Abstract
The sustainable use of scarce natural resources is a significant challenge for the aquaculture industry’s future development. There are several obstacles to future expansion, including problems with fish nutrition and disease management. Aquaculture is a rapidly growing industry that faces challenges in maintaining the sustainability and environmental friendliness of production. Aquatic environments make fish especially susceptible to widespread pathogens, making disease and health management important for sustainable development. The use of probiotics has been proposed as a potential solution to these challenges. Probiotics are live microorganisms that when administered in adequate amounts, confer a health benefit on the host. In aquaculture, probiotics have been shown to improve growth rates, disease resistance, and water quality. Using probiotics to modulate intestinal microbiota is an alternative method of reducing pathogen adhesion and colonization in larval, fry, and juvenile intestines. To increase the proportion of healthy bacteria in the gut, they are administered into a fish diet or water. However, the research on the applications of probiotics in sustainable and environmentally friendly aquaculture is still not fully completed. Further research is needed to address the knowledge gap and to investigate the potential benefits and limitations of probiotics in different aquaculture systems. Areas that need to be addressed include the selection and optimization of probiotics for specific aquaculture species and production systems, the development of effective delivery methods, and the assessment of the long-term effects of probiotic use on the environment and the host. By addressing these areas, probiotics can be integrated into sustainable and environmentally friendly aquaculture practices to support the growth of the industry while minimizing its impact on the environment. This review aims to provide a comprehensive overview of the sources and standards of assortment and actions of microorganisms used in aquaculture as probiotics, whether native or commercial. Additionally, this review examines the mechanisms of action by which probiotics promote fish health and the current state of knowledge on the efficacy of probiotics in immunity, and the interchange between the reproduction and metabolism of probiotics in aquaculture. Overall, this review provides valuable insights for researchers and industry professionals seeking to effectively utilize probiotics in aquaculture.
1. Introduction
An aquaculture industry that is sustainable relies heavily on aquafeeds and aquaculture health management. Approximately, half of the global fish production was accounted for by aquaculture in 2019, and the industry remains the fastest-growing sector (Wei et al., Citation2022). Due to consumer market demand and technological advancements, the industry has dramatically shifted to more intensive farming systems to increase yields, causing stress to farmed fish. A significant challenge for the industry’s future development is the sustainable use of scarce natural resources. There are several obstacles to future expansion, including problems with fish nutrition and disease management. Aquatic environments make fish especially susceptible to widespread pathogens, making disease and health management important for sustainable development (Wuertz et al., Citation2021). A great concern is the degradation of the rearing environment, including water quality, as the well-being of aquatic organisms is greatly dependent on it (Br¨onmark & Hansson, Citation2017; Hura et al., Citation2018). When water quality deteriorates rapidly due to high stocking density, stress increases, resulting in an environment conducive to the proliferation of pathogenic microorganisms, and eventual mortality among cultured species (Lieke et al., Citation2019). Fish production can decrease due to poor feed conversion ratios and high mortality rates (Ghori et al., Citation2022). In aquaculture, antibiotic treatment has been linked to the emergence of resistant bacteria (Allameh et al., Citation2016; Ghori et al., Citation2022). Scientists have been searching for alternate controls due to a rise in antibiotic resistance and concerns about consumer safety (Ghori et al., Citation2022; Pandiyan et al., Citation2013). Aquaculture management studies are therefore geared toward using probiotics (Hlordzi et al., Citation2020; Hura et al., Citation2018). As probiotics improve both water quality and give added benefits to cultured fish, they are becoming increasingly popular for the remediation of aquaculture. In addition to immuno-stimulating the immune system, probiotics provide nutrients and enzymes, improve feed utilization and growth, and inhibit pathogens directly (Hlordzi et al., Citation2020; Hoseinifar et al., Citation2018; Kuebutornye et al., Citation2019; Ringø et al., Citation2018; Van Doan et al., Citation2019). It has been shown that probiotics have the potential to improve water quality, thus, allowing aquatic organisms to grow and thrive (Hlordzi et al., Citation2020; Hura et al., Citation2018). In order to fight against diseases and increase growth, weight gain, and size (Amenyogbe et al., Citation2020), a range of valuable diet supplements, probiotics that are advantageous to aquatic organisms, especially for fish, are used in fish farming. In some cases, probiotics can be used as an alternative to antibiotics (Irianto & Austin, Citation2002a), these measures encourage the immune response of the organism. Probiotics can confer host health, so Food and Agriculture Organization (FAO) defines probiotics as live microbial feed supplements. Because these probiotic-rich additives can change the gastrointestinal microbial community, they are widely used in bacterial food additives (Food and Agricultural Organization 815 of the United Nations and World Health Organization, Citation2001; X. Wang et al., Citation2017). Probiotics have received widespread attention by inhibiting or preventing pathogen production, improving health, and resistance of aquatic organisms (especially fish) (Dimitroglou et al., Citation2011).
Probiotics are widely used for various reasons, including promoting economic development, stimulating digestion, and preventing infectious disease outbreaks (Balcazar et al., Citation2006; Nayak, Citation2010). Microorganisms known as probiotics are considered beneficial and healthy. They are usually used to improve water quality by removing ammonia and nitrate from an aquaculture system as well as feed additives for improving host health, increasing growth, boosting immunity, and boosting disease resistance (Wei et al., Citation2022). Using probiotics to modulate intestinal microbiota is an alternative method of reducing pathogen adhesion and colonization in larval, fry, and juvenile intestines (Ringø et al., Citation2022). To increase the proportion of healthy bacteria in the gut, these probiotics species can be added to a fish diet or water. In the early stages of fish development, vaccination by injection is not practical. Vaccination method has the advantage of being implemented in the early stages of development. Various literature available has demonstrated the ability of probiotics to prevent gut colonization of pathogens, improve immunity, and produce inhibitory substances (Cordero et al., Citation2015; Guardiola et al., Citation2017; Jakhar et al., Citation2013a; Nayak, Citation2010; Ringø, Citation1999; Ringø & Vadstein, Citation1998; Ringø et al., Citation1996; Stienstra et al., Citation2017; Strøm & Ringø, Citation1993). While the use of probiotics in aquaculture has gained attention, there is still a significant knowledge gap regarding their comprehensive application, efficacy, and potential environmental benefits. The existing literature primarily focuses on the individual effects of probiotics on specific species or environmental parameters, limiting our understanding of their broader application across diverse aquaculture systems. Further research is needed to address the current knowledge gap and guide future directions. By focusing on strain selection, understanding mechanisms of action, ecological impact assessment, and integration with other management practices, we can unlock the full benefits of probiotics in ensuring the long-term sustainability of aquaculture systems. Hence, this review aims to provide a comprehensive overview of the sources and standards of assortment and actions of microorganisms used in aquaculture as probiotics, whether native or commercial. Additionally, this review examines the mechanisms of action by probiotics which promote fish health and the current state of knowledge on the efficacy of probiotics in immunity, and the interchange between the reproduction and metabolism of probiotics in aquaculture. Overall, this review provides valuable insights for researchers and industry professionals seeking to effectively utilize probiotics in aquaculture.
