Abstract
This study determined the effect of Vachellia karroo leaf meal (VKLM) in diets on feed intake (FI), digestibility, feed conversion ratio (FCR), body weight gain (BWG), final live weight (FLW) and meat quality parameters of male Ross 308 broiler chickens fed from 22 to 42 days. One hundred sixty 22-day-old male chickens were assigned to four treatments with four replicates of 10 chickens. VKLM inclusion levels were at 0, 0.5, 1.0 and 1.5 g/kg dry matter (DM). The total FI and BWG were recorded and used to calculate FCR. Meat pH, shear force and cooking loss of chicken breast meat samples were measured for stability over refrigerated storage periods of 0, 7 and 14 days at −4°C. Data were analysed using general linear model procedures (SAS, 2013) as Completely Randomized Design (CRD) with Initial Body Weight (IBW) as a covariate. VKLM inclusion levels in the diets did not affect (P > 0.05) FI, FCR and nutrient digestibility of male broilers. Breast meat pH, shear force and cooking loss were similar (P > 0.05) after slaughter (day 0). However, after 7 days, meat from chickens on VKLM treatment diets had lower pH than a diet without VKLM (control). Meat samples from broilers fed 1.5 g VKLM had higher (P < 0.05) shear force and lower (P < 0.05) cooking loss than control. After 14 days, VKLM inclusion had no effect (P > 0.05) on shear force. It was concluded that VKLM could be included in broiler diets at up to 1.5 g/kg DM to improve meat quality and shelf life without any adverse effects on performance and productivity.
1. Introduction
Poultry production is one of the main animal protein contributors in the world as it contributes more than 30% of protein from meat, eggs and meat products for consumption (Al-Hijazeen et al., Citation2016; Hafiz et al., Citation2015). The poultry industry, especially broiler production, continues to grow every year and is considered one of the largest livestock industries due to its high demand for consumption (Mbajiorgu et al., Citation2007). Currently, there is a demand for high-quality poultry meat products by consumers. Therefore, due to the rapid growth in poultry meat consumption, there is a need for poultry industries to produce high-quality meat with prolonged shelf life (Tan et al., Citation2018). Genetic selection of better-performing chickens has been reported to reduce their growth periods, thus improving production. However, broiler chicken breeds have high intake and feed conversion ratio (Hafeni, Citation2013). This leads to rapid growth with high accumulation of fat deposition and low meat yield, which is associated with the regulation of feed intake (FI) (Musa et al., Citation2006) and reduction of meat quality in broiler chickens (Ng’ambi et al., Citation2009). This is because a high amount of fat content, especially unsaturated fatty acids, exposes the meat to lipid oxidation, which negatively affects meat quality and shelf life during storage (Wapi et al., Citation2013). Lipid oxidation process in meat occurs when the unsaturated fatty acids start to accumulate in the membrane phospholipids and become oxidized (Nikmaram et al., Citation2018). This process further causes undesirable smell, texture, flavour and discolouration of the meat, consequently leading to poor meat quality with low shelf life (Cunha et al., Citation2018; Tshabalala et al., Citation2003).
In previous years, synthetic antioxidants have been commonly used to retard lipid oxidation in chickens; however, they are considered harmful to humans health (Gheisari et al., Citation2017; Kang et al., Citation2001; Lorenzo et al., Citation2018). Therefore, recently, there has been a shift in preference by consumers towards meat produced naturally rather than meat produced using synthesized chemicals and hormones aimed to improve the stability and shelf life of livestock meat (Cunha et al., Citation2018; Lorenzo et al., Citation2018; Nikmaram et al., Citation2018; Shah et al., Citation2014; Tshabalala et al., Citation2003). Thus, there is a need to find alternative natural antioxidants from plant material to reduce and delay lipid oxidation and increase the stability of the meat during refrigerated storage. It has been observed that plants such as taninniferous (leguminous) trees contain antioxidants that aid in improving meat quality and shelf life of meat and meat products (Mbajiorgu et al., Citation2007; Sanchez et al., Citation2006; Tshabalala et al., Citation2003; Wapi et al., Citation2013), hence reducing lipid oxidation and degradation of meat (Falowo et al., Citation2014). Examples of plant antioxidants present in tanniniferous leaves include tocopherols, polyphenols, flavonoids, tannins and terpenoids (Hygreeva et al., Citation2014).
It has been reported that Vachellia karroo tree leaves contain high tannin levels (Ng’ambi et al., Citation2009; Ng’ambu et al., Citation2013), which could potentially have a positive influence on meat stability by reducing meat deterioration (Mbajiorgu et al., Citation2007, Citation2007). Although high levels of dietary tannins are considered antinutritional factors that reduce animal performance when included in livestock diets (Al-Hijazeen et al., Citation2016; Mapiye et al., Citation2011), several studies have reported that the inclusion of low levels as feed additives could result in improved growth and meat quality in poultry, sheep and goats (Ng’ambu et al., Citation2013; Vasta et al., Citation2008; Wapi et al., Citation2013). However, information on the use of tanniniferous Vachellia karroo leaf meal (VKLM) as an additive in broiler chicken diets is limited. Therefore, there is a need to determine the effect of low incremental levels of VKLM on broiler performance and meat quality under the refrigerated storage period. The objectives of this study are to evaluate the responses in growth performance and meat quality from male Ross 308 broiler chickens fed incremental levels of VKLM.
