Abstract
Currently, the occurrence of diseases in the digestive system in swine has increased. Environmental stress and gastric bacteria were associated with body imbalance and stress ulcer, which are related to pathological changes in the stomach. This study aimed to investigate stomach histological characteristics of beta-agonist free, hygienic, and natural swine in different farming methods. Three types of swine’s stomachs were bought from three different farms. The samples in each part of the stomach, including the fundus, body, antrum, and pylorus, were processed through histopathological techniques and dyed with haematoxylin and eosin. The results showed that in the fundus of the stomach, beta agonist-free swine had the thinnest mucosa and submucosa layers with the highest number of inflammatory cells in both layers (p < 0.001, all). The hygienic swine showed the highest amount of red blood cells in the body, antrum, and pylorus of the stomachs (p < 0.001). In conclusion, we showed the histological characteristics of stomachs in three kinds of swine, which presents swine stomach health status. This could be information for consumers.
PUBLIC INTEREST STATEMENT
Variations of living environment, feeding, and housing in different swine farm types might lead to variable stomach health of swine. In Thailand, there are three distinct kinds of swine farming including beta-agonist free, hygienic, and natural swine. Histological changes of swine stomach might refer to the stomach’s health. We aimed to investigate stomach histological characteristics of beta-agonist free, hygienic, and natural swine in three different farming methods. We found that the fundus region of stomachs of beta agonist-free swine had the thinnest mucosa and submucosa layers with the highest number of inflammatory cells in both layers. The hygienic swine had the highest amount of red blood cells in the body, antrum, and pylorus of the stomachs. The thickness of mucosa and submucosa layers and the appearance of inflammatory and red blood cells might suggest an abnormal status, inflammatory condition, and genetic variation, respectively, occurring in the swine.
1. Introduction
Porcine gastric ulceration is a common disease associated with increased mortality of pigs (Melnichouk, Citation2002) and important economic losses (De Witte et al., Citation2018). There was a sudden death of swine from gastric ulcer bleeding during the growing stage in some farms, resulting in significant losses of economic income (Melnichouk, Citation2002).
A swine’s stomach has four distinct areas, including the esophageal, cardiac, fundic, and pyloric regions (Bauer et al., Citation2020). Gastric ulcer lesions in swine are most frequently found in the pars esophageal of the stomach (Hessing et al., Citation1992; Queiroz et al., Citation1996) which are the non-glandular region unprotected by a mucus coating (Omotosho et al., Citation2016) and occasionally found in the fundus which is rare and questioned the cause (Omotosho et al., Citation2016). Gross anatomy and histology of swine’s stomach with gastric ulcer were strikingly similar to those of ones observed in humans (Kernkamp, Citation1945). Generally, the gross appearance of ulcerated swine stomach is directly associated with the histological appearance of various degrees of ulceration, erosion, and parakeratosis (Kopinski & McKenzie, Citation2007), which is the nuclei retention in the stratum corneum seen as rough surface. Moreover, in the pars esophagea of pig’s stomach, there was an association between gastric ulcers and a change in the stratified squamous epithelium, leading to the formation of excess keratin on the epithelial surface known as hyperkeratosis (Roels et al., Citation1997). The ulcers may be recovered with epithelium and healed uneventfully in some swine. A previous study revealed that nutritional factors and method of feeding had an impact on gastric ulcer status (Reese et al., Citation1966), and the disease status might affect feed consumption (Juan Miguel J. M. Peralvo-Vidal, N. R. Weber, J. P. Nielsen, J. K. Bache, S. Haugegaard, & A. Pedersen, Citation2021). A decrease in feed intake is associated with a delayed growth rate of normal-birth-weight pigs (van Erp et al., Citation2018). The means of swine farming might affect the swine’s gut health, which is fundamental to the swine’s holistic health and well-being. Housing environment is correlated with the prevalence and severity of gastric ulcers in slaughter pigs (Amory et al., Citation2006). Preventing or reducing gastric ulcer in swine could be achieved by feed quality, including feed particle fineness, feed nutrient content, and concurrent gastric bacterial infections (Doster, Citation2000).
In Thailand, there are different kinds of swine farming, including beta-agonist free, hygienic, and natural swine. The beta-agonist free pork is the pork from farms with no beta-agonist using the farm housing supervised by the Department of Livestock Development and feeding an exact recipe to control the daily intake of nutrients. Hygienic pork is from sanitary pig farms that do not use beta-agonist and antibiotics; they are raised in a closed system with temperature and pathogens controlled by using detergent and drugs for farm disinfection. Hygienic swine is housed and farmed if they pass the standard certification from the Department of Livestock Development. Natural swine is raised naturally without restriction on places; they are raised in farms certified by the Department of Livestock Development. Herbs such as rhizome of Curcuma longa L. and the leaf of Psidium guajava L. have been used when swine get sick. Information across beta-agonist free, hygienic, and natural swine farming methods is summarized in Table .