2. Diseases in fish farming (bacterial infections)
In fish diseases, pathogens, hosts, and the environment interact to cause their appearance and development. Aquaculture production is severely constrained by diseases. Aquaculture establishments suffer from mortality caused by diseases (Sihag & Sharma, Citation2012). To control diseases in aquaculture, chemical compounds have traditionally been used. Recent years have also seen the use of probiotic microorganisms and vaccinations or other forms of immuno-stimulation (Jakhar et al., Citation2013b; Sihag & Sharma, Citation2012; Dahiya, Gahlawat, et al., Citation2012, b, c). It is only through multidisciplinary studies that we will be able to apply appropriate measures to prevent and control the main diseases limiting fish production (Sharma, Sihag, & Minakshi, Citation2013). A relatively small number of pathogenic bacteria are responsible for important economic losses worldwide in cultured fish, despite their widespread presence throughout the majority of existing taxonomic groups (Toranzo et al., Citation2005). Vibrio, Aeromonas, Pseudomonas, and Streptococcus are among the bacteria that can infect fish (Bekele et al., Citation2019). There are species of the genera Pseudomonas, Aeromonas, Plesiomonas, and Pseudomonas that may also be present in healthy fish (Newaj-Fyzul et al., Citation2008). Fish are generally susceptible to opportunistic bacteria, such as Aeromonas hydrophila, infected by stress factors like a suboptimal environment, for instance, poor water quality (Awan et al., Citation2018; El-Attar & Moustafa, Citation1996; Zaher et al., Citation2021). There are also many factors involved in the development of bacterial diseases (Gorgoglione et al., Citation2020). In trying to find a cure for bacterial fish diseases, scientist should keep this in mind (Haenen et al., Citation2023) Streptococcosis, Aeromonas, Francisellosis, columnaris disease, and vibriosis are the current bacterial fish diseases of importance (to fish welfare, economy, and society). Usually in freshwater aquacultures, diseases such as furunculosis (Aeromonas salmonicida), bacterial kidney disease (BKD) (Renibacterium salmoninarum) and some forms of streptococcosis are now becoming major problems in marine culture as well. Depending on the host species, fish age, and disease stage (acute, chronic, subclinical carrier), each pathogen causes different clinical signs (external and internal). Conversely, other diseases with relatively low mortality rates (i.e., flexibacteriosis, winter ulcer syndrome, some streptococci) cause significant external lesions, including ulcers, necrosis, and exophthalmia which render fish unmarketable (Toranzo et al., Citation2005). There are usually pathogenic agents present in wild fish populations that are similar to those described in cultured systems. Since they are not subjected to the stressful conditions that normally occur in culture facilities, they seldom cause mortality in natural environments. Compared to other industries, fish farming comes with a wide range of economic losses due to bacterial disease, as costs vary by species, production system, country, currency, and so on. There are not enough data collected consistently across studies, countries, fish species, or production systems to allow comparisons. An analysis of % of fish production loss might be meaningful, however (Haenen et al., Citation2023).
In the wild and captivity, bacterial diseases pose a significant threat to fishes’ lives (Abdelsalam et al., Citation2023; EI-Jakee et al., Citation2020; El-Adawy et al., Citation2020; Elgendy et al., Citation2016, Citation2021). The prevalence of mixed infections is higher than that of single bacterial infections (Moustafa et al., Citation2015). Infections caused by bacterial pathogens in fish occur simultaneously with infections caused by viruses, mycotic agents, and parasites (Austin, Citation2019). When more than one genetically different microbial pathogen infects the same host at the same time, it is called a coinfection (Abdel Latif & Khafaga, Citation2020; Abdelsalam et al., Citation2023). It is possible to change the susceptibility of the host to specific pathogens by coinfection. By amplifying the severity, duration, and progression of infection, it can significantly change the dynamics of fish disease outbreaks. Furthermore, coinfection can have a detrimental effect on the affected host’s clinical outcomes and pathology (Elgendy et al., Citation2016).
Infectious pathogens may interact synergistically or antagonistically with each other (Bradley & Jackson, Citation2008). As a result of synergistic coinfection, the primary pathogen suppresses the immune system of the infected host, allowing the secondary pathogen to increase disease severity and fish mortalities (Dong et al., Citation2016). One of these listed pathogens is Aeromonas hydrophila, which increases mortality and worsens disease severity in tilapia when combined with the tilapia lake virus (TilV) (Abdelsalam et al., Citation2023; Nicholson et al., Citation2017, Citation2020). An antagonistic coinfection occurs when a primary pathogen modifies the immune response, delaying the entry of a secondary pathogen (Kotob et al., Citation2016). Due to the presence of more than one pathogen, bacterial coinfections can cause complications during diagnostic procedures (Austin, Citation2019). Moreover, bacterial coinfections can impair fish vaccination efficacy and retard immuno-prophylaxis (Abdelsalam et al., Citation2023; Figueroa et al., Citation2017).
Veterinary medicines or drugs are essential for semi-intensive and intensive aquaculture operations to succeed. Improper use of antibiotics, however, may result in ineffective treatment and undesirable residue levels in aquaculture products, resulting in bans on importation, rejections, and detentions (FAO, Citation2019). Antimicrobial resistance may be caused by misuse of veterinary medicines because antibacterial-resistant genes may be developed in bacteria. All sectors that produce food, including aquaculture, suffer from this consequence. An antibiotic resistance rate of 97% to ampicillin, erythromycin, and oxytetracycline in 173 bacterial isolates from Trinidad tilapia is one example (Newaj-Fyzul et al., Citation2008). The choice of antibiotics, therefore, should always be based on the results of an antibiogram, in order to ensure that the therapy is effective. A number of assemblies, conferences, and meetings have highlighted the importance and growth of antimicrobial resistance. During the 68th World Health Assembly in 2015, FAO and World Organisation for Animal Health (OIE) (formerly the Office International des Épizooties) contributed to the Global Action Plan (GAP) on antimicrobial resistance (WHO, Citation2015).
There is now an urgent need for aquaculture countries to pay attention to the emergence of antimicrobial-resistant organisms that may result from imprudent and irresponsible use of antimicrobials (specifically antibiotics) in aquaculture, especially those with substantial aquaculture production and food security objectives through aquaculture. A number of biosecurity measures have been suggested by FAO (Citation2019) as a means of reducing or eliminating antimicrobial resistance. Among them are avoiding infectious bacteria, keeping clean facilities, using immuno-stimulants to boost innate immunity, and adding probiotics to feeds. Several studies indicate that probiotics can be a viable alternative to antibiotics in aquaculture, as they combat diseases, improve growth, and stimulate the host’s immune system in order to fight infections (Newaj- Fyzul et al., Citation2014; N. V. Hai, Citation2015).
3. Probiotics utilization in aquaculture
In addition to preventing and treating diseases in humans and animals, antibiotics have played a significant role since Fleming discovered penicillin in 1928 (Fleming, Citation1944). Food animals and aquaculture also benefit from antibiotics, either as prophylactics or for growth stimulation (Tan et al., Citation2016; Marshall and Levy, Citation2011). Aquaculture is thus heavily dependent on antibiotics in order to develop intensively and on a large scale. A rise in antibiotic resistance is, however, a consequence of the indiscriminate and uncontrolled use of antibiotics (Huang et al., Citation2015; Letchumanan et al., Citation2015); additional research suggests that aquaculture ponds also harbor antibiotic resistance genes (Tomova et al., Citation2015; Xiong et al., Citation2015). A horizontal gene transfer mechanism can allow human and animal pathogens to acquire antibiotic-resistance genes (Tomova et al., Citation2015), leading to difficulty in treating diseases caused by bacteria. Recent evidence indicates that residual antibiotics in rearing species might pose health risks to humans (Chen et al., Citation2015; Pereira et al., Citation2015; Pham et al., Citation2015).
To prevent and treat diseases and improve aquaculture production’s quality and sustainability, it is urgently necessary to develop an alternative to antibiotics to counteract the continuous emergence of antibiotic-resistant pathogens due to the abuse of antibiotics in aquaculture. The occurrence of frequent diseases in aquaculture and the need for sustainability of aquaculture have stimulated the exploration of the use of probiotics on aquaculture species. Initially, attention was paid to their use to promote growth and enhance the overall well-being of these aquaculture species; nevertheless, their influence on stress tolerance and reproduction is being exploited lately, even though further scientific development is essential in these areas. Probiotics are utilized through feeding on supplemented pellets, live food, and direct addition to the water column and injection.