2. Materials and methods
2.1. Study site
This study was conducted at the University of Limpopo Animal Unit (1,282 m altitude, 23°53′24″S latitude and 29°45′25″E longitude), Limpopo Province, South Africa. The experimental procedures and protocol for proper animal handling were conducted in accordance with the guidelines of the University of Limpopo (UL) Animal Research Ethics Committee, Number: AREC/08/2020: PG. The ambient temperature around the study area ranged between 16°C and 27°C in summer.
2.2. Feed ingredients
The Vachellia karroo tree leaves were hand-harvested in August 2019 at the University of Limpopo experimental farm. They were then cleaned and stored under a shaded room indoors to dry off according to the method used by Brown et al. (Citation2017). The leaves along with other purchased feed ingredients such as yellow maize, soya bean and limestone were then crushed and ground into a fine meal to go through a 2 mm screen with a grinder (Staalmeester 2121 Hammer Mill Electric machine), and then stored in airtight bags prior to chemical analysis (AOAC, Citation2010) and diet formulation. The yellow maize and wheat offals were acquired from NTK (PTY) Ltd., Polokwane. Full-fat soya was purchased from Midfeeds (PTY) Ltd., Nelspruit, Mpumalanga. Fish meal, bone meal and limestone were acquired from Irvine’s Africa (PTY) Ltd., Pretoria, Gauteng. All purchased feed ingredients used in diet formulation were in mash or powder form.
2.3. Experimental diets, designs and procedures
This study was divided into two experiments. Experiment 1 (22–42 days): The experiment determined the responses in FI, feed conversion ratio (FCR), body weight gain (BWG) and live weight (LW) of male Ross 308 broiler chickens to dietary VKLM levels. A total of 320 unsexed Ross 308 broiler chicks with an initial live weight (ILW) of 40 ± 1.6 g/bird were fed commercial starter diet until 21 days. At day 22, a total number of 160 male Ross 308 broiler chickens with an ILW of 840 ± 5 g/bird were assigned to four treatments (dietary treatment levels) in a Completely Randomized Design (CRD), replicated four times per pen. The VKLM inclusion levels were at 0, 0.5, 1.0 and 1.5 g/kg dry matter (DM). The chickens' diets as shown in Table and described in Table were formulated to meet the nutrient requirements of broiler chickens as recommended by the National Research Council (1994). Two experimental houses were divided into 16 floor pens of equal sizes (each measuring approximately 1.5 × 1.5 m holding 10 chickens). All the equipments such as drinkers, feeders and wire separators will also be cleaned thoroughly and disinfected with Virkon (disinfectant). Feeds and water were offered ad libitum. The birds were reared under natural (sunlight) and artificial (light bulb) lights for 23 hours daily throughout the experiment with 1 hour of darkness to rest for better health and productivity. For biosecurity measures, a footbath containing disinfectant was used on a daily basis. Sick chickens were isolated and treated accordingly by a certified veterinarian. Broiler male chickens were used due to limited funds. Apart from that, they grow faster and perform better than females.
Table 1. Ingredients and nutrient composition of the experimental diet in grower phase
Table 2. Dietary treatments for the experiment (22–42 days)
Experiment 2 (42–52 days): The experiment determined the responses in physico-chemical shelf-life indicators (pH, shear force and cooking loss) and meat stability of breast meat from male Ross 308 broiler chickens to dietary VKLM levels. A total of 36 carcass samples, according to their treatments (four treatment groups), were assigned to three storage times of 0, 7 and 14 days (having three replicates) in a completely randomized design arrangement. The physico-chemical shelf-life indicators were measured by randomly selecting breast meat from each treatment at slaughter (day 0), then every after 7 days under refrigerated storage period of 14 days.
2.4. Data collection
ILWs were recorded at the start of the experiment (day 22) and subsequently on a weekly basis using an electronic weighing balance. The live weights were used to calculate the chickens’ BWG. Firstly, mean live weights were calculated by dividing the total weight with the number of chickens in each pen. BWGs were obtained by subtracting the ILW of the chicken from the FLW. The voluntary FI per male chicken was determined by subtracting the weight of feed leftovers from feed offered per day and the difference was then divided by the total number of chickens per pen. The weighed feed was offered in the morning, refusal was collected the following day at the same time, and FI was obtained as the difference. Subsequently, FI and BWG were used to determine FCR (McDonald et al., Citation2010).