Table 1. Information across beta-agonist free, hygienic, and natural swine farming methods
These different farming methods might lead to different pigs’ gut health that may be reflected by the morphology and histology of swine gastrointestinal specimens, especially their stomach. In this context, this study aimed to investigate stomach histological characteristics of beta-agonist free, hygienic, and natural swine methods. Knowledge from this study will illustrate the stomach’s health status of three kinds of swine for the consumers’ reference.
2. Materials and Methods
2.1. Ethical Considerations
This study was consistent with the Laboratory Animal Use Convention published by the National Institutes of Health. All animal experimental procedures were approved by the animal ethics committee of the Center for Animal Research, Naresuan University (Approval no. NU-AEE620505).
2.2. Sample collection and histological analysis
Twenty-seven pieces of swine stomachs collected from four-month-old pigs were bought from beta-agonist free, hygienic, and natural farm enterprises in Nakhon Pathom, Thailand. Three pieces of tissues from each part of the stomach, including the fundus, body, antrum, and pylorus in each swine, were cut into 0.5 × 1 cm size. Then, the cut tissues were fixed in 10% neutral buffered formalin for 7 days. After that, the fixed tissues were processed and embedded in paraffin. A thickness of 3 µm per section was cut by rotary microtome in superficial, middle, and deep parts from each tissue paraffin block. Hematoxylin and eosin staining were performed in all 324 tissue slides, and then all slides were mounted. Photos were taken in three fields of all slides by an Olympus B×51 light microscope connected to a digital camera, and then all images were recorded by a ZEN 2012 (blue edition) program. For all swine stomach tissue slides, the histological characteristics of three collected fields were microscopically determined. Morphological and quantitative evaluation to measure the thickness of the stomach wall, including mucosa, submucosa, and muscularis externa layers, were determined by 2.5× field. The quantification of the inflammatory cell number of mucosa and submucosa layers was determined by 40× field. To view the characteristic of gastric gland in the mucosa layer, all slides were evaluated by 10× field. The quantitative results for the thickness of mucosa, submucosa, and muscularis externa layers were analyzed by the ImageJ program.
2.3. Statistical analysis
The quantities of the stomach wall’s thickness and the histological characteristics of swine stomach in beta-agonist free, hygienic, and natural swine farming methods were determined. For the quantitative data, the comparisons among groups were analyzed by one-way analysis of variance method followed by the Bonferroni post-hoc test. The data in each experiment were calculated using the mean ± standard deviation (SD). A p value of <0.05 was considered a statistically significant difference.
3. Results
3.1. Thickness of mucosa, submucosa, and muscularis layers of swine’s stomach
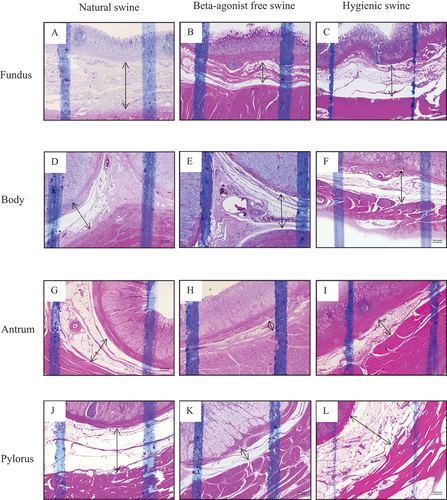
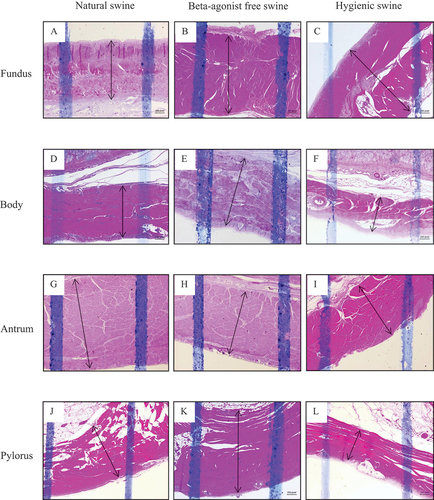
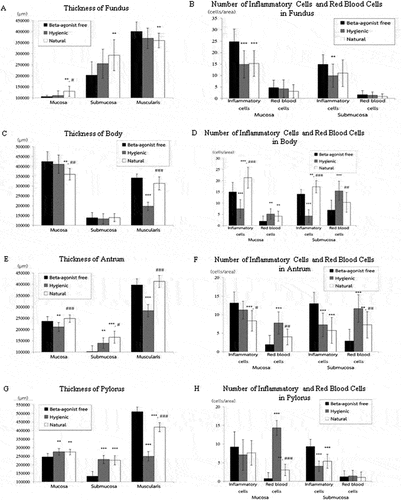