4. Probiotics sources and standards of assortment
Consumers and the research community have become acquainted with probiotics over the past few decades (Milner et al., Citation2021). As a category of microorganisms, “probiotics” encompass a wide variety of microorganisms that can have beneficial effects on the body after consumption, i.e., strengthening the gut barrier function, improving immunity, alleviating gastrointestinal infections, inhibiting the growth of pathogenic bacteria, strengthening the barrier function of the gut, improving immunity, assimilation of serum cholesterol, prevention of irritable bowel syndrome, reducing hypertension, preventing diarrhea, improvement of mental health, etc (O. S. Papadopoulou et al., Citation2023; Soomro et al., Citation2001). In addition to improving growth, immunity, disease resistance, sensory characteristics, and safety aspects, probiotics are also used as additives in new products with high value (O. Papadopoulou et al., Citation2018). The health-promoting effects of native probiotics have been linked to the complex microbial communities that contribute to these fish species’ growth and the substances they produce, according to several studies on native probiotics. Health benefits have long been associated with their consumption. Probiotic strains are incorporated into commercial feeds and formulated feeds (O. S. Papadopoulou et al., Citation2023). Therefore, native probiotic strains are used in the aquaculture industry, and research into these native isolates is on the rise.
Aquaculture species have benefited from using several microorganisms identified, characterized, and applied as probiotics in recent decades. Probiotics used in these species include bacteria (Gram-positive and Gram-negative), yeasts, microalgae, and bacteria (Amenyogbe et al., Citation2020, Citation2021; Caipang et al., Citation2020; Chauhan & Singh, Citation2019; Hoseinifar et al., Citation2018, Ray, Citation2017; Ringø et al., Citation2020; Simo´n et al., Citation2021; Zorriehzahra et al., Citation2016). Despite this, aquaculture offers fewer commercially available probiotics (Hasan & Banerjee, Citation2020). Probiotic microorganisms are obtained from various sources, such as the intestines of healthy fish, rearing environment water, sediments in culture tanks, other animals, or fermented foods (Shefat, Citation2018). Generally, the main purpose of a probiotic is to establish a relationship with beneficial and harmful bacteria found in fish intestines. Most commonly, aquatic animals (Lazado et al., Citation2015; S. Sharifuzzaman & Austin, Citation2017; S. Tapia-Paniagua et al., Citation2012) and the guts of aquatic animals provide the best source of microorganisms for probiotic use. Despite the fact that probiotics could originate outside a host, it is preferable to use microorganisms derived from the host, as they can play an important role in the natural defense system of the host (Gomez et al., Citation2013; S. Sharifuzzaman et al., Citation2014; Simo´n et al., Citation2021). Additionally, probiotics native to the environment can survive spontaneously and perform their functions physiologically at their best (S. Sharifuzzaman & Austin, Citation2017). As opposed to terrestrial animals, aquatic species have a highly dependent gastrointestinal microbiota because continuous water flows through their digestive tracts, making them highly dependent on the external environment. Therefore, most bacteria colonizing the tract are transient and could change in response to environmental changes (Martı́nez-Cruz et al., Citation2012; Simo´n et al., Citation2021).
The characterization and screening of fish probiotic strains have been the subjects of numerous scientific papers in recent years (Alonso et al., Citation2019; Aly et al., Citation2008; Amenyogbe et al., Citation2021; Amenyogbe, Yang, et al., Citation2022; Amenyogbe, Zhang, et al., Citation2022; Balcázar et al., Citation2008; Chabrillón et al., Citation2006; Muthukumar & Kandeepan, Citation2015; Nikoskelainen et al., Citation2001; Sica et al., Citation2012; Thankappan et al., Citation2015; Wanka et al., Citation2018). The potential for finding ideal probiotics is assessed through multiple screening steps after they are isolated from different sources. The safety of these products (Verschuere et al., Citation2000) and their nonpathogenicity (Chythanya et al., Citation2002) are essential to demonstrating their safety. Probiotic candidates must also meet certain criteria to succeed. Merrifield et al. (Citation2010) divided the criteria into either essential or favorable. New findings have led to adding new criteria over the last several decades (Ray, Citation2017, Chauhan & Singh, Citation2019). As a result of all of this, a microorganism must meet the following criteria before it can be considered as a probiotic: being nonpathogenic, both for the host species and aquatic animals as a whole, as well as for human consumers; lacking antibiotic resistance genes encoded by plasmids; and tolerant of low to high pH as well as bile salt concentrations (>2.5%). Alternatively, merely advantageous characteristics include: should or must have the ability to adhere to and grow fantastically in the guts mucus; colonizing intestinal epithelial surfaces; being approved for utilization as a feed supplementation; exhibiting favorable growth characteristics; exhibiting anti-pathogen activity ranging from moderate to aggressive; providing extracellular digestive enzymes or vitamins that are beneficial to the host or the rearing environment; being native to both the host and the culturing environment; being robust enough to survive industrial processes and being viable under normal storage conditions; possessing better sensorial properties, possessing action of fermentation, a tolerance to freeze-drying and viability in feed during packaging and storage; promoting fish growth, boosting immunity and protecting fish from disease-causing microorganisms (Simo´n et al., Citation2021). A probiotic is more likely to be a promising candidate if at least some of these characteristics are met, though it is unlikely to find one that fulfills all of them. Among different candidates, potential probiotics were chosen based on their inhibitory activity against target pathogens in vitro or in vivo (Amenyogbe et al., Citation2020; Balcázar et al., Citation2007, Citation2008; Chahad Bourouni et al., Citation2007; Chauhan & Singh, Citation2019; Gatesoupe, Citation1999; N. V. Hai, Citation2015; Hoseinifar et al., Citation2018; Medina et al., Citation2020; S. Sharifuzzaman & Austin, Citation2017; Simo´n et al., Citation2021; Verschuere et al., Citation2000).
5. Means of exploit and improvement of the immune reaction; impacts of probiotics
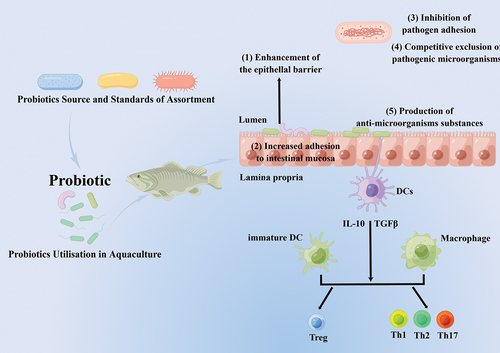
The brain and functions of the gastric could be affected by pathogens and impede development and feeding (Butt & Volkoff, Citation2019). A suggestion has been made that the microbiota protects the host from the proliferation and colonization of conservational pathogens, a process known as “colonization resistance” (S. Kim et al., Citation2017; Lawley & Walker, Citation2013; S. Sharifuzzaman & Austin, Citation2017). The relationship between hosts and probiotic microbes is very multifaceted; therefore, methods of probiotic activity still need to be fully understood. However, the effects of probiotics on disease reduction could be linked to an amalgamation of factors, as shown in Figure . The opinion on probiotics involves a competitive separation of prospective pathogens when the host enters the digestive tract; inhibitory molecules are produced by beneficial microbes and strive/fight/compete for nutrients, adhesion sites, and sources of vitality that may interfere with growth and pathogen action (Balcazar et al., Citation2006; Brunt et al., Citation2007; Chabrillón, Rico, Arijo, et al., Citation2005; Decamp et al., Citation2008; Irianto & Austin, Citation2002b; Verschuere et al., Citation2000; Vine, Leukes, Kaiser, Daya, et al., Citation2004).
Figure 1. Mechanisms of action of Probiotics in Fish and other organisms (Bermudez-Brito et al., Citation2012; Shefat, Citation2018).