The apparent digestibility (AD) trial of the diets was performed when the chickens were between 36 and 42 days old. It was conducted in specially designed metabolic cages having separated watering and feeding troughs. For measurement of AD, one bird was randomly selected from each replicate and transferred to the metabolic cages in which clean containers were placed underneath them. The chickens were given 3 days of acclimatization prior to digestibility trials. Droppings voided by each bird were collected by gloves into small sample trays on a daily basis for 7 days at 08.00 hours. The collected samples were then weighted and oven-dried at 105°C for 24 hours and then cooled, weighted and grounded through a sieve sized 2 mm (Staalmeester 2121 Hammer Mill Electric machine) following the procedures used by Manyelo et al. (Citation2019) for chemical analysis. Care was taken to avoid contamination from feathers, scales, debris and feeds. The AD of nutrients was calculated as follows: AD(%) = (amount of nutrient ingested − amount of nutrient excreted)/(amount of nutrient ingested) × 100. N-retention was calculated according to the procedures used by McDonald et al. (Citation2010). The formula is as follows: N-retention = (N-intake − N-excreted)/0.1 × N-intake.
At day 42, three chickens per replicate were humanely slaughtered using cervical dislocation method followed by Manyelo et al. (Citation2019) to determine physico-chemical shelf-life indicators (pH, shear force and cooking loss). This slaughtering procedure involves applying pressure to the neck and dislocating the spinal column from the skull or brain in accordance with the guidelines of the University of Limpopo Ethics Committee, thus separating the spinal cord from the brain to provide the animal with a fast and painless death. This helped in avoiding discomfort, pain, distress, fear and anxiety to the chickens. After slaughter, the carcasses were then hanged upside down to completely bleed out and defeathered by putting them in boiled water for few seconds to ease hand-defeathering. The carcasses were cut open to remove the breast meat that will be used for meat quality analysis. At day 0 of storage period, three breast meat samples from each replicate were evaluated for meat quality parameters including meat pH, shear force and cooking loss. The other remaining samples were marked as per treatment, put in separate plastics, and then stored in a refrigerator at −4°C following the procedure used by Babiker et al. (Citation2019). At day 7 of storage period, three samples from each treatment were randomly removed from the refrigerator to evaluate changes (shelf life) in breast meat pH, shear force and cooking loss. At day 14 of storage period, three remaining breasts per treatment were removed from the refrigerator to evaluate changes in breast meat pH, shear force and cooking loss.
The digital pH meter (Crison, Basic 20 pH meter) with a piercing electrode was used to measure breast meat pH. The shear force used to assess meat tenderness was measured using a Warner–Bratzler Shear Force device mounted on a Universal Instron Apparatus (cross head speed = 200 mm/min, one shear in the centre of each core) following the procedures by Van Ngo et al. (Citation2017), where three cylindrical samples (12.5 mm core diameter) from each breast were cored parallel to the grain of the meat and sheared perpendicular to the fibre direction. Further modifications on determining breast meat sample shear force were performed following the procedures used by Manyelo et al. (Citation2019). The cooking losses for each breast meat sample per treatment were measured by recording weight before cooking (WBC) and weight after cooking (WAC). Cooking loss (%) = [(WBC − WAC)/WBC] × 100 (Ng’ambu et al., Citation2013). However, prior to determining shear force and WAC values, the samples were initially (removed from the refrigerator at days 7 and 14) tagged and cooked in a water bath at an internal temperature of 35°C, and then turned and finished at 70°C in a cylindrical pot on an electric stove for 25 minutes.
2.5. Chemical analysis
2.5.1. Laboratory analysis
Prior to feed formulation, 500 g of VKLM and experimental feeds, as well as faecal samples (obtained during the experiment), were analysed (AOAC, Citation2010). DM of the diet, faecal and meat samples was determined by oven-drying at 105°C for 24 hours. The ash content of meat, faeces and diets was analysed by ashing the samples overnight at 600°C in a muffle furnace. The gross energy value of feeds and faeces was determined using a bomb calorimeter at the University of Limpopo Animal Nutrition Laboratory (AOAC, Citation2010). The nitrogen content of the feed was determined using Kjeldahl method (AI Scientific Automatic Kjeldahl Analyzer Machine) at Animal Nutrition Research Laboratory, University of Pretoria, Gauteng, South Africa (AOAC, Citation2010). The crude protein and energy of the feeds were determined at the Pietermaritzburg Laboratory, Kwa-Zulu Natal, South Africa, also according to AOAC (Citation2010).
2.6. Statistical analysis
The effect of VLKM inclusion levels on growth performance and meat quality parameters was analysed using general linear model by means of analysis of variance from SAS version 9.4 software program (SAS Institute Inc, Citation2013) as a CRD with initial body weight as a covariate. The significant differences (P < 0.05) among the treatment means were determined using least significant difference test at 5% level of probability (SAS Institute Inc, Citation2013).
3. Results
3.1. Growth performance parameters
The VKLM inclusion levels in the diets did not affect (P > 0.05) FI, FCR and nutrient digestibility of male broiler experimental birds (Table ). However, VKLM significantly affected (P < 0.05) their BWG and FLW. Chickens on a diet having 0.5 g of VKLM recorded highest (P < 0.05) BWG and FLW than those on a diet having 0, 1.0 and 1.5 g VKLM inclusion levels. However, broilers fed a diet having 0, 1.0 and 1.5 g of VKLM had the same (P > 0.05) BWG and FLW values. Similarly, chickens on diets having 0, 0.5 and 1.0 g of VKLM had similar (P > 0.05) BWG, whereas those on diets having 0 and 0.5 g of VKLM also had the same (P > 0.05) FLW.