We first examined the thickness of the mucosa, submucosa, and muscularis layers of beta-agonist free, hygienic, and natural swine’s stomach. Figures showed morphology reflecting the thickness of mucosa, submucosa, and muscularis layers of stomach structures, including the fundus, body, antrum, and pylorus sections, respectively. We found that the three layers of stomach structures, including the fundus, body, antrum, and pylorus sections, differed among beta-agonist free, hygienic, and natural swine (Figure ). In the fundus region, the mucosa layer of natural swine was significantly thicker than that of beta-agonist free and hygienic swine (p < 0.01 and p < 0.05, respectively, Figure ). Submucosa and muscularis layers of natural swine were significantly thicker than those of beta-agonist free swine (p < 0.01, all, Figure ) but did not differ from that of hygienic swine. In the body region, the mucosa layer of natural swine was significantly thinner than that of beta-agonist free and hygienic swine (p < 0.01, all, Figure ). There was a comparable thickness of the submucosa layer among beta-agonist free, hygienic, and natural swine (Figure ). The muscularis layer of hygienic swine was thinner than beta-agonist free and natural swine (p < 0.001, all), while there was no difference in the muscularis layer between beta-agonist free and natural swine (Figure ). In the antrum region, the mucosa layer of natural and beta-agonist free swine was significantly thicker than that of hygienic swine (p < 0.001 and p < 0.01, respectively, Figure ). The submucosa layer of natural swine was significantly thicker than that of beta-agonist free and hygienic swine (p < 0.001 and p < 0.05, Figure ). The submucosa layer of hygienic swine was significantly thicker than that of beta-agonist free swine (p < 0.01, Figure ). The muscularis layer of hygienic swine was thinner than that of beta-agonist free and natural swine (p < 0.001, all, Figure ). In the pylorus region (Figure ), the mucosa layer of hygienic and natural swine was significantly thicker than beta-agonist free swine (p < 0.01, Figure ). The submucosa layer of hygienic and natural swine was significantly thicker than that of beta-agonist free swine (p < 0.001, Figure ). For the muscularis layer, hygienic, and natural swine had a significantly thinner layer than beta-agonist free swine (p < 0.001, Figure ). Lastly, when compared between hygienic and natural swine, the muscularis layer of natural swine was significantly thicker than that of hygienic swine (p < 0.001, Figure ).
Figure 1. The histology presenting thickness of mucosa layer of swine’s stomachs in various regions including fundus, body, antrum, and pylorus of natural, beta-agonist free, and hygienic swine. Determined by scanning objective of 2.5× of microscope.
4. Gastric glands and number of inflammatory and red blood cells in the stomach of natural, beta-agonist free, and hygienic swine
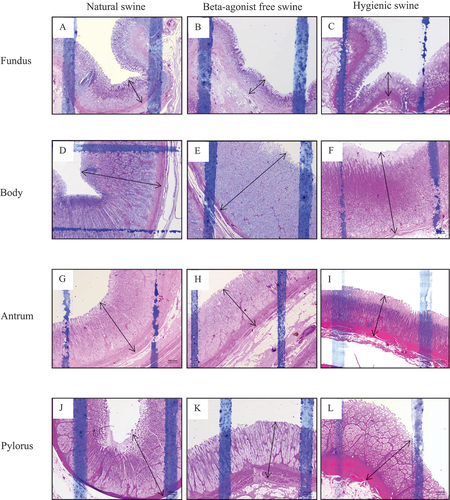
We further investigated the histology of gastric glands and inflammatory and red blood cells in the stomach of beta-agonist free, hygienic, and natural swine. We observed similar characteristics of gastric gland histology in the mucosa layer of the fundus, body, antrum, and pylorus when compared among beta-agonist free, hygienic, and natural swine (Figure ). In the mucosa layer, the simple columnar epithelium lining with the surface mucous cells of gastric pits and parietal cells was found. Chief cells and enteroendocrine cells of gastric glands were also observed under the light microscope. We also found connective tissues and the thin layer of smooth muscle cells as lamina propria and muscularis mucosae layers, respectively. For the red blood cells, we observed the scattered non-nucleated round to oval-shaped cells within the mucosal layer. In addition, the large condensed nucleated cells were also observed and identified as inflammatory cells. Moreover, we determined the number of inflammatory and red blood cells in the mucosa (Figure ) and submucosa (Figure ) layers of the different parts of the stomach, including the fundus, body, antrum, and pylorus. Our