, A., Nisar, M. S., & Khan, K. A. (2021). Insect-fungal-interactions: A detailed review on entomopathogenic fungi pathogenicity to combat insect pests. Microbial Pathogenesis, 159, 105122. https://doi.org/10.1016/j.micpath.2021.105122
- Jaber, L. R., & Araj, S.-E. (2018). Interactions among endophytic fungal entomopathogens (Ascomycota: Hypocreales), the green peach aphid Myzus persicae Sulzer (Homoptera: Aphididae), and the aphid endoparasitoid Aphidius colemani Viereck (Hymenoptera: Braconidae). Biological Control, 116, 53–61. https://doi.org/10.1016/j.biocontrol.2017.04.005
- Jaber, L. R., & Ownley, B. H. (2018). Can we use entomopathogenic fungi as endophytes for dual biological control of insect pests and plant pathogens? Biological Control, 116, 36–45. https://doi.org/10.1016/j.biocontrol.2017.01.018
- Jankielsohn, A. (2018). The Importance of Insects in Agricultural Ecosystems. Advances in Entomology, 06(2), 62–73. https://doi.org/10.4236/ae.2018.62006
- Jessica, J. J., Peng, T. L., Sajap, A. S., Lee, S. H., & Syazwan, S. A. (2018). Evaluation of the virulence of entomopathogenic fungus, Isaria fumosorosea isolates against subterranean termites Coptotermes spp. (Isoptera: Rhinotermitidae). Journal of Forestry Research 2018 30:1, 30(1), 213–218. https://doi.org/10.1007/S11676-018-0614-9
- Jordan, C., Dos Santos, P. L., Oliveira, L. R., dos, S., Domingues, M. M., Gêa, B. C. C., Ribeiro, M. F., Mascarin, G. M., & Wilcken, C. F. (2021). Entomopathogenic fungi as the microbial frontline against the alien Eucalyptus pest Gonipterus platensis in Brazil. Scientific Reports, 11(1), 1–13. https://doi.org/10.1038/s41598-021-86638-9
- Kaiser, D., Bacher, S., Mène-Saffrané, L., & Grabenweger, G. (2019). Efficiency of natural substances to protect Beauveria bassiana conidia from UV radiation. Pest Management Science, 75(2), 556–563. https://doi.org/10.1002/ps.5209
- Kaya, H. K., & Vega, F. E. (2012). Scope and Basic Principles of Insect Pathology. Insect Pathology, (pp. 1–12); Elsevier. https://doi.org/10.1016/B978-0-12-384984-7.00001-4
- Klass, J. I., Blanford, S., & Thomas, M. B. (2007). Development of a model for evaluating the effects of environmental temperature and thermal behaviour on biological control of locusts and grasshoppers using pathogens. Agricultural and Forest Entomology, 9(3), 189–199. https://doi.org/10.1111/j.1461-9563.2007.00335.x
- Klieber, J., & Reineke, A. (2016). The entomopathogen B eauveria bassiana has epiphytic and endophytic activity against the tomato leaf miner T uta absoluta. Journal of Applied Entomology, 140(8), 580–589. https://doi.org/10.1111/jen.12287
- Kushiyev, R., Tuncer, C., Erper, I., Ozdemir, I. O., & Saruhan, I. (2018). Efficacy of native entomopathogenic fungus, Isaria fumosorosea, against bark and ambrosia beetles, Anisandrus dispar Fabricius and Xylosandrus germanus Blandford (Coleoptera: Curculionidae: Scolytinae). Egyptian Journal of Biological Pest Control, 28(1), 1–6. https://doi.org/10.1186/s41938-018-0062-z