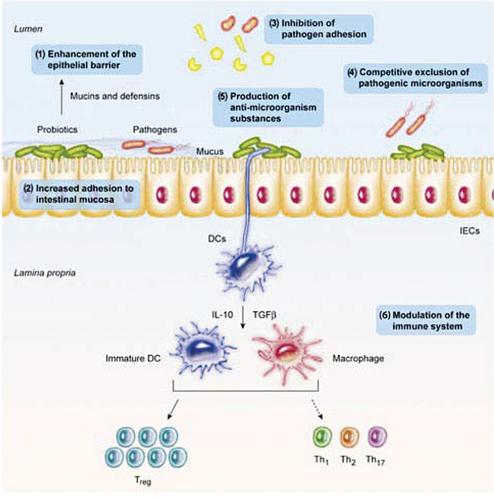
Parenthetically, the isolation of useful probiotics from the gut of freshwater and marine species has shown antagonistic action against several species of pathogens. For instance, five candidate probiotics extracellular products isolates from the gut and stomach of common clownfish (Amphiprionpercula) were inhibitory against A. hydrophila, A. salmonicida, V. harveyi, V. anguillarum, V. damsela [= Photobacteriumdamselae], V.alginolyticusand C. piscicola (Balcazar et al., Citation2006; Vine, Leukes, & Kaiser, Citation2004). Aeromonas media (strain A199) that was recovered in the culturing water of eel was similarly inhibitory to Saprolegniasp (Lategan et al., Citation2004). Diffusible inhibitors were found to be produced by B. amyloliquefaciensto V. parahaemolyticustarda, V. harveyi, and A. hydrophila, Ed. (Das et al., Citation2013). Among several others, known substances that persuade the influences of bacteriostatic comprise iderophores, bacteriocins, carbon dioxide, lysozymes, proteases, and hydrogen peroxide. Also, the production of volatile fatty acids and organic acids, such as propionic, acetic, butyric, and lactic acids, could change the pH of the intestine (Chabrillón, Rico, Balebona, et al., Citation2005; Gram & Melchiorsen, Citation1996; Ringø & Gatesoupe, Citation1998; Sugita et al., Citation1998; Verschuere et al., Citation2000; Vine et al., Citation2006). This means that probiotics could out-challenge the propagation of pathogenic microbes that are opportunists in vivo.
Selected probiotic microbes can prevent pathogens from developing or growing on the gut surface through challenging adhesion sites (Balcázar et al., Citation2008; Gueimonde et al., Citation2006; Mukai et al., Citation2002). This is similar to the results that confirmed fish pathogens’ adhesion of the V. anguillarum, A. salmonicida, A. hydrophila, and Y. ruckeri to rainbow trout gastric mucus was lessened via L. fermentum, L. Plantarum, and La. Lactis are all lactic acid bacteria, respectively, in conditions in vitro. The antagonistic adhesion action could be elucidated via the antimicrobial constituent’s secretion, like antibiotics (Balcazar et al., Citation2006; Balcázar et al., Citation2008; Gram et al., Citation1999). Certainly, through rivaling aimed at free iron, the growth of V. anguillarum was inhibited by sideropho regenerating probiont Ps. Fluorescens (S. Sharifuzzaman et al., Citation2014). Also, the colonization of the gut of aquatic hosts by probiotics has been reported through an oral application. The colonization of Rhodococcus SM2 and Kocuria SM1 in rainbow trout intestines was reported by S. Sharifuzzaman et al. (Citation2014) after feeding for 14 days, of which these microbes accounted for ∼90–100% of the entire culturable microbial populace.
The culture of probiotics does not demonstrate impulsive crucial/primary occupation/colonization in the intestines or gut. Rather, a transient state is sustained as long as microorganisms are presented/introduced through feeding and disappear when switched to normal feed (De Schryver et al., Citation2012; Newaj-Fyzul et al., Citation2007; Robertson et al., Citation2000). Nonetheless, the probiotic’s capacity to grow in mucous, stick to epithelial cells, and colonize the intestinal tract is well thought out as an immediate point of defense against pathogens invasion (Newaj-Fyzul & Austin, Citation2015).
Probiotics have the ability to modulate immune responses through the gut lymphoid tissue at the gut level (Standen et al., Citation2013). An improved goblet cells number (Picchietti et al., Citation2007), lysozyme and phagocytic cells, T-cells, acidophilic granulocytes, and immunoglobulin positive (Ig+) actions have been defined (Balcazar et al., Citation2006; V. N. Hai & Fotedar, Citation2009; Picchietti et al., Citation2008; Salinas et al., Citation2008). Again, there were reported enhancements in superoxide dismutase, lysozyme, hemocyte count, plasma protein concentration, phagocytosis, phenoloxidase, and antibacterial actions/activities in prawn/shrimp (Abbass et al., Citation2010; Li et al., Citation2009; NavinChandran et al., Citation2014; Pattukumar et al., Citation2014; Rahiman et al., Citation2010; Rengpipat et al., Citation2000; Y. B. Wang & Gu, Citation2010). The vertebrate’s nonspecific defense has developed in the direction of appreciation of preserved bacteriological components (Arijo et al., Citation2008). Therefore, it must be pointed out that many probiotics can have an immune-modulatory effect on aquatic organisms (Michael et al., Citation2014; Sakai, Citation1999; S. M. Sharifuzzaman et al., Citation2011).
Nonspecific (also known as innate) and specific immune systems different from complex vertebrates are the two basic parts of aquaculture species with immune systems. The first-line resistance in the nonspecific immune system is composed of cells and machinery used to protect the host from infection by pathogens (Amenyogbe et al., Citation2020). According to Michael et al. (Citation2014), immune system modulation is significant remunerations of probiotics between the several positive influences of probiotics. According to them, the immune systems of shrimps, fish larvae, and other invertebrates rely largely on nonspecific immune responses to resist or fight infection. It was also established by Sakai et al. (Citation1995) that Clostridiumbutyricum microbes improved resistance to vibriosis by developing leukocytosis phagocytosis when administered to rainbow trout.
In several types of fish, certain probiotics can greatly raise the digit of erythrocytes, lymphocytes, granulocytes, and macrophages (Akhter et al., Citation2015). That is, through research, it was found that the interaction between probiotics and immune cells like natural killer cells, mononuclear phagocytes, and polymorphonuclear leukocytes can improve nonspecific immune responses (Akhter et al., Citation2015; Balcázar et al., Citation2007; Hellio et al., Citation2007; Irianto & Austin, Citation2003; D. H. Kim & Austin, Citation2006; Kumar et al., Citation2008; Nayak et al., Citation2007). Many humoral dynamics have been described in stimulating specific and nonspecific immune systems. The resistance mechanism refers to the activation of aquaculture immune response species by damaging the adverse effects of chemotherapeutic agents, thereby combating pathological agents and probiotics supported by antibiotic agents. The substances produced by the beneficial bacteria can enhance the immune response of fish and crustaceans (S. M. Sharifuzzaman et al., Citation2011). The antibacterial peptides in fish serum are lysozyme and immunoglobulin, which can resist the development of pathogenic microorganisms to avoid disease; it is essential for its existence as a defense mechanism (Talpur et al., Citation2014). According to Geovanny et al. (Citation2007), lysozyme enhances the pathogenicity of microorganisms of fish after this test. As discussed in (Sakai et al., Citation1995), several studies have also witnessed, for example, in the snakehead fish’s serum, the lysozyme content is very high when supplemented with a lactic acid bacteria diet. Immunoglobulin also plays a vital role in resisting pathogenic bacteria, another important humoral immune component in fish. Although the application of probiotics in aquaculture has many beneficial effects, the immune system of shrimp, fish larvae, and several other invertebrate organisms depends largely on the resistance to nonspecific immune responses. Facts have proven that the regulation of immune structure is the biggest benefit of probiotics to the aquaculture industry in addition (Akhter et al., Citation2015; Biagi et al., Citation2017; Kumar et al., Citation2008). The immune system changes originate from the physiological changes in the gastrointestinal tract, which are related to the changes in the intestinal microbiome and the changes in dietary behaviors related to the stage (Carnevali et al., Citation2017). The intestinal homeostasis of tough and strong animals results from the mutual interference between the immune system and intestinal flora. At the same time, the formation of protracted disease is due to changes in the intestinal environment caused by the disease, which leads to changes in the balance of the gastrointestinal tract within the intestinal flora (Akhter et al., Citation2015; Carnevali et al., Citation2017).