results showed that the number of inflammatory cells in the mucosa layer of the fundus of hygienic and natural swine were significantly lower than those of beta-agonist free swine (p < 0.001, Figure ). In the submucosal layer of the fundus, the number of inflammatory cells of hygienic swine were lower than those of beta-agonist free swine (p < 0.01) but did not differ from those of the natural swine (Figure ). When compared among beta-agonist free, hygienic, and natural swine, there was a comparable number of red blood cells in the mucosa and submucosa layers of the fundus (Figure ). In the mucosa layer of the body, the number of inflammatory cells of natural swine were higher than those of beta-agonist free and hygienic swine (p < 0.001, Figure ). Then when compared between beta-agonist free and hygienic swine, the inflammatory cells were significantly higher in the mucosa layer of beta-agonist free and hygienic swine (p < 0.001, Figure ). The number of red blood cells in the mucosa layer were significantly greater in hygienic and natural swine when compared with those of beta-agonist free swine (p < 0.01, Figure ). In the submucosa layer, number of inflammatory cells in natural swine were higher than those of beta-agonist free (p < 0.01) and hygienic swine (p < 0.001). Moreover, the number of inflammatory cells in natural swine were higher than those of hygienic swine (p < 0.001, Figure ). For red blood cells in the submucosa layer, the hygienic swine showed the highest number of red blood cells when compared with those of beta-agonist free (p < 0.001) and natural swine (p < 0.01, Figure ). In the mucosa layer of the antrum, the number of inflammatory cells of natural swine were lower than in beta-agonist free (p < 0.001) and hygienic swine (p < 0.05, Figure ). For red blood cells in the mucosal layer of the antrum, hygienic swine exhibited a higher number of red blood cells than in beta-agonist free (p < 0.001) and natural swine (p < 0.01, Figure ). In the submucosa layer of the antrum, the number of inflammatory cells in beta-agonist free swine were higher than those of hygienic and natural swine (p < 0.001, all, Figure ). Regarding red blood cells in submucosa layer of the antrum, beta-agonist free swine had the fewest red blood cells, followed by those of natural swine and hygienic swine (p < 0.01, all, Figure ). In the pylorus, the number of inflammatory cells in the mucosa layer were comparable among beta-agonist free, hygienic, and natural swine (Figure ). For red blood cells in mucosal layer of the pylorus, hygienic swine showed the highest number of red blood cells, followed by those of natural and beta-agonist free swine, respectively (p < 0.01, all, Figure ). In the submucosa layer of the pylorus, the number of inflammatory cells of beta-agonist free swine was significantly higher than those of hygienic and natural swine (p < 0.001, all, Figure ). For red blood cells in the submucosal layer of the pylorus, there was a comparable number of red blood cells among beta-agonist free, hygienic, and natural swine (Figure ).
Figure 4. Histology of gastric glands in mucosa layer of swine’s stomachs in various regions including fundus, body, antrum, and pylorus of natural, beta-agonist free, and hygienic swine. Determined by scanning objective of 10× of microscope.
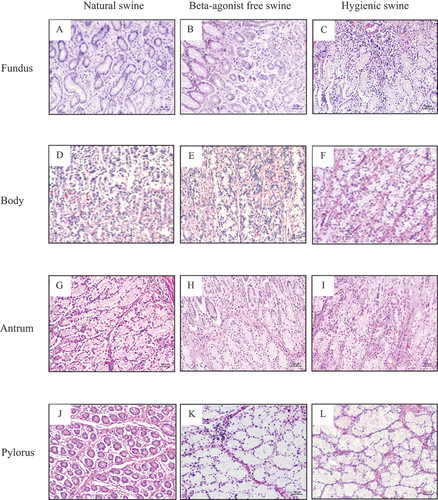
Figure 5. Morphology indicating inflammatory cells indicated as red circle and red blood cells indicated as black arrow in mucosa layer of swine’s stomachs in various regions including fundus, body, antrum, and pylorus of natural, beta-agonist free, and hygienic swine. Determined by scanning objective of 40x of microscope.