- Li, Z., Alves, S. B., Roberts, D. W., Fan, M., Jr, I, D., Tang, J., Lopes, R. B., Faria, M., & Rangel, D. E. (2010). Biological control of insects in Brazil and China: History, current programs and reasons for their successes using entomopathogenic fungi. Biocontrol Science and Technology, 20(2), 117–136. https://doi.org/10.1080/09583150903431665
- Li, W., & Sheng, C. F. (2007). Occurrence and distribution of entomophthoralean fungi infecting aphids in mainland China. Biocontrol Science and Technology, 17(4), 433–439. https://doi.org/10.1080/09583150701213802
- Litwin, A., Nowak, M., & Rózalska, S. (2020). Entomopathogenic fungi: Unconventional applications. In Reviews in Environmental Science and Biotechnology (Vol. 19, No. 1, pp. 23–42). Springer. https://doi.org/10.1007/s11157-020-09525-1
- Mascarin, G. M., & Jaronski, S. T. (2016). The production and uses of Beauveria bassiana as a microbial insecticide. World Journal of Microbiology & Biotechnology, 32(11), 1–26. https://doi.org/10.1007/s11274-016-2131-3
- McKinnon, A. C., Glare, T. R., Ridgway, H. J., Mendoza-Mendoza, A., Holyoake, A., Godsoe, W. K., & Bufford, J. L. (2018). Detection of the entomopathogenic fungus Beauveria bassiana in the rhizosphere of wound-stressed Zea mays plants. Frontiers in Microbiology, 9, 1161. https://doi.org/10.3389/fmicb.2018.01161
- Moran, P. J., Patt, J. M., Cabanillas, H. E., Adamczyk, J. L., Jackson, M. A., Dunlap, C. A., Hunter, W. B., & Avery, P. B. (2011). Localized Autoinoculation and Dissemination of Isaria fumosorosea for Control of the Asian Citrus Psyllid in South Texas. Subtropical Plant Science, 63, 23–35. https://pubag.nal.usda.gov/catalog/54210
- Mwamburi, L. A. (2020). Chapter 37—Beauveria. In N. Amaresan, M. Senthil Kumar, K. Annapurna, K. Kumar, & A. Sankaranarayanan (Eds.), Beneficial Microbes in Agro-Ecology (pp. 727–748). Academic Press. https://doi.org/10.1016/B978-0-12-823414-3.00037-X
- Niassy, S., Diarra, K., Ndiaye, S., & Niassy, A. (2011). Pathogenicity of local Metarhizium anisopliae var. Acridum strains on Locusta migratoria migratorioides Reiche and Farmaire and Zonocerus variegatus Linnaeus in Senegal. African Journal of Biotechnology, 10(1), 28–33. https://www.ajol.info/index.php/ajb/article/view/137916
- Nicoletti, R., & Becchimanzi, A. (2020). Endophytism of Lecanicillium and Akanthomyces. Agriculture, 10(6). https://doi.org/10.3390/agriculture10060205
- Nielsen, C., & Wraight, S. P. (2009). Exotic aphid control with pathogens. In Hajek, A.E., Glare, T.R., O’Callaghan, M. (Eds.), Use of Microbes for Control and Eradication of Invasive Arthropods (Vol. 6, pp. 93–113). Springer. https://doi.org/10.1007/978-1-4020-8560-4_6
- Oliveira, D. G. P., De, Lopes, R. B., Rezende, J. M., & Delalibera, I. (2018). Increased tolerance of Beauveria bassiana and Metarhizium anisopliae conidia to high temperature provided by oil-based formulations. Journal of Invertebrate Pathology, 151, 151–157. https://doi.org/10.1016/j.jip.2017.11.012
- Ortiz-Urquiza, A., & Keyhani, N. O. (2013). Action on the surface: Entomopathogenic fungi versus the insect cuticle. Insects, 4(3), 357–374. https://doi.org/10.3390/insects4030357
- Ownley, B. H., Griffin, M. R., Klingeman, W. E., Gwinn, K. D., Moulton, J. K., & Pereira, R. M. (2008). Beauveria bassiana: Endophytic colonization and plant disease control. Journal of Invertebrate Pathology, 98(3), 267–270. https://doi.org/10.1016/J.JIP.2008.01.010