6. Influences of probiotic use on the interchange between reproduction and metabolism
Neuropeptide sequences free from hypothalamic nucleus neurons play a vital role in the satisfactory stability that links reproduction and energy stores (Shefat, Citation2018). Circumstances of energy deficiency and acute metabolic stress usually disrupt the initiation of puberty. This rapidly costly process significantly impacts fat metabolism, an essential source of resilience or energy accumulation in organisms (Won & Borski, Citation2013). Recently, there has been a significant improvement in clarifying the pathways of neurohormones responsible for the gonadotropic function and metabolic regulation of the beginning of sexual maturity. Neuropeptides and hormones that are signaling characteristics are produced and free in the hypothalamus, some of which are proopiomelanocortin, kisspeptins, and neuropeptide Y, and peripheral organs (ghrelin and leptin) (Shefat, Citation2018), all of which play a role in creating a network of signals responsible for the mechanism or cycle of reproduction, usually by passing “afferent inputs to the Gonadotropin-Releasing Hormone (GnRH) neurons and thereby regulating their secretion”. Neuropeptide Y (Npy) is an abundant peptide found in the nervous system. It is primarily engaged in the homeostasis of energy (Shefat, Citation2018).
The working mechanism of Neuropeptide Y neurons becomes operational in response to starving stimuli. Their messenger ribonucleic acid production is also regulated by flowing levels of insulin and leptin (Zohar et al., Citation2009); aside from being directly convoluted in gonadotropins and gonadotropin-releasing hormone, luteinizing hormone (LH), secretion and follicle-stimulating hormone (FSH) (Gioacchini et al., Citation2010; Levavi-Sivan et al., Citation2010; Tang et al., Citation2015). There are currently scarce data on the impacts of probiotic application on the central nervous system. For example, the outcomes accessible, exposure of the adult zebrafish to the L. rhamnosus IMC501 strain of probiotic (Qin et al., Citation2014), have indicated that probiotic application downregulated brain and the gut npy messenger ribonucleic acid (mRNA). This agrees with the hypothesis that such neuropeptide’s peripheral synthesis, besides already acknowledged at the central level, permits assumptions on the anorexigenic role of probiotic application (Palermo et al., Citation2011; Qin et al., Citation2014; Shefat, Citation2018). Research has been carried out in the course of the larval development of S. solea. The objective of the examination is to know whether the application of the autochthonous probiotic E. faecium IMC 511 strain may have a functional impact on the interaction between the endocannabinoid system and the hypothalamus-pituitary-thyroid (HPT) axis, all of which play a role in food intake and adaptive modulation responses (Migliarini et al., Citation2011).
The important role played by proopiomelanocortin in the regulation of homeostasis of energy by teleosteans has been extensively discussed. Proopiomelanocortin is a precursor to several molecules and generally regulates energy homeostasis, controlling food ingestion (Cheung et al., Citation1997) through the interchange between leptin and α-MSH (Tena-Sempere, Citation2007). Between the target genes studied, cannabinoid receptor 1a (cb1a) and proopiomelanocortin messenger ribonucleic acid levels were down-regulated. However, the mRNA level of thyroid hormone receptor α (trα) has increased. Generally, the results of the larval development of S. Solea indicated that the usage of Enterococcus faecium IMC511 stimulates a compensatory system that enhances the development and growth of food intake control (Migliarini et al., Citation2011; Shefat, Citation2018).
Although several peptides are involved in the interchange between the rate of metabolism, appetite, and energy storage, leptin is unquestionably the most valuable peptide for the central control system (Copeland et al., Citation2011; Sanchez-Garrido & Tena-Sempere, Citation2013). Research on its systems of action and nature has recently begun, particularly in the case of fish species (Carnevali et al., Citation2013; Gorissen et al., Citation2009; Mechaly et al., Citation2013; Ohga et al., Citation2015; Salmerón et al., Citation2015). The most energy-abundance marker is leptin, and the circulating levels of leptinapprise tissue in the energy reserves ultimately hinder or improve reproduction. It is a difference in fish compared to other animals; the expression of leptin is largely in the liver (Picchietti et al., Citation2009) and to a small degree in the brain (Pfundt et al., Citation2009), adipose tissue (Gioacchini et al., Citation2014), and intestine (Qin et al., Citation2014). According to Gioacchini et al. (Citation2014), the utilization of the L. rhamnosus strain of probiotic upregulates leptin expression in adult zebrafish at both the brain and intestinal level and was related to upregulation of kiss1, kiss2, and kisspeptins. This is an indication that probiotics are useful for reproduction (Fernandez-Fernandez et al., Citation2006). The reported anorexogenic effect of Leptin and its increased levels of mRNA supported the suitability of energy stores for a costly process like reproduction. Also, as Fernandez-Fernandez et al. (Citation2006) have stated, “peripheral hormones from strategic metabolic tissues, such as the gastrointestinal tract, the pancreas, and the adipose, have been suggested as putative regulators of the gonadotropic axis” and consist of the intestinal hormone ghrelin that alienates leptin in the control of feed ingestion (Piccinetti et al., Citation2010; Unniappan, Citation2010), by or exigenic-marked exploitation (Singh et al., Citation2011; Tinoco et al., Citation2014).
Ghrelin thwarts the occurrence of sexual maturity or puberty and pushes inhibitory signals into the hypothalamic-pituitary-gonadal axis and adulthood. Other signals are peroxisome proliferator-activated receptors (PPARs) and a member of the family of nuclear receptors. The PPARs’ responsibilities include the circulating approval of lipids for the regulation of lipid accumulation and adipocyte differentiation (Huang, Citation2008; Poulsen et al., Citation2012) and also function in the gamete maturation and embryo growth (Avella et al., Citation2010; Falcinelli et al., Citation2015). The puberty of several species appears to be related to a mass of characteristic hypodermal body fat that is autonomous either at the age of the species at the beginning of puberty or to an existing close link between the amount of energy stored and gonadal maturation in the adipose tissue form. The indication of the vital role played by fat storage at the beginning or starting stage of puberty. Concerning such links, results from species of different fish showed the positive capacity of probiotics regulates lipid metabolism. For example, according to Falcinelli et al. (Citation2015), there was a downregulation of genes involved in triglycerides and metabolism in larvae of zebrafish when L. rhamnosus IMC 501 was utilized (Shefat, Citation2018). The decreased overall content of triglyceride and cholesterol in the body and the observed circumstantial growth levels of fatty acid indicated the potential utilization of a strain of probiotics in the cure of lipid disorder (Falcinelli et al., Citation2016; T. S. Tapia-Paniagua et al., Citation2014). A similar trend occurred in juvenile Soleasenegalensis when Shewanellabaltica Pdp13 and Shewanellaputrefaciens Pdp11 strains of probiotics were administered (DiCesare et al., Citation2012), and a potential role of L. rhamnosus in the glucose metabolism was reported by Falcinelli et al. (Citation2015).
7. Further benefits of probiotics to fish
In addition to producing beneficial substances, probiotics can also enhance the performance and immunity of the host by converting feeds and improving growth. A potential probiotic candidate should have the ability to produce extracellular enzymes. This includes proteases, amylases, cellulases, phytases, chitinases, lipases, etc. Among the endogenous enzymes produced by fish are those listed above (Alarcón et al., Citation2001; German et al., Citation2004; Krogdahl et al., Citation2005; Ray et al., Citation2012), but their activity and quantity are insufficient to complete the metabolic process of the ingested materials. From a nutritional perspective, enzymes secreted by permanent gut endosymbionts and potential probiotics are critical (S. P. Banerjee et al., Citation2013) to facilitating digestion (Simo´n et al., Citation2021). Over the past few years, there has been extensive research into the extracellular enzyme production of several fish probiotic strains (Ringø et al., Citation2020; Ray, Citation2017). For instance, Dawood et al. (Citation2019) demonstrated that Lactobacillus plantarum significantly increased Nile tilapia’s amylase, lipase, and protease activities. Bacillus sp. and L. plantarum supplementation of olive flounder were both successful and beneficial by increasing the activities of enzymes such as amylase, trypsin, and lipase (Jang et al., Citation2019). Lactobacillus casei combined with b-glucan and mannan oligosaccharide significantly increased the production of these enzymes and proteases in carp (Cyprinus carpio) (Mohammadian, Nasirpour, Tabandeh, & Mesbah, Citation2019). Citrobacter, Lactobacillus bulgaricus, and Lactobacillus acidophilus also increase rainbow trout amylase, trypsin, and alkaline phosphatase (Mohammadian, Nasirpour, Tabandeh, & Mesbah, Citation2019). A study by Tarkhani et al. (Citation2020) reported an increase in intestinal digestive enzyme activity in Caspian roaches (Rutilus caspicus) following the administration of E. faecium. Amenyogbe, Yang, et al (Citation2022, Citation2022, Citation2022). also reported increased intestinal digestive enzyme activity in juvenile cobia (Rachycentron canadum) after supplementing indigenous isolates Pantoea agglomerans RCS2, indigenous isolates Bacillus sp. RCS1 and Bacillus cereus RCS3, and a mixture of P. agglomerans RCS2, Bacillus sp. RCS1 and Bacillus cereus RCS3. Although these enzymes have been reported to contribute to fish metabolism, their exact contribution is still unclear.