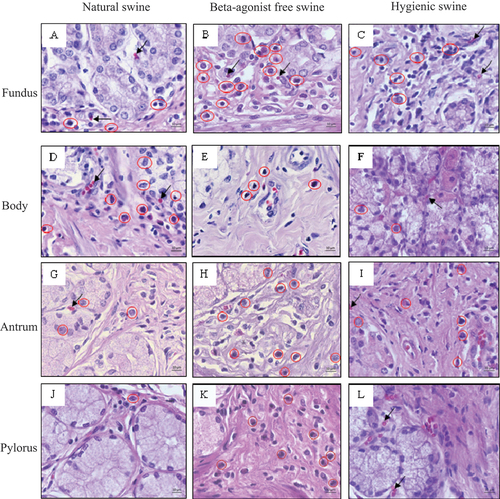
Figure 6. Morphology indicating inflammatory cells indicated as red circle and red blood cells indicated as black arrow in submucosa layer of swine’s stomachs in various regions including fundus, body, antrum, and pylorus of natural, beta-agonist free, and hygienic swine. Determined by scanning objective of 40x of microscope.
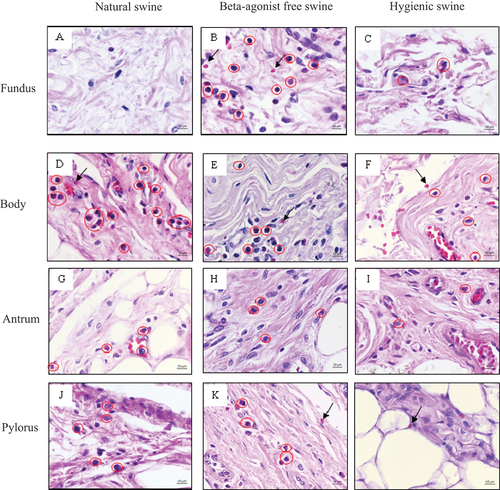
Figure 7. Comparisons of the thickness of fundus, body, antrum, and pylorus regions on mucosa, submucosa, and muscularis layers in swine. Comparisons of number of inflammatory and red blood cells in fundus, body, antrum, and pylorus regions on mucosa and submucosa layers in swine. Data are presented as mean ± S.D. **p < 0.01, ***p < 0.01, #p < 0.05, ##p < 0.01, ###p < 0.001
5. Discussion
To the best of our knowledge, there has been no study on the histology of swine stomachs with different farming methods, whether they will lead to different micro-organistic appearances. To fill this gap, our study is the first one to investigate the stomach’s histological characteristics of beta-agonist free, hygienic, and natural swine. We revealed the novel finding that the stomach of beta-agonist free swine had the thinnest mucosa layer in the fundus and pylorus regions and submucosa layer in the fundus, antrum, and pylorus regions. Moreover, beta-agonist free swine also showed the highest number of inflammatory cells in the mucosa layer of the fundus and antrum and in the submucosa layer of the fundus, antrum, and pylorus of the stomach. Our results suggest that beta-agonist free swine might have some conditions resulting in some inflammatory process in the stomach and the changes in the thickness of the mucosa and submucosa layers. Indeed, the high number of inflammatory cells found in the stomach of beta-agonist free swine strongly suggests the inflammatory condition occurring in the swine. Hereditary, autoimmune processes, inflammatory, and infectious diseases can affect the thickness of the mucosa layer of the stomach. The processes of these diseases involve primary defenses of the stomach wall, leading to changes in the mucosa layer (Sikiric & Brzozowski, Citation2020). For example, Helicobacter spp. infection in swine was found to be strongly associated with naturally occurring ulcer and pre-ulcer lesions of the pars esophagea (Knezević et al., Citation2007). This might lead to changes in morphological manifestations of the region of pig’s stomachs. Moreover, environmental stress is one of the factors creating a negative effect on swine welfare, such as increased secretion of inflammatory substances (Martínez-Miró et al., Citation2016). Slatted floors and pelleted feed were associated with increased occurrence of gastric ulcers and severity of ulcers. Amory et al. (Citation2006) suggested that housing environment and feeding influenced the increase in the prevalence of gastric ulcers. We previously showed that different farming methods resulted in diverse histological characteristics of the hepatocytes. Indeed, we reported high inflammatory cell infiltration and collagen fiber accumulation in the liver samples of beta-agonist free swine (Chayanisa Chaicharoen et al., Citation2019). A previous study revealed that herd characteristics (i.e., the numbers of pigs and herd staff) were associated with antimicrobial use in pig herds (Yun et al., Citation2021). The number of sows negatively correlated with antimicrobial use and the number of sows and the total number of pigs in the herds had strongly positive correlations with the number of pigs per staff. Also, enhancing internal biosecurity measures could reduce the spread of antimicrobial resistance in pig herds (Yun et al., Citation2021). These might suggest that the pigs’ quality of life and welfare can be associated with farm condition with antimicrobial treatments and nursery methods. Hence, improving biosecurity and living environment in farms with antimicrobial use are the major factors that may reduce the number of antimicrobial treatments due to musculoskeletal diseases, joint infections, and respiratory diseases of pigs (Stygar et al., Citation2020). In summary, farming methods and living environment impact pigs’ welfare, which might lead to differences in their organ histology. Here, our data suggested that beta-agonist free farming might associate with thin mucosa/submucosa layers and inflammation of the swine’s stomach.