Table 3. Effect of Vachellia karroo leaf meal inclusion levels on feed intake (FI) (g), body weight gain (BWG) (g), feed conversion ratio (FCR), final live weight (FLW) (g), dry matter (DM) digestibility (%), crude protein (CP) digestibility (%), metabolisable energy (ME) (MJ/kg DM) and nitrogen retention (N-retention) (g/bird/day) of male Ross 308 broiler chickens aged 22–42 days
3.2. Meat pH
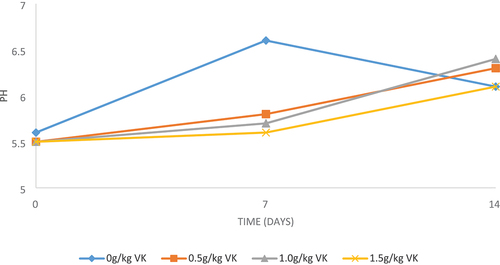
As represented in Table , VKLM inclusion levels in the diets did not affect (P > 0.05) broiler chicken breast meat pH values at slaughter (day 0). However, VKLM significantly affected (P < 0.05) meat pH after 7 and 14 days of refrigerated storage. Meat samples from chickens on treatment had lower (P < 0.05) breast meat pH than meat from chickens on a control diet, after 7 days of storage. Also, the pH decreases with an increase in VKLM inclusion levels. However, breast meat samples from chickens on a diet having 1.0 g of VKLM had higher (P < 0.05) pH values than control diet, after 14 days storage. Meat from chickens on diets having 0, 0.5 and 1.5 g of VKLM had similar (P > 0.05) pH values.
Table 4. Effect of Vachellia karroo leaf meal inclusion levels in a diet on pH, shear force and cooking loss of male Ross 308 broiler chicken breast meat at different storage time
3.3. Shear force
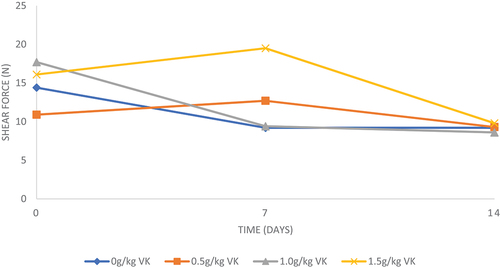
The VKLM inclusion levels did not affect (P > 0.05) breast meat shear force at day 0 and after 14 days of storage (Table ). After 7 days, meat from broilers on treatment diets had higher (P < 0.05) shear force values than control diet. The breast meat from chickens on diet having 1.5 g of VKLM had the highest (P < 0.05) shear force values after 7 days. However, meat from chickens on diets having 0 and 1.0 g of VKLM had similar (P > 0.05) shear force.
3.4. Cooking loss
The VKLM inclusion levels in the diets did not affect (P > 0.05) broiler chicken breast meat cooking loss values after slaughter (Table ). Meat from chickens on a diet having 1.5 g of VKLM had lower (P < 0.05) cooking loss values than those on diets having 0, 0.5 and 1.0 g of VKLM after 7 days of storage. However, meat from chickens on a diet having 0 and 1.0 g of VKLM as well as those on diets having 0 and 0.5 g of VKLM had the same (P > 0.05) cooking loss values. Similarly, meat from chickens on diets having 0.5 and 1.5 g also had the same (P > 0.05) cooking losses. After 14 days of storage, meat from chickens on diets with 0.5 and 1.5 g of VKLM had higher (P < 0.05) cooking losses than the control diet. However, meat from chickens on diets having 0, 1.0 and 1.5 g had similar (P > 0.05) breast meat cooking loss values.
4. Discussion
The present study showed that VKLM inclusion levels in diets had no effect on FI, FCR and nutrient digestibility of male Ross 308 broiler experimental chickens (Table ). This is in agreement with Ng’ambi et al. (Citation2009), Cui et al. (Citation2018) and Wapi et al. (Citation2013) who reported that supplementation of tanniniferous leaf meals such as Vachellia karroo and Moringa oleifera in broiler chicken diets had no effect on their growth performance parameters at finisher phase, indicating that VKLM levels used in this study could be safely included in the diet of broiler chickens without causing any adverse effects on diet digestibility and performance of broiler chickens. The non-significant differences may be due to low inclusion levels of VKLM in diets. However, the inclusion levels of VKLM significantly affected BWG and FLW of male broilers. Chickens fed on control diet had a significantly higher BWG than chickens fed on 1.5 level of VKLM, whereas chickens fed on 0 g level of VKLM had a significantly higher FLW than those fed on 1.0 and 1.5 levels of VKLM. Similar results were observed by Kolobe et al. (Citation2022) who reported significant effect in LW and growth rate when broilers were fed on different levels of VKLM after 6 weeks of growth period. In contrast, Hafeni (Citation2013) and Gheisari et al. (Citation2017) reported non-significant difference in chicken BWG when VKLM is included in broiler diets. Moreover, Ncube et al. (Citation2018) observed an increased BWG when including Vachellia leaf meals in hen diets after 18 weeks. The contradiction may be due to different inclusion levels utilized in diets as well as the length of experimental trials. The observed reduction in BWG and LW might be due to elevated fibre and content in treatment diets, which tends to increase as VKLM inclusion levels increased, thus inhibiting protein and energy digestibility which consequently reduces broiler performance (Gudiso et al., Citation2019).