As a general rule, fish are incapable of producing vitamins. They are supplied with vitamins by endosymbionts and probiotics. The host is therefore supplied with vitamins, fatty acids, and essential amino acids by many probiotics (Balcázar et al., Citation2006; Panigrahi & Azad, Citation2007; Sugita et al., Citation1991, Citation1992). There have been studies in which a variety of yeasts have been used as dietary supplements in fish in addition to bacterial probiotics (Navarrete & Tovar-Ramı́rez, Citation2014). Intriguingly, polyamines produced by yeasts stimulate intestinal maturation (X. Wang et al., Citation2000). In this context, probiotics might also be regarded as complementary foods due to their ability to provide vital nutrients such as biotin, vitamins, and fatty acids (Verschuere et al., Citation2000).
Additionally, probiotics positively affect host growth rate as they contribute to feed consumption and nutrient absorption (Nath et al., Citation2019; Sharma, Sihag, & Gahlawat, Citation2013). This increases survival rates as well as growth performance with probiotics. Among the bacteria studied for their impact on growth, Lactobacillus is the most studied. A diet containing L. plantarum induced growth enhancements in several fish species (carp, Nile tilapia, brown trout, Salmo trutta caspius) (Dawood et al., Citation2019; Hamdan et al., Citation2016; Soltani et al., Citation2017; Van Doan et al., Citation2020; Yu et al., Citation2017; Zhai et al., Citation2017). In addition, combining L. plantarum with natural immuno-stimulants and probiotics also enhances growth rates. Consequently, Alishahi et al (Alishahi et al., Citation2018). showed a weight gain increase in carp after supplementing its diet with L. bulgaricus. and L. plantarum. It has been demonstrated that Nile tilapia growth performance is enhanced by administering the fungus Cordyceps militaris with L. plantarum together (Van Doan et al., Citation2017). Several farmed fish species have shown positive growth effects from L. lactis (Feng et al., Citation2019; Nguyen et al., Citation2017, Citation2018; Sun et al., Citation2018), along with immuno-stimulant monosaccharides (Mohammadian, Nasirpour, Tabandeh, & Mesbah, Citation2019); as well as other Lactobacillus species (Xia et al., Citation2018). Several Lactobacillus species have been shown to increase growth rates (Jang et al., Citation2019; Sewaka et al., Citation2019; Xia et al., Citation2018; Zhang et al., Citation2017) and other bacteria species (Ashouri et al., Citation2018; Baños et al., Citation2019; Mohammadian, Nasirpour, Tabandeh, & Mesbah, Citation2019; Rahimnejad et al., Citation2018; Tarkhani et al., Citation2020). As an example, the growth of Asian seabass was increased by a commercial probiotic containing Lactobacillus spp., E. faecium, B. subtilis, and Saccharomyces cerevisiae. In addition, Streptococcus faecium, in combination with Lactobacillus acidophilus and S. cerevisiae provides growth stimulation to Nile tilapia (Lara-Flores et al., Citation2003; Xie et al., Citation2017). Also, Pantoea agglomerans RCS2, Bacillus sp. RCS1 and Bacillus cereus RCS3, and a mixture of P. agglomerans RCS2, Bacillus sp. RCS1 and Bacillus cereus RCS3 all stimulated growth of juvenile cobia (Amenyogbe, Luo, et al., Citation2022; Amenyogbe, Yang, et al., Citation2022; Amenyogbe, Zhang, et al., Citation2022). Several species of farmed fish have been reported to benefit from Bacillus probiotics as growth promoters. Hence, feeding B. subtilis to Nile tilapia (Liu et al., Citation2017; Xie et al., Citation2017), carp (Bisht et al., Citation2012), and grass carp (Guo et al., Citation2016) enhances their growth. A combination of Bacillus species and other probiotic species has benefited growth (e.g. Cobia, rohu, Labeo rohita, rainbow trout, catfish, carp, cobia, and tilapia) (Abareethan & Amsath, Citation2015; Amenyogbe, Luo, et al., Citation2022; Amenyogbe, Zhang, et al., Citation2022; Han et al., Citation2015, Giannenas et al., Citation2015; Lin et al., Citation2012; Thy et al., Citation2017).
8. Conclusion
Innovatively combining feed additives helped the industry by boosting aquaculture species’ immune systems, disease resistance, and growth performance. It is without a doubt urgent to develop alternative eco-friendly methods that can be used to prevent and treat diseases and improve growth performance in the context of rapidly increasing aquaculture production globally. Probiotics have gained attention as an alternative to antibiotics in aquaculture, as they can help improve the growth, survival, and overall health of fish and shellfish. Probiotics are live microorganisms that, when administered in adequate amounts, confer a health benefit to the host. In aquaculture, probiotics are used as a feed additive or a water supplement to promote the growth of beneficial bacteria that can help control harmful pathogens and improve nutrient utilization. Studies have shown that the use of probiotics in aquaculture can improve growth performance, enhance disease resistance, and improve water quality. Additionally, probiotics can also reduce the need for antibiotics, which can help mitigate the development of antibiotic resistance in aquatic animals and their surrounding environment. In aquaculture, native species have only been tested in a limited number of studies, despite previous studies showing promising results. The probiotic nature of native species in preventing disease and enhancing the growth of aquaculture animals still needs to be established in extensive trials before native species can be fully included or accepted as commonly used biological control agents in aquaculture. In addition, it is necessary to understand better the exact mechanism by which native species act to promote probiotic effects. In order to elucidate how native probiotics function in aquaculture settings, future research could focus more on molecular techniques. However, more research is needed to fully understand the mechanisms of probiotics’ action and their effects on the environment. For instance, the impact of probiotics on the microbial community of the aquatic environment is not yet fully understood, and there is a need for further investigation into the potential ecological risks associated with the use of probiotics in aquaculture. Moreover, the efficacy of probiotics may be influenced by various factors, such as the type of probiotic strain used, the dose, and the method of application, and further research is needed to optimize their application. Candidate strains must be rigorously evaluated by a global standard before being formulated into fish probiotics. In summary, while probiotics hold great promise for improving the sustainability and environmental friendliness of aquaculture, further research is needed to fully understand their benefits and risks. Addressing these research gaps can help maximize the potential of probiotics to promote sustainable and environmentally friendly aquaculture practices.
Competing interests
The author confirms that there are no conflicts of interest.
Availability of data and materials
To undertake this study, the search database using Web of Science, Google Scholar, and subscribe journals were used as Core Collections to detect or identify papers. Initially, amalgamations of several keywords were used as hunt criteria to salvage papers published up to 2023. Furthermore, studies found from the preliminary search were distinguished based on the relevance of material regarding fish probiotics used in aquaculture.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Notes on contributors
Eric Amenyogbe
Eric Amenyogbe (PhD) is a researcher with multidisciplinary expertise in aquaculture and a keen interest in aquatic microbiology and diseases. He has several scientific research articles as the first author or co-author and a corresponding author in Scientific Index Citation peer-reviewed journals with over seven years of research and working experience. He works as a Lecturer at the University of Environment and Sustainable Development (UESD), Somanya, Ghana. Eric Amenyogbe’s research interests and areas of expertise are aquaculture - Fish Culture – Fish Physiology – Fish Biology - Microbiology – Biochemistry- Gene Expression - Antioxidant Enzymes - Digestive Enzymes - Immune Responses - Gut Microbiota- Fish Diseases.