Another main finding of this study is that hygienic swine exhibited the highest number of red blood cells in the mucosa layer of the body, antrum, and pylorus, and the submucosa layer of the body and antrum of the stomach. The expressions of red blood cells in the mucosa and submucosa layers of the different regions of the swine stomach can be related to stomach hemorrhage and/or swine hereditary characteristics. Early stages of hemorrhage might lead to enhanced bone marrow activity, which compensates for red blood cell production (Penington, Citation1967). However, blood examination could not be performed in this study; hence, further studies with blood investigation and/or a complete blood count are recommended. Additionally, the highest expressions of red blood cells in hygienic swine can be associated with the highest erythrocyte genetic traits compared to the natural and beta-agonist free swine. A previous study showed that red blood cell traits were regulated by multiple genes (Reiner et al., Citation2007). Another study of young pigs with different genetics revealed that the pigs with low residual feed intake had greater hemoglobin concentration and red blood cell volume than those with high residual feed intake (Mpetile et al., Citation2015). This difference suggests that hemorrhage and genetics might be linked to the existence of red blood cells. However, further research should be performed regarding the complete blood count and genetic identification of swine in these three farming methods to determine expressions of red blood cells in swine. Moreover, we found that hygienic swine had the thinnest muscularis layer in their stomach’s body, antrum, and pylorus regions. These results imply that a hygienic environment can be connected to the alteration of the muscularis layer of the swine’s stomach. Muscularis propria atrophy and fibrosis or degenerative changes of myocytes might be because of gastrointestinal tract pathology (Angkathunyakul et al., Citation2015). A previous study in pigs showed that the thickness of muscularis propria was affected by the gastric ulcer, and this was healed by covering it with hexanoyl group-modified alkaline-treated gelatin porous film (Maeda et al., Citation2021). Thus, the environment, management, and feeding conditions can indicate the risk factors predisposing to increased prevalence of gastric ulceration in swine (J. M. Peralvo-Vidal, N. R. Weber, J. P. Nielsen, J. K. Bache, S. Haugegaard, & A. Pedersen, Citation2021). Here, we observed that hygienic farming methods influence gastric ulceration, which trended to the reduced muscularis layer of the hygienic swine.
Therefore, among the three different kinds of swine, we revealed the novel finding that beta-agonist free swine had the thinnest mucosa layer in the fundus and pylorus regions and the submucosa layer in the fundus, antrum, and pylorus regions. Beta-agonist free swine also exhibited the highest number of inflammatory cells in the mucosa layer of the fundus and antrum and in the submucosa layer of the fundus, antrum, and pylorus of the stomach. Hygienic swine showed the highest number of red blood cells in the mucosa layer of the stomach’s body, antrum, and pylorus and the submucosa layer of the stomach’s body and antrum. Finally, hygienic swine had the thinnest muscularis layer in their stomach’s body, antrum, and pylorus regions. These illustrate histological characteristics and stomach health status of the three kinds of swine and provide essential information to consumers. However, our study still has some limitations, including (1) revealing the disease status of the swine could not be provided; (2) the inflammatory and red blood cells were measured only in the stomach, which might not represent their existence in the whole body; (3) the sex of the swine could not be controlled, so samples collected from them might have a confounding factor notably regarding hormonal variation; (4) immunohistochemistry that can be used to determine the subtype of inflammatory cells could not be performed because of the limitation on the budget and scarcity of the samples; and (5) the genetic studies that explore swine generalities in different farming methods were not performed. Further studies that identify swine diseases, collect samples from the same sex of swine, measure the inflammatory substances and red blood cell counts in the circulation of swine, and use immunohistochemical staining to determine the subtype of inflammatory cells are suggested. Moreover, genetic studies that explore swine generalities based on the production systems in Thailand are recommended. These studies may provide further information on the overall health status of pigs raised under different conditions and their stomach.
6. Geolocation information
This work was performed at Department of Anatomy, Faculty of Medical Science, Naresuan University, Phitsanulok 65000, Thailand and at Princess Srisavangavadhana College of Medicine, Chulabhorn Royal Academy, Bangkok 10210, Thailand.