- Posada, F., Aime, M. C., Peterson, S. W., Rehner, S. A., & Vega, F. E. (2007). Inoculation of coffee plants with the fungal entomopathogen Beauveria bassiana (Ascomycota: Hypocreales). Mycological Research, 111(6), 748–757. https://doi.org/10.1016/j.mycres.2007.03.006
- Qasim, M., Islam, S. U., Islam, W., Noman, A., Khan, K. A., Hafeez, M., Hussain, D., Dash, C. K., Bamisile, B. S., & Akutse, K. S. (2020). Characterization of mycotoxins from entomopathogenic fungi (Cordyceps fumosorosea) and their toxic effects to the development of asian citrus psyllid reared on healthy and diseased citrus plants. Toxicon, 188, 39–47. https://doi.org/10.1016/j.toxicon.2020.10.012
- Qasim, M., Ronliang, J., Islam, W., Ali, H., Khan, K. A., Dash, C. K., Jamal, Z. A., & Wang, L. (2021). Comparative pathogenicity of four entomopathogenic fungal species against nymphs and adults of citrus red mite on the citrus plantation. International Journal of Tropical Insect Science, 41(1), 737–749. https://doi.org/10.1007/s42690-020-00263-z
- Qayyum, M. A., Saeed, S., Wakil, W., Nawaz, A., Iqbal, N., Yasin, M., Chaurdhry, M. A., Bashir, M. A., Ahmed, N., & Riaz, H. (2021). Diversity and correlation of entomopathogenic and associated fungi with soil factors. Journal of King Saud University-Science, 33(6), 101520. https://doi.org/10.1016/j.jksus.2021.101520
- Quesada-Moraga, E., Ruiz-García, A., & Santiago-Alvarez, C. (2006). Laboratory evaluation of entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae against puparia and adults of Ceratitis capitata (Diptera: Tephritidae). Journal of Economic Entomology, 99(6), 1955–1966. https://doi.org/10.1093/jee/99.6.1955
- Quesada-Sojo, K. A., & Rivera-Méndez, W. (2016). Hirsutella as biological controller agent of mites and insects of agricultural importance. Revista Tecnología En Marcha, 29, 85–93. https://doi.org/10.18845/tm.v29i7.2709
- Reddy, S. G. E. (2020). Lecanicillium spp. For the management of aphids, whiteflies, thrips, scales and mealy bugs. In Eduardo, R., & Ranz, R. (Eds.), Arthropods-Are They Beneficial for Mankind?. https://doi.org/10.5772/intechopen.94020
- Reddy, N., Mahesh, G., Priya, M., Singh, R. U. S., & Manjunatha, L. (2020). Chapter 43—Hirsutella. In N. Amaresan, M. Senthil Kumar, K. Annapurna, K. Kumar, & A. Sankaranarayanan (Eds.), Beneficial Microbes in Agro-Ecology (pp. 817–831). Academic Press. https://doi.org/10.1016/B978-0-12-823414-3.00043-5
- Resquín-Romero, G., Garrido-Jurado, I., Delso, C., Ríos-Moreno, A., & Quesada-Moraga, E. (2016). Transient endophytic colonizations of plants improve the outcome of foliar applications of mycoinsecticides against chewing insects. Journal of Invertebrate Pathology, 136, 23–31. https://doi.org/10.1016/j.jip.2016.03.003
- Rijal, J. P., & Godfrey, L. D. (2018). Efficacy of selected bio-and reduced-risk insecticides on mint root borer, 2014. Arthropod Management Tests, 43(1), tsx134. https://doi.org/10.1093/amt/tsx134