The meat quality parameters, such as meat pH and shear force, could aid in determining the meat’s toughness and softness, which is highly associated with meat tenderness and water-holding capacity, consequently affecting cooking and drip loss (Northcutt et al., Citation1994; Przywitowski et al., Citation2016; Wattanachant, Citation2008). In the current study, VKLM inclusion levels did not affect breast meat pH, shear force and cooking loss of male Ross 308 broiler chicken meat at day 0 after slaughter (Table ). Similar results were reported by Al-Hijazeen et al. (Citation2016), Przywitowski et al. (Citation2016) and Gheisari et al. (Citation2017). This may be attributed to very low inclusion levels of tanniniferous feed ingredients, including VKLM in broiler diets.
However, VKLM inclusion levels in the diets significantly affected breast meat pH after days 7 and 14 of storage. Meat samples from chickens on treatment had lower breast meat pH than meat from chickens on a control diet after 7 days of storage (Figure ). The results are in agreement with those of Ng’ambu et al. (Citation2013). However, the pH values of the breast meat fall within the recommended range (5.5–6.5) (Cunha et al., Citation2018). After day 7, breast meat pH tended to reduce with an increased VKLM inclusion level (Table ). Similar findings were reported by Ebrahim et al. (Citation2015). This may be due to the production of high lactic acid that tends to elevate meat pH after slaughter (Ng’ambu et al., Citation2013). After 14 days, breast meat from chickens on a diet having 1.0 g of VKLM had slightly higher pH values than the control diet. However, meat samples from chickens on diets having 0, 0.5 and 1.5 g of VKLM had the same pH values.
Figure 1. Effect of incremental levels of Vachellia karroo leaf meal over time (days) on pH levels of breast meat.
The current study showed that VKLM inclusion levels in the diets significantly affected breast meat shear force values after 7 days of storage. The breast meat samples from broilers having 1.5 g of VKLM had higher shear force compared to those fed diets having 0, 0.5 and 1.0 g (Figure ). Therefore, this suggests that VKLM inclusion level in the diet tended to harden the meat. The results agree with those obtained by Ng’ambu et al. (Citation2013), who further concluded that higher meat tenderness is highly associated with low pH and cooking loss. This may be very important in preserving the shelf life of the meat and could be attributed to higher dietary natural antioxidants such as tannins present in VKLM, which tend to improve the oxidative stability of the meat (Al-Hijazeen et al., Citation2016, Citation2016). However, VKLM inclusion levels had no effect on broiler breast meat shear force values after 14 days. Similar results were obtained by Hafeni (Citation2013) and Liu et al. (Citation2009) who found non-significant differences in meat tenderness when including VKLM and dietary tannin natural extracts in monogastric animals. Hence, this suggests that VKLM inclusion levels could be included in broiler diets without any adverse effect on meat shear force over 14 days of refrigerated storage.
Figure 2. Effect of incremental levels of Vachellia karroo leaf meal over time (days) on shear force (N) levels of breast meat.
VKLM inclusion levels in the diets significantly affected breast meat cooking loss values after 7 and 14 days (Table and Figure ). The findings of the current results contradict those of Lorenzo et al. (Citation2018) and Cornale et al. (Citation2011) who observed that plant phenolic compounds such as those found in dietary tanniniferous VKLM did not have any significant effect on cooking and drip loss of broiler meat. This contradiction may be attributed to different inclusion levels, or the type of ingredients used when mixing the diet. However, meat from chickens fed 0.5 and 1.5 g of VKLM had lower cooking losses after 7 days of refrigerated storage than meat from broilers fed control diet. The findings are in agreement with those made by Ng’ambu et al. (Citation2013) who reported lower meat cooking losses when increasing VKLM levels in goat diets. This may be due to lower meat pH observed in the current study, which mainly aids the water holding capacity of the meat, hence improving cooking loss, tenderness and juiciness.
Figure 3. Effect of incremental levels of Vachellia karroo leaf meal over time (days) on cooking loss (%) levels of breast meat.