References
- Abareethan, M., & Amsath, A. (2015). Characterisation and evaluation of probiotic fish feed. Int J Pure Appl Zool, 3(2), 148–23.
- Abbass, A., Sharifuzzaman, S. M., & Austin, B. (2010). Cellular components of probiotics control Yersinia ruckeri infection in rainbow trout, Oncorhynchus mykiss (Walbaum). Journal of Fish Diseases, 33(1), 31–37. https://doi.org/10.1111/j.1365-2761.2009.01086.x
- Abdel Latif, H. M. R., & Khafaga, A. F. (2020). Natural co-infection of cultured Nile tilapia Oreochromis niloticus with Aeromonas hydrophila and Gyrodactylus cichlidarum experiencing high mortality during summer. Aquaculture Research, 51(5), 1880–1892. https://doi.org/10.1111/are.14538
- Abdelsalam, M., Elgendy, M. Y., Elfadadny, M. R., Ali, S. S., Sherif, A. H., & Abolghait, S. K. (2023). A review of molecular diagnoses of bacterial fish diseases. Aquaculture International, 31(1), 417–434. https://doi.org/10.1007/s10499-022-00983-8
- Akhter, N., Wu, B., Memon, A. M., & Mohsin, M. (2015). Probiotics and prebiotics associated with aquaculture: A review. Fish & Shellfish Immunology, 45(2), 733–741. https://doi.org/10.1016/j.fsi.2015.05.038
- Alarcón, F., Martinez, T., Dı́az, M., & Moyano, F. (2001). Characterisation of digestive carbohydrase activity in the gilthead seabream (Sparus aurata). Hydrobiologia, 445(1–3), 199–204. https://doi.org/10.1023/A:1017521900442
- Alishahi, M., Tulaby Dezfuly, Z., Mohammadian, T., & Mesbah, M. (2018). Effects of two probiotics, Lactobacillus plantarum and Lactobacillus bulgaricus on growth performance and intestinal lactic acid bacteria of Cyprinus carpio. Iranian Journal of Veterinary Medicine, 12(3), 207–218. https://doi.org/10.22059/IJVM.2018.235444.1004816
- Allameh, S., Yusoff, F., Ringø, E., Daud, H., Saad, C., & Ideris, A. (2016). Effects of dietary mono- and multiprobiotic strains on growth performance, gut bacteria and body composition of Javanese carp (Puntius gonionotus Bleeker 1850). Aquaculture Nutrition, 22(2), 367–373. https://doi.org/10.1111/anu.12265
- Alonso, S., Castro, M. C., Berdasco, M., de la Banda IG, Moreno-Ventas, X., & de Rojas, A. H. (2019). Isolation and partial characterisation of lactic acid bacteria from the gut microbiota of marine fishes for potential application as probiotics in aquaculture. Probiotics and Antimicrobial Proteins, 11(2), 569–579. https://doi.org/10.1007/s12602-018-9439-2
- Aly, S. M., Abd-El-Rahman, A. M., John, G., & Mohamed, M. F. (2008). Characterisation of some bacteria isolated from Oreochromis niloticus and their potential use as probiotics. Aquaculture, 277(1–2), 1–6. https://doi.org/10.1016/j.aquaculture.2008.02.021
- Amenyogbe, E., Chen, G., Wang, Z., Huang, J., Huang, B., & Li, H. (2020). The exploitation of probiotics, prebiotics and synbiotics in aquaculture: Present study, limitations and future directions: A review. Aquaculture International, 28(3), 1017–1041. https://doi.org/10.1007/s10499-020-00509-0
- Amenyogbe, E., Huang, J.-S., Chen, G., & Wang, W.-Z. (2021). Probiotic Potential of Indigenous (Bacillus sp. RCS1, Pantoea agglomerans RCS2, and Bacillus cereus strain RCS3) Isolated from cobia fish (rachycentron canadum) and their antagonistic effects on the growth of pathogenic vibrio alginolyticus, vibrio harveyi, streptococcus iniae, and streptococcus agalactiae. Front Mar Science, 8, 672213. https://doi.org/10.3389/fmars.2021.672213
- Amenyogbe, E., Luo, J., Fu, W.-J., Abarike, E. D., Wang, Z.-L., Huang, J.-S., Ayisi, C. L., & Chen, G. (2022). Effects of autochthonous strains mixture on gut microbiota and metabolic profile in cobia (Rachycentron canadum). Scientific Reports, 12(1), 17410. https://doi.org/10.1038/s41598-022-19663-x
- Amenyogbe, E., Yang, E. J., Xie, R. T., Huang, J. S., & Chen, G. (2022). Influences of indigenous isolates Pantoea agglomerans RCS2 on growth, proximate analysis, haematological parameters, digestive enzyme activities, serum biochemical parameters, antioxidants activities, intestinal morphology, disease resistance, and molecular immune response in juvenile’s cobia fish (Rachycentron canadum). Aquaculture, 551, 737942. https://doi.org/10.1016/j.aquaculture.2022.737942
- Amenyogbe, E., Zhang, J. D., Huang, J. S., & Chen, G. (2022). The efficiency of indigenous isolates Bacillus sp. RCS1 and Bacillus cereus RCS3 on growth performance, blood biochemical indices and resistance against Vibrio harveyi in cobia fish (Rachycentron canadum) juveniles. Aquaculture Reports, 25, 101241. https://doi.org/10.1016/j.aqrep.2022.101241
- Arijo, S., Brunt, J., Chabrillón, M., Diaz-Rosales, P., & Austin, B. (2008). Subcellular components of Vibrio harveyi and probiotics induce immune responses in rainbow trout, Oncorhynchus mykiss (Walbaum), against V. harveyi. Journal of Fish Diseases, 31(8), 579–590. https://doi.org/10.1111/j.1365-2761.2008.00932.x
- Ashouri, G., Soofiani, N. M., Hoseinifar, S. H., Jalali, S. A. H., Morshedi, V., Van Doan, H., & Torfi Mozanzadeh, M. (2018). Combined effects of dietary low molecular weight sodium alginate and Pediococcus acidilactici MA18/5M on growth performance, haematological and innate immune responses of Asian sea bass (Lates calcalifer) juveniles. Fish & Shellfish Immunology, 79, 34–41. https://doi.org/10.1016/j.fsi.2018.05.009
- Austin, B. (2019). Methods for the diagnosis of bacterial fish diseases. Marine Life Science & Technology, 1(1), 41–49. https://doi.org/10.1007/s42995-019-00002-5
- Avella, M. A., Olivotto, I., Silvi, S., Place, A. R., & Carnevali, O. (2010). Effect of dietary probiotics on clownfish: A molecular approach to define how lactic acid bacteria modulate development in a marine fish. American Journal of Physiology-Regulatory, Integrative & Comparative Physiology, 298(2), R359–R371. https://doi.org/10.1152/ajpregu.00300.2009
- Awan, F., Dong, Y., Wang, N., Liu, J., Ma, K., & Liu, Y. (2018). The fight for invincibility: Environmental stress response mechanisms and Aeromonas hydrophila. Microbial Pathogenesis, 116, 135–145. https://doi.org/10.1016/j.micpath.2018.01.023
- Balcázar, J. L., De Blas, I., Ruiz-Zarzuela, I., Vendrell, D., Gironés, O., & Muzquiz, J. L. (2007). Enhancement of the immune response and protection induced by probiotic lactic acid bacteria against furunculosis in rainbow trout (Oncorhynchus mykiss). FEMS Immunology and Medical Microbiology, 51(1), 185–193. https://doi.org/10.1111/j.1574-695X.2007.00294.x
- Balcazar, J. L., de Blas, I., Ruiz-Zazuela, I., Cunningham, D., Vandrell, D., & Muzquiz, J. L. (2006). The role of probiotics in aquaculture. Veterinary Microbiology, 114(3–4), 173–186. https://doi.org/10.1016/j.vetmic.2006.01.009