Correction
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Authors’ contributions
SC and CP participated in the conceptualization, methodology, investigation, data analysis, and writing of the original draft. SC, CP, and AM review and editing of the manuscript. NK, AI, and SJ participated in the data analysis and investigation. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
The authors would like to acknowledge Asst. Prof. Dr. Sukanya Horpaopan, Asst. Prof. Dr. Pichaya Jumnongprakhon, Lect. Dr. Kaemisa Srisen, Mr. Phisid Saenganantakarn, and Ms. Sureeporn Nakung for beneficially scientific and experimental suggestions. We also thank Department of Anatomy and Medical Science Academic Service Center, Faculty of Medical Science, Naresuan University for experimental facilities supporting.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request http://dx.doi.org/10.1080/23311932.2023.2208248.
Additional information
Funding
Notes on contributors

Charkriya Promsuban
Our team has interested in one health. Here, we have studied both structural and functional aspects of swine. We had started from a gastrointestinal organ of the swine which is stomach by staining it with Hematoxylin and Eosin. Our present study found that thickness and numbers of both inflammatory cells and red blood cells in different layers of beta-agonist free, hygienic, and natural swine were different. We also expect to explore the swine project in functional and genomic fields for our further studies.
References
- Amory, J. R., Mackenzie, A. M., & Pearce, G. P. (2006). Factors in the housing environment of finisher pigs associated with the development of gastric ulcers. The Veterinary Record, 158(8), 260–13. https://doi.org/10.1136/vr.158.8.260
- Angkathunyakul, N., Treepongkaruna, S., Molagool, S., & Ruangwattanapaisarn, N. (2015). Abnormal layering of muscularis propria as a cause of chronic intestinal pseudo-obstruction: A case report and literature review. World Journal of Gastroenterology: WJG, 21(22), 7059–7064. https://doi.org/10.3748/wjg.v21.i22.7059
- Bauer, M., Morales-Orcajo, E., Klemm, L., Seydewitz, R., Fiebach, V., Siebert, T., & Böl, M. (2020). Biomechanical and microstructural characterisation of the porcine stomach wall: Location- and layer-dependent investigations. Acta Biomaterialia, 102, 83–99. https://doi.org/10.1016/j.actbio.2019.11.038
- Chayanisa Chaicharoen, O. H., Thorsuwan, S., Jaikua, W., Monteil, A., & Promsuban, C. (2019). Histological study of natural, beta-agonist free, and hygienic pork livers. International Journal of Applied Biomedical Engineering, 12(2), 1–7.
- De Witte, C., Ducatelle, R., & Haesebrouck, F. (2018). The role of infectious agents in the development of porcine gastric ulceration. Veterinary Journal (London, England: 1997), 236, 56–61. https://doi.org/10.1016/j.tvjl.2018.04.015
- Doster, A. R. (2000). Porcine Gastric Ulcer. The Veterinary Clinics of North America Food Animal Practice, 16(1), 163–174. https://doi.org/10.1016/S0749-0720(15)30141-9
- Hessing, M. J., Geudeke, M. J., Scheepens, C. J., Tielen, M. J., Schouten, W. G., & Wiepkema, P. R. (1992). Mucosal lesions in the pars esophagus in swine: Prevalence and the effect of stress. Tijdschrift voor diergeneeskunde, 117(15–16), 445–450.
- Kernkamp, H. C. (1945). Gastric Ulcer in Swine. The American Journal of Pathology, 21(1), 111–113.
- Knezević, M., Aleksić-Kovacević, S., & Aleksić, Z. (2007). Cell proliferation in pathogenesis of esophagogastric lesions in pigs. International Review of Cytology, 260, 1–34. https://doi.org/10.1016/s0074-7696(06)60001-6
- Kopinski, J. S., & McKenzie, R. A. (2007). Oesophagogastric ulceration in pigs: A visual morphological scoring guide. Australian Veterinary Journal, 85(9), 356–361. https://doi.org/10.1111/j.1751-0813.2007.196_1.x
- Maeda, H., Sasaki, F., Morinaga, Y., Kabayama, M., Iwaya, H., Komaki, Y., Arima, S., Nasu, Y., Tanoue, S., Hashimoto, S., Kanmura, S., Nishiguchi, A., Taguchi, T., & Ido, A. (2021). Covering post-endoscopic submucosal dissection ulcers in miniature swine with hexanoyl (Hx: C6) group-modified alkaline-treated gelatin porous film (hag) induces proper healing by decreasing inflammation and fibrosis. Digestion, 102(3), 415–427. https://doi.org/10.1159/000509056
- Martínez-Miró, S., Tecles, F., Ramón, M., Escribano, D., Hernández, F., Madrid, J., Orengo, J., Martínez-Subiela, S., Manteca, X., & Cerón, J. J. (2016). Causes, consequences and biomarkers of stress in swine: An update. BMC Veterinary Research, 12(1), 171. https://doi.org/10.1186/s12917-016-0791-8
- Melnichouk, S. I. (2002). Mortality associated with gastric ulceration in swine. The Canadian Veterinary Journal La Revue Veterinaire Canadienne, 43(3), 223–225.