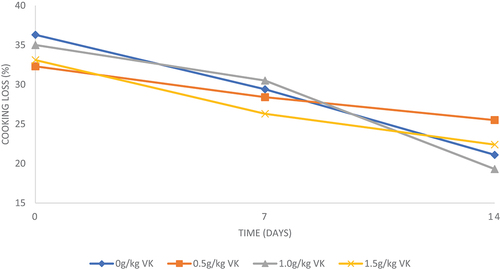
However, after 14 days, meat from broilers on diets having 0.5 and 1.5 g had higher cooking loss trend compared to control (Figure ). The results contradict with findings made by Babiker et al. (Citation2019), who reported that Vachellia extracts improved cooking loss of meat, hence prolonged shelf life until 15 days of refrigerated storage. This may be due to the inclusion of low levels of VKLM since higher tanniniferous leaf meal inclusions increase moisture loss in cooked broiler meat, consequently resulting in drier meat compared to low inclusion levels (Gheisari et al., Citation2017). Therefore, based on the results, low inclusion of VKLM stabilized meat quality parameters, including pH, shear force and cooking loss over refrigerated storage without adverse effect on growth performance. However, further research is required to ascertain the findings.
5. Conclusion
It is concluded that VKLM inclusion level of up to 1.5 g/kg DM could be included in male broiler chicken diets to improve meat quality and shelf life without any adverse effects on chicken performance and productivity.
Acknowledgments
Authors would like to Acknowledge the National Research Foundation and VLIR-OUS project 7 for funding the study. The late Mr Johan Theron as well as all University of Limpopo Eperiential farm workers are also appreciated for aquisation of Acacia leaves and mixing of experimental diets.
Disclosure statement
The authors have no conflicts of interest to declare.
Data availability statement
The data that support the findings of this study are available in “figshare” at https://doi.org/10.6084/m9.figshare.22683412
Additional information
Funding
Notes on contributors

S.D. Kolobe
Sekobane Daniel Kolobe is a PhD student in animal sicence at University of South Africa, Gauteng, South Africa. His research focus is on the utilisation of insects and insect meals in poultry diets.
T.G. Manyelo
Tlou Grace Manyelo (PhD) is a senior lecture and researcher at the University of Limpopo, Limpopo, University of Limpopo. She has authored many Journal articles in poultry science, mainly broiler and indigenous chickens.
J.W. Ng’ambi
Jones Wilfred Ng’ambi (PhD) is a professor in animal nutrition at University of Limpopo. He has authored many journal articles on poultry and ruminant nutrition and contributed a lot to the development of the field. He has been supervising many MSc and PhD students in animal nutrition at University of Limpopo.
E. Malematja
Emmanuel Malematja is a PhD student in animal science at the University of South Africa.
M.F.D. Nemauluma
Munyadziwa Felicia Dorcus Nemauluma is a PhD student in animal science at the University of South Africa.
References
- Al-Hijazeen, M., Lee, E. J., Mendonca, A., & Ahn, D. U. (2016). Effects of tannic acid on lipid and protein oxidation, color, and volatiles of raw and cooked chicken breast meat during storage. Antioxidants, 5(2), 19. https://doi.org/10.3390/antiox5020019
- AOAC. (2010). Association of Analytical Chemist, Official Method of Analysis (17th ed) (Vol. 1). Association of Official Analytical Chemists (AOAC).
- Ao, T., Cantor, A. H., Pescatore, A. J., & Pierce, J. L. (2008). In vitro evaluation of feed-grade enzyme activity at pH levels simulating various parts of the avian digestive tract. Animal Feed Science and Technology, 140(3–4), 462–12. https://doi.org/10.1016/j.anifeedsci.2007.04.004
- Babiker, E. E., Al-Juhaimi, F. Y., Alqah, H. A., Adisa, A. R., Adiamo, O. Q., Mohamed Ahmed, I. A., Alsawmahi, O. N., Ghafoor, K., & Ozcan, M. M. (2019). The effect of Acacia nilotica seed extract on the physicochemical, microbiological, and oxidative stability of chicken patties. Journal of Food Science and Technology, 56(8), 3910–3920. https://doi.org/10.1007/s13197-019-03862-y
- Brown, D., Ng’ambi, J. W., & Norris, D. (2017). Effect of tanniniferous Vachellia karroo leaf meal inclusion level on feed intake, digestibility and live weight gain of goats fed a Setaria verticillata grass hay-based diet. Journal of Applied Animal Research, 46(1), 248–253. https://doi.org/10.1080/09712119.2017.1289939
- Cornale, P., Parantola, M., Lussiana, C., Tassone, S., Castellina, C., & Battaglini, L. M. (2011). Effects of ginger (Zingiber officinale) and European Stoneseed (Lithospermum officinale) extracts on performances, meat quality and fatty acid composition of finishing bulls. Annalen Warsaw University Life Science SGGW Animal Science, 49, 11–20. https://doi.org/10.3923/javaa.2011.1127.1132
- Cui, Y. M., Wang, J., Lu, W., Zhang, H. J., Wu, S. G., & Qi, G. H. (2018). Effect of dietary supplementation with Moringa oleifera leaf on performance, meat quality, and oxidative stability of meat in broilers. Poultry Science, 97(8), 2836–2844. https://doi.org/10.3382/ps/pey122