- Rivas, F., Nuñez, P., Jackson, T., & Altier, N. (2014). Effect of temperature and water activity on mycelia radial growth, conidial production and germination of Lecanicillium spp. Isolates and their virulence against Trialeurodes vaporariorum on tomato plants. BioControl, 59(1), 99–109. https://doi.org/10.1007/s10526-013-9542-y
- Roberts, D. W., & St Leger, R. J. (2004). Metarhizium spp., cosmopolitan insect-pathogenic fungi: Mycological aspects. Advances in Applied Microbiology, 54(1), 1–70. https://doi.org/10.1016/S0065-2164(04)54001-7
- Roy, H. E., Baverstock, J., & Chamberlain, K. (2005). Do aphids infected with entomopathogenic fungi continue to produce and respond to alarm pheromone? Biocontrol Science and Technology, 15(8), 859–866. https://www.tandfonline.com/doi/abs/10.1080/09583150500136170
- Russo, M. L., Pelizza, S. A., Vianna, M. F., Allegrucci, N., Cabello, M. N., Toledo, A. V., Mourelos, C., & Scorsetti, A. C. (2019). Effect of endophytic entomopathogenic fungi on soybean Glycine max (L.) Merr. Growth and yield. Journal of King Saud University-Science, 31(4), 728–736. https://doi.org/10.1016/j.jksus.2018.04.008
- Sahayaraj, K., & Namasivayam, S. K. R. (2008). Mass production of entomopathogenic fungi using agricultural products and by products. African Journal of Biotechnology, 7, 12. https://doi.org/10.5897/AJB07.778
- Sandhu, S. S., Sharma, A. K., Beniwal, V., Goel, G., Batra, P., Kumar, A., Jaglan, S., Sharma, A. K., & Malhotra, S. (2012). Myco-biocontrol of insect pests: factors involved, mechanism, and regulation. Journal of Pathogens, 2012, 1–10. https://doi.org/10.1155/2012/126819
- Senthil-Nathan, S. (2015). A review of biopesticides and their mode of action against insect pests. In Thangavel, P., & Shridevi, G. (Eds.), Environmental Sustainability (pp. 49–63). https://doi.org/10.1007/978-81-322-2056-5_3
- Sharma, L., Bohra, N., Rajput, V. D., Quiroz-Figueroa, F. R., Singh, R. K., & Marques, G. (2021). Advances in entomopathogen isolation: A case of bacteria and fungi. Microorganisms, 9(1), 1–25. https://doi.org/10.3390/microorganisms9010016
- Shin, T. Y., Lee, M. R., Park, S. E., Lee, S. J., Kim, W. J., & Kim, J. S. (2020). Pathogenesis-related genes of entomopathogenic fungi. Archives of Insect Biochemistry and Physiology, 105(4), e21747. https://doi.org/10.1002/arch.21747
- Shinohara, S., Fitriana, Y., Satoh, K., Narumi, I., & Saito, T. (2013). Enhanced fungicide resistance in Isaria fumosorosea following ionizing radiation-induced mutagenesis. FEMS Microbiology Letters, 349(1), 54–60. https://doi.org/10.1111/1574-6968.12295
- Silva, A. C. L., Silva, G. A., Abib, P. H. N., Carolino, A. T., & Samuels, R. I. (2020). Endophytic colonization of tomato plants by the entomopathogenic fungus Beauveria bassiana for controlling the South American tomato pinworm, Tuta absoluta. CABI Agriculture and Bioscience, 1(1), 1–9. https://doi.org/10.1186/s43170-020-00002-x
- Sinha, K. K., Choudhary, A. K., & Kumari, P. (2016). Ecofriendly Pest Management for Food Security Entomopathogenic Fungi. Academic Press.https://doi.org/10.1016/B978-0-12-803265-7.00015-4
- Sorokin, N. (1883). The parasites of plants, man and animals. Rastitelnye Oarazty Ceoveska I Zitotnyh, 2, 268–290.