- Mpetile, Z., Young, J. M., Gabler, N. K., Dekkers, J. C., & Tuggle, C. K. (2015). Assessing peripheral blood cell profile of Yorkshire pigs divergently selected for residual feed intake. Journal of Animal Science, 93(3), 892–899. https://doi.org/10.2527/jas.2014-8132
- Omotosho, O. O., Emikpe, B. O., Lasisi, O. T., & Jarikre, T. A. (2016). Prevalence, distribution and pattern of gastric lesions in slaughtered pigs in south-western Nigeria. The Onderstepoort Journal of Veterinary Research, 83(1), a1063. https://doi.org/10.4102/ojvr.v83i1.1063
- Penington, D. G. (1967). Regulation of red cell and platelet production. Proceedings of the Royal Society of Medicine, 60(10), 1032–1036. https://doi.org/10.1177/003591576706001043
- Peralvo-Vidal, J. M., Weber, N. R., Nielsen, J. P., Bache, J. K., Haugegaard, S., & Pedersen, A. (2021). Risk factors for gastric ulceration in nursery pigs. Preventive Veterinary Medicine, 189, 105298. https://doi.org/10.1016/j.prevetmed.2021.105298
- Queiroz, D. M., Rocha, G. A., Mendes, E. N., De Moura, S. B., De Oliveira, A. M., & Miranda, D. (1996). Association between Helicobacter and gastric ulcer disease of the pars esophagea in swine. Gastroenterology, 111(1), 19–27. https://doi.org/10.1053/gast.1996.v111.pm8698198
- Reese, N. A., Muggenburg, B. A., Kowalczyk, T., Grummer, R. H., & Hoekstra, W. G. (1966). Nutritional and environmental factors influencing gastric ulcers in swine. Journal of Animal Science, 25(1), 14–20. https://doi.org/10.2527/jas1966.25114x
- Reiner, G., Fischer, R., Hepp, S., Berge, T., Köhler, F., & Willems, H. (2007). Quantitative trait loci for red blood cell traits in swine. Animal Genetics, 38(5), 447–452. https://doi.org/10.1111/j.1365-2052.2007.01629.x
- Roels, S., Ducatelle, R., & Broekaert, D. (1997). Keratin pattern in hyperkeratotic and ulcerated gastric pars oesophagea in pigs. Research in Veterinary Science, 62(2), 165–169. https://doi.org/10.1016/s0034-5288(97)90140-5
- Sikiric, P., & Brzozowski, T. (2020). Physiological and pharmacological mechanisms in gastrointestinal protection, ulcer healing and mucosal repair - an update. Current Pharmaceutical Design, 26(25), 2933–2935. https://doi.org/10.2174/138161282625200629111007
- Stygar, A. H., Chantziaras, I., Toppari, I., Maes, D., & Niemi, J. K. (2020). High biosecurity and welfare standards in fattening pig farms are associated with reduced antimicrobial use. Animal, 14(10), 2178–2186. https://doi.org/10.1017/s1751731120000828
- van Erp, R. J. J., van Hees, H. M. J., Zijlstra, R. T., van Kempen, T., van Klinken, J. B., & Gerrits, W. J. J. (2018). Reduced feed intake, rather than increased energy losses, explains variation in growth rates of normal-birth-weight piglets. The Journal of Nutrition, 148(11), 1794–1803. https://doi.org/10.1093/jn/nxy200
- Yun, J., Muurinen, J., Nykäsenoja, S., Seppä-Lassila, L., Sali, V., Suomi, J., Tuominen, P., Joutsen, S., Hämäläinen, M., Olkkola, S., Myllyniemi, A. L., Peltoniemi, O., & Heinonen, M. (2021). Antimicrobial use, biosecurity, herd characteristics, and antimicrobial resistance in indicator Escherichia coli in ten Finnish pig farms. Preventive Veterinary Medicine, 193, 105408. https://doi.org/10.1016/j.prevetmed.2021.105408