- Cunha, L. C. M., Monteiro, M. L. G., Lorenzo, J. M., Munekata, P. E. S., Muchenje, V., de Carvalho, F. A. L., & Conte-Junior, C. A. (2018). Natural antioxidants in processing and storage stability of sheep and goat meat products. Food Research International, 111, 379–390. https://doi.org/10.1016/j.foodres.2018.05.041
- Ebrahim, R., Liang, J. B., Jahromi, M. F., Shokryazdan, P., Ebrahimi, M., Li Chen, W., & Goh, Y. M. (2015). Effects of tannic acid on performance and fatty acid composition of breast muscle in broiler chickens under heat stress. Italian Journal of Animal Science, 14(4), 3956. https://doi.org/10.4081/ijas.2015.3956
- Falowo, A. B., Fayemi, P. O., & Muchenjie, V. (2014). Natural antioxidants against lipid-protein oxidative deterioration in meat and meat products. Food Research International, 64(14), 171–181. https://doi.org/10.1016/j.foodres.2014.06.022
- Gheisari, A. A., le Saheb Fosoul, S. S., Pourali, S., Nasre Esfahani, E., & Mohammadrezaei, M. (2017). Blood lipid metabolites and meat lipid peroxidation responses of broiler chickens to dietary lecithinized palm oil. South African Journal of Animal Science, 47(4), 526–534. https://doi.org/10.4314/sajas.v47i4.11
- Gudiso, X., Hlatini, V., Chimonyo, M., & Mafongoya, P. (2019). Response of broiler (Gallus gallus domesticus) performance and carcass traits to increasing levels of Acacia angustissima leaf meal as a partial replacement of standard protein sources. The Journal of Applied Poultry Research, 28(1), 13–22. https://doi.org/10.3382/japr/pfx068
- Hafeni, S., 2013. Performance of broiler chickens fed Pearl Millet as an energy source and Acacia karroo leaf meal as an additive ( Doctoral dissertation). http://hdl.handle.net/11070/872
- Hafiz, A., Zaiton Hassan, Z., & Manap, M. N. A. (2015). Effect of slaughtering methods on meat quality. indicators, chemical changes and microbiological quality of broiler chicken meat during refrigerated storage. J. Agricultural Veterinary ScienceJournal of Agriculture and Veterinary Science, 8(9), 12–17.
- Hygreeva, D., Pandey, M. C., & Radhakrishna, K. (2014). Potential applications of plant-based derivatives as fat replacers, antioxidants, and antimicrobials in fresh and processed meat products: Review. Meat Science, 98(1), 47–57. https://doi.org/10.1016/j.meatsci.2014.04.006
- Kang, K. R., Cherian, G., & Sim, J. S. (2001). Dietary palm oil alters the lipid stability of polyunsaturated fatty acid-modified poultry products. Poultry Science, 80(2), 228–234. https://doi.org/10.1093/ps/80.2.228
- Kolobe, S. D., Manyelo, T. G., Ngambi, J. W., Nemauluma, M. F. D., & Malematja, E. (2022). Effect of Acacia karroo leaf meal inclusion levels on performance and gut morphology of broiler chickens. Advances in Animal and Veterinary Sciences, 10(11), 2347–2355. https://doi.org/10.17582/journal.aavs/2022/10.11.2347.2355
- Liu, H. W., Gai, F., Gasco, L., Brugiapaglia, A., Lussiana, C., Guo, K. J., Tong, J. M., & Zoccarato, I. (2009). Effects of chestnut tannins on carcass characteristics, meat quality, lipid oxidation and fatty acid composition of rabbits. Meat Science, 83(4), 678–683. https://doi.org/10.1016/j.meatsci.2009.08.003
- Lorenzo, J. M., Pateiro, M., Domínguez, R., Barba, F. J., Putnik, P., Kovačević, D. B., Shpigelman, A., Granato, D., & Franco, D. (2018). Berries extracts as natural antioxidants in meat products. A review. Food Research International, 106, 1095–1104. https://doi.org/10.1016/j.foodres.2017.12.005
- Manyelo, T. G., Ng’ambi, J. W., Mabelebele, M., & Mabelebele, M. (2019). Effects of replacing maize meal with a low tannin white sorghum meal, macia on productivity of Ross 308 broilers aged 1-42 days. South African Journal of Animal Science, 49(3), 478–484. https://doi.org/10.4314/sajas.v49i3.8
- Mapiye, C., Chimonyo, M., Marufu, M. C., & Dzama, K. (2011). Utility of Acacia karroo for beef production in Southern African smallholder farming systems: A review. Animal Feed Science and Technology, 164(3–4), 135–146. https://doi.org/10.1016/j.anifeedsci.2011.01.006
- Mbajiorgu, C. A., Ng’ambi, J. W., & Norris, D. (2007). Effect of time of initiation of feeding after hatching and influence of dietary lysine supplementation on productivity and carcass characteristics of Ross 308 broiler chickens in South Africa. International Journal of Poultry Science, 6(8), 2–12. https://doi.org/10.3923/ijps.2007.583.591
- McDonald, P. R., Edwards, A., Greenhalgh, J. F. D., Morgan, C. A., Sinclair, L. A., & Wilkinson, R. G. (2010). Animal Nutrition (7th ed.). Prentice Hall. U.K.