- Srygley, R. B. (2012). Ontogenetic changes in immunity and susceptibility to fungal infection in Mormon crickets Anabrus simplex. Journal of Insect Physiology, 58(3), 342–347. https://doi.org/10.1016/j.jinsphys.2011.12.005
- St. Leger, R. J. (2008). Studies on adaptations of Metarhizium anisopliae to life in the soil. Journal of Invertebrate Pathology, 98(3), 271–276. https://doi.org/10.1016/j.jip.2008.01.007
- Su, L., Zhu, H., Guo, Y., Du, X., Jianguo, G., Zhang, L., & Qin, C. (2019). Lecanicillium coprophilum (Cordycipitaceae, Hypocreales), a new species of fungus from the feces of Marmota monax in China. Phytotaxa, 387(1), 55–62. https://doi.org/10.11646/phytotaxa.387.1.4
- Thomas, M. B. (2000). Development of a myco-insecticide for biological control of locusts in Southern Africa. Workshop on Research Priorities for Migrant Pests of Agriculture in Southern Africa, 3, 173. https://www.cabdirect.org/cabdirect/abstract/20188100108
- Thungrabeab*, M., & Tongma, S. (2007). Effect of entomopathogenic fungi, Beauveria bassiana (Balsam) and Metarhizium anisopliae (Metsch) on non target insects. Current Applied Science And Technology, 7(1–1). https://li01.tci-thaijo.org/index.php/cast/article/view/86784
- Trandem, N., Bhattarai, U. R., Westrum, K., Knudsen, G. K., & Klingen, I. (2015). Fatal attraction: Male spider mites prefer females killed by the mite-pathogenic fungus Neozygites floridana. Journal of Invertebrate Pathology, 128, 6–13. https://doi.org/10.1016/J.JIP.2015.04.002
- Uma Devi, K., Padmavathi, J., Uma Maheswara Rao, C., Khan, A. A. P., & Mohan, M. C. (2008). A study of host specificity in the entomopathogenic fungus Beauveria bassiana (Hypocreales, Clavicipitaceae). Biocontrol Science and Technology, 18(10), 975–989. https://doi.org/10.1080/09583150802450451
- Usman, M., Gulzar, S., Wakil, W., Wu, S., Piñero, J. C., Leskey, T. C., Nixon, L. J., Oliveira-Hofman, C., Toews, M. D., & Shapiro-Ilan, D. (2020). Virulence of entomopathogenic fungi to rhagoletis pomonella (Diptera: Tephritidae) and Interactions With Entomopathogenic Nematodes. Journal of Economic Entomology, 113(6), 2627–2633. https://doi.org/10.1093/JEE/TOAA209
- Usta, C. (2013). Microorganisms in biological pest control—A review (bacterial toxin application and effect of environmental factors). Current Progress in Biological Research, 13, 287–317. https://doi.org/10.5772/55786
- Uziel, A., Levy, K., & Yuval, B. (2003). Infection of Ceratitis capitata by two species of the Entomophthora muscae species complex (Zygomycetes: Entomophthorales) in the field. Phytoparasitica, 31(2), 204–206. https://doi.org/10.1007/BF02980791
- Valero-Jiménez, C. A., Wiegers, H., Zwaan, B. J., Koenraadt, J. M., & Van Kan, J. A. L. (2016). Genes Involved in Virulence of the Entomopathogenic Fungus Beauveria Bassiana. Journal of Invertebrate Pathology, 133, 41–49. https://doi.org/10.1016/j.jip.2015.11.011
- Valizadeh, H., Abbasipour, H., Mahmoudvand, M., Askary, H., & Reza Moniri, V. (2011). Ensayos de Laboratorio de Metarhizium anisopliae var. acridum (Green muscle®) contra la Langosta de Saxaul, Dericorys albidula Serville (Orthoptera: Dericorythidae). Chilean Journal of Agricultural Research, 71(4), 549–553. https://doi.org/10.4067/S0718-58392011000400008
- Vandermeer, J., Perfecto, I., & Liere, H. (2009). Evidence for hyperparasitism of coffee rust (Hemileia vastatrix) by the entomogenous fungus, Lecanicillium lecanii, through a complex ecological web. Plant Pathology, 58(4), 636–641. https://doi.org/10.1111/j.1365-3059.2009.02067.x
- Vega, F. E. (2018). The use of fungal entomopathogens as endophytes in biological control: A review. Mycologia. 110(1), 4–30. https://doi.org/10.1080/00275514.2017.1418578