- Musa, H. H., Chen, G. H., Cheng, J. H., Li, B. C., & Mekki, D. M. (2006). Study on carcass characteristics of chicken breeds raised under the intensive condition. International Journal of Poultry Science, 5(6), 530–533. https://doi.org/10.3923/ijps.2006.530.533
- Ncube, S., Halimani, T. E., Chikosi, E. V. I., & Saidi, P. T. (2018). Effect of Acacia angustissima leaf meal on performance, yield of carcass components and meat quality of broilers. South African Journal of Animal Science, 48(2), 271–283. https://doi.org/10.4314/sajas.v48i2.8
- Ng’ambi, J. W., Nakalebe, P. M., Norris, D., Malatje, M. S., & Mbajiorgu, C. A. (2009). Effects of dietary energy level and tanniferous Vachellia karroo leaf meal level of supplementation at finisher stage on performance and carcass characteristics of Ross 308 broiler chickens in South Africa. International Journal of Poultry Science, 8(1), 40–46. https://doi.org/10.3923/ijps.2009.40.46
- Ng’ambu, S., Muchenje, V., & Marume, U. (2013). Effect of Vachellia karroo supplementation on growth, ultimate pH, colour and cooking losses of meat from indigenous Xhosa lop-eared goats. Asian-Australasian Journal of Animal Sciences, 26(1), 128–133. https://doi.org/10.5713/ajas.2012.12046
- Nikmaram, N., Budaraju, S., Barba, F. J., Lorenzo, J. M., Cox, R. B., Mallikarjunan, K., & Roohinejad, S. (2018). Application of plant extracts to improve the shelf-life, nutritional and health-related properties of ready-to-eat meat products. A review. Meat Science, 145, 245–255. https://doi.org/10.1016/j.meatsci.2018.06.031
- Northcutt, J. K., Foegeding, E. A., & Edens, F. W. (1994). Water-holding properties of thermally preconditioned chicken breast and leg meat. Poultry Science, 73(2), 308–316. https://doi.org/10.3382/ps.0730308
- Przywitowski, M., Mikulski, D., Zdunczyk, Z., Rogiewicz, A., & Jankowski, J. (2016). The effect of dietary high-tannin and low-tannin faba bean (Vicia faba L.) on the growth performance, carcass traits and breast meat characteristics of finisher turkeys. Animal Feed Science and Technology, 221, 124–136. https://doi.org/10.1016/j.anifeedsci.2016.08.027
- Sanchez, N. R., Sporndly, E., & Ledin, I. (2006). Effect of feeding different levels of foliage of Moringa oleifera to creole dairy cows on intake, digestibility, milk production and composition. Livestock Science, 101(1–3), 24–31. https://doi.org/10.1016/j.livprodsci.2005.09.010
- SAS Institute Inc. (2013). Base SAS® 9.4 Procedures Guide: Statistical Procedures (Second ed.).
- Shah, M. A., Bosco, S. J. D., & Mir, S. A. (2014). Plant extracts as natural antioxidants in meat and meat products. A review. Meat Science, 98(1), 21–33. https://doi.org/10.1016/j.meatsci.2014.03.020
- Tan, S. M., de Kock, H. L., Dykes, G. A., Coorey, R., & Buys, E. M. (2018). Enhancement of poultry meat: Trends, nutritional profile, legislation, and challenges. South African Journal of Animal Science, 48(2), 1–14. https://doi.org/10.4314/sajas.v48i2.1
- Tshabalala, P. A., Strydom, P. E., Webb, E. C., & de Kock, H. L. (2003). Meat quality of designated South African indigenous goat and sheep breeds P.A. Meat Science, 65(1), 563–570. https://doi.org/10.1016/S03091740(02)00249-8
- Van Ngo, T., Scarlett, C. J., Bowyer, M. C., Ngo, P. D., & Van Vuong, Q. (2017). Impact of different extraction solvents on bioactive compounds and antioxidant capacity from the root of Salacia chinensis L. Journal of Food Quality, 1–8. https://doi.org/10.1155/2017/9305047
- Vasta, V., Nudda, A., Cannas, A., Lanza, M., & Priolo, A. (2008). Alternative feed resources and their effects on the quality of meat and milk from small ruminants: Review. Animal Feed Science and Technology, 147(1–3), 223–246. https://doi.org/10.1016/j.anifeedsci.2007.09.020
- Wapi, C., Nkukwana, T. T., Hoffman, L. C., Dzama, K., Pieterse, E., Mabusela, T., & Muchenjie, V. (2013). Physico-chemical shelf-life indicators of meat from Ross 308 broilers given Moringa oleifera leaf meal. South African Journal of Animal Science, 43(5), 1–5. https://doi.org/10.4314/sajas.v43i5.8
- Wattanachant, S. (2008). Factors affecting the quality characteristics of Thai indigenous chicken meat. Warasan Technology Suranaree, 15(4), 317–322. http://sutir.sut.ac.th:8080/jspui/bitstream/123456789/3788/1/v15n4p317.pdf