- Vega, F. E., Goettel, M. S., Blackwell, M., Chandler, D., Jackson, M. A., Keller, S., Koike, M., Maniania, N. K., Monzon, A., & Ownley, B. H. (2009). Fungal entomopathogens: New insights on their ecology. Fungal Ecology, 2(4), 149–159. https://doi.org/10.1016/j.funeco.2009.05.001
- Vega, F. E., Meyling, N. V., Luangsa-Ard, J. J., & Blackwell, M. (2012). Fungal entomopathogens. In Vega, F., & Kaya, H.K. (Eds.), Insect Pathology (pp. 171–220). Elsevier Inc. https://doi.org/10.1016/B978-0-12-384984-7.00006-3
- Vega, F. E., Posada, F., Aime, M. C., Pava-Ripoll, M., Infante, F., & Rehner, S. A. (2008). Entomopathogenic fungal endophytes. Biological Control, 46(1), 72–82. https://doi.org/10.1016/j.biocontrol.2008.01.008
- Vinayaga Moorthi, P., Balasubramanian, C., Selvarani, S., & Radha, A. (2015). Efficacy of sub lethal concentration of entomopathogenic fungi on the feeding and reproduction of Spodoptera litura. SpringerPlus, 4(1), 1–12. https://doi.org/10.1186/S40064-015-1437-1
- Wakil, W., Kavallieratos, N. G., Ghazanfar, M. U., Usman, M., Habib, A., & El-Shafie, H. A. (2021). Efficacy of different entomopathogenic fungal isolates against four key stored-grain beetle species. Journal of Stored Products Research, 93, 101845. https://doi.org/10.1016/j.jspr.2021.101845
- Wang, J., Lovett, B., & Leger, R. J. S. (2019). The secretome and chemistry of Metarhizium; a genus of entomopathogenic fungi. Fungal Ecology, 38, 7–11. https://doi.org/10.1016/j.funeco.2018.04.001
- Weng, Q., Zhang, X., Chen, W., & Hu, Q. (2019). Secondary metabolites and the risks of Isaria fumosorosea and Isaria farinosa. Molecules 2019, Vol. 24, Page 664, 24(4), 664. https://doi.org/10.3390/MOLECULES24040664
- Wheelis, M. (2002). Biological warfare at the 1346 siege of Caffa. Emerging Infectious Diseases, 8(9), 971. https://doi.org/10.3201/eid0809.010536
- Woo, R. M., Park, M. G., Choi, J. Y., Park, D. H., Kim, J. Y., Wang, M., Kim, H. J., Woo, S. D., Kim, J. S., & Je, Y. H. (2020). Insecticidal and insect growth regulatory activities of secondary metabolites from entomopathogenic fungi, Lecanicillium attenuatum. Journal of Applied Entomology, 144(7), 655–663. https://doi.org/10.1111/jen.12788.
- Xie, T., Jiang, L., Li, J., Hong, B., Wang, X., & Jia, Y. (2019). Effects of Lecanicillium lecanii strain JMC-01 on the physiology, biochemistry, and mortality of Bemisia tabaci Q-biotype nymphs. PeerJ, 7, e7690. https://doi.org/10.7717/peerj.7690
- Xu, J., Xu, X., Shakeel, M., Li, S., Wang, S., Zhou, X., Yu, J., Xu, X., Yu, X., & Jin, F. (2017). The entomopathogenic fungi isaria fumosorosea plays a vital role in suppressing the immune system of plutella xylostella: RNA-Seq and DGE analysis of immunity-related genes. Frontiers in Microbiology, 8(JUL), 1421. https://doi.org/10.3389/FMICB.2017.01421
- Yan, X., Scherphof, G. L., & Kamps, J. A. A. M. (2005). Liposome Opsonization. Journal of Liposome Research, 15(1), 109–139. https://doi.org/10.1081/LPR-64971
- Zare, R., & Gams, W. (2001). A revision of Verticillium section Prostrata. IV. The genera Lecanicillium and Simplicillium gen. Nov. Nova Hedwigia, 73(1), 1–50. https://doi.org/10.1127/nova.hedwigia/73/2001/1
- Zhang, L., Fasoyin, O. E., Molnár, I., & Xu, Y. (2020). Secondary metabolites from hypocrealean entomopathogenic fungi: Novel bioactive compounds. Natural Product Reports, 37(9), 1181–1206. https://doi.org/10.1039/C9NP00065H
- Zimmermann, G. (2008). The entomopathogenic fungi Isaria farinosa (formerly Paecilomyces farinosus) and the Isaria fumosorosea species complex (formerly Paecilomyces fumosoroseus). Biology, Ecology and Use in Biological Control, 18(9), 865–901. https://doi.org/10.1080/09583150802471812
- Zou, C., Li, L., Dong, T., Zhang, B., & Hu, Q. (2014). Joint action of the entomopathogenic fungus Isaria fumosorosea and four chemical insecticides against the whitefly Bemisia tabaci. Biocontrol Science and Technology. 24(3), 315–324. https://doi.org/10.1080/09583157.2013.860427