- Balcázar, J. L., Vendrell, D., de Blas, I., Ruiz-Zarzuela, I., Gironés, O., & Muzquiz, J. L. (2006). Immune modulation by probiotic strains: Quantification of phagocytosis of Aeromonas salmonicida by leukocytes isolated from gut of rainbow trout (Oncorhynchus mykiss) using a radiolabelling assay. Comparative Immunology, Microbiology and Infectious Diseases, 29(5), 335–343. https://doi.org/10.1016/j.cimid.2006.09.004
- Balcázar, J. L., Vendrell, D., de Blas, I., Ruiz-Zarzuela, I., Muzquiz, J. L., & Girones, O. (2008). Characterisation of probiotic properties of lactic acid bacteria isolated from intestinal microbiota of fish. Aquaculture, 278(1–4), 188–191. https://doi.org/10.1016/j.aquaculture.2008.03.014
- Banerjee, S. P., Dora, K. C., & Chowdhury, S. (2013). Detection, partial purification and characterisation of bacteriocin produced by Lactobacillus brevis FPTLB3 isolated from freshwater fish. Journal of Food Science and Technology, 50(1), 17–25. https://doi.org/10.1007/s13197-011-0240-4
- Baños, A., Ariza, J. J., Nuñez, C., Gil-Martı́nez, L., Garcı́a-López, J. D., Martı́nez- Bueno, M., & Valdivia, E. (2019). Effects of Enterococcus faecalis UGRA10 and the enterocin AS-48 against the fish pathogen Lactococcus garvieae. Studies in vitro and in vivo. Food Microbiology, 77, 69–77. https://doi.org/10.1016/j.fm.2018.08.002
- Bekele, B., Workagegn, K. B., & Natarajan, P. (2019). Prevalence and antimicrobial susceptibility of pathogenic bacteria in Nile tilapia, Oreochromis niloticus L. International Journal of Aquaculture and Fishery Sciences, 5(4), 22–26. https://doi.org/10.17352/2455-8400.000047
- Bermudez-Brito, M., Plaza-Díaz, J., Muñoz-Quezada, S., Gómez-Llorente, C., & Gil, A. (2012). Probiotic mechanisms of action. Annals of Nutrition and Metabolism, 61(2), 160–174. https://doi.org/10.1159/000342079
- Biagi, E., Rampelli, S., Turroni, S., Quercia, S., Candela, M., & Brigidi, P. (2017). The gut microbiota of centenarians: Signatures of longevity in the gut microbiota profile. Mechanisms of Ageing and Development, 165, 180–184. https://doi.org/10.1016/j.mad.2016.12.013
- Bisht, A., Singh, U. P., & Pandey, N. (2012). Bacillus subtilis as a potent probiotic for enhancing growth in fingerlings of common carp (Cyprinus carpio Linnaeus). Indian Journal Fish, 59(3), 103–107.
- Br¨onmark, C., & Hansson, L.-A. (2017). The Biology of Lakes and Ponds. Oxford University Press. https://doi.org/10.1093/oso/9780198713593.001.0001
- Bradley, J. E., & Jackson, J. A. (2008). Measuring immune system variation to help understand host-pathogen community dynamics. Parasitology, 135(7), 807–823. https://doi.org/10.1017/S0031182008000322
- Brunt, J., Newaj-Fyzul, A., & Austin, B. (2007). The development of probiotics for the control of multiple bacterial diseases of rainbow trout, Oncorhynchus mykiss (Walbaum). Journal of Fish Diseases, 30(10), 573–579. https://doi.org/10.1111/j.1365-2761.2007.00836.x
- Butt, R. L., & Volkoff, H. (2019). Gut microbiota and energy homeostasis in fish. Frontiers in Endocrinology, 10. https://doi.org/10.3389/fendo.2019.00009
- Caipang, C., Suharman, I., Avillanosa, A., & Bargoyo, V. (2020). Host-derived Probiotics for Finfish Aquaculture. In IOP Conf Ser: Earth Environ Sci (Vol. 430. p. 012026). IOP Publishing. https://doi.org/10.1088/1755-1315/430/1/012026
- Carnevali, O., Avella, M. A., & Gioacchini, G. (2013). Effects of probiotic administration on zebrafish development and reproduction. General & Comparative Endocrinology, 188, 297–302. https://doi.org/10.1016/j.ygcen.2013.02.022
- Carnevali, O., Maradonna, F., & Gioacchini, G. (2017). Integrated control of fish metabolism, well-being and reproduction: The role of probiotic. Aquaculture, 472, 144–155. https://doi.org/10.1016/j.aquaculture.2016.03.037
- Chabrillón, M., Arijo, S., Dı́az-Rosales, P., Balebona, M. C., & Moriñigo, M. A. (2006). Interference of Listonella anguillarum with potential probiotic microorganisms isolated from farmed gilthead seabream (Sparus aurata L.). Aquaculture Research, 37(1), 78–86. https://doi.org/10.1111/j.1365-2109.2005.01400.x
- Chabrillón, M., Rico, R. M., Arijo, S., Díaz-Rosales, P., Balebona, M. C., & Moriñigo, M. A. (2005). Interactions of microorganisms isolated from gilthead sea bream, Sparus aurata L., on Vibrio harveyi, a pathogen of farmed Senegalese sole, Solea senegalensis (Kaup). Journal of Fish Diseases, 28(9), 531–537. https://doi.org/10.1111/j.1365-2761.2005.00657.x
- Chabrillón, M., Rico, R. M., Balebona, M. C., & Moriñigo, M. A. (2005). Adhesion to sole, Solea senegalensis Kaup, mucus of microorganisms isolated from farmed fish, and their interaction with Photobacterium damselae subsp. piscicida. Journal of Fish Diseases, 28(4), 229–237. https://doi.org/10.1111/j.1365-2761.2005.00623.x
- Chahad Bourouni, O., El Bour, M., Mraouna, R., Abdennaceur, H., & Boudabous, A. (2007). Preliminary selection study of potential probiotic bacteria from aquacultural area in Tunisia. Annals of Microbiology, 57(2), 185. https://doi.org/10.1007/BF03175205
- Chauhan, A., & Singh, R. (2019). Probiotics in aquaculture: A promising emerging alternative approach. Symbiosis, 77(2), 99–113. https://doi.org/10.1007/s13199-018-0580-1
- Chen, H., Liu, S., Xu, X., Liu, S., Zhou, G., Sun, K., Zhao, J., & Ying, G. (2015). Antibiotics in typical marine aquaculture farms surrounding Hailing Island, South China: Occurrence, bioaccumulation and human dietary exposure. Marine Pollution Bulletin, 90(1–2), 181–187. https://doi.org/10.1016/j.marpolbul.2014.10.053
- Cheung, C. C., Clifton, D. K., & Steiner, R. A. (1997). Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology, 138, 4489–4492. http://doi.org/10.1210/endo.138.10.5570
- Chythanya, R., Karunasagar, I., & Karunasagar, I. (2002). Inhibition of shrimp pathogenic vibrios by a marine Pseudomonas I-2 strain. Aquaculture, 208(1–2), 1–10. https://doi.org/10.1016/S0044-8486(01)00714-1
- Copeland, D. L., Duff, R. J., Liu, Q., Prokop, J., & Londraville, R. L. (2011). Leptin in teleost fishes: An argument for comparative study. Frontiers in Physiology, 2, 26. https://doi.org/10.3389/fphys.2011.00026
- Cordero, H., Guardiola, F. A., Tapia-Paniagua, S. T., Cuesta, A., Meseguer, J., Balebona, M. C., Moriñigo, M. Á., & Esteban, M. Á. (2015). Modulation of immunity and gut microbiota after dietary administration of alginate encapsulated Shewanella putrefaciens Pdp11 to gilthead seabream (Sparus aurata L.). Fish & Shellfish Immunology, 45(2), 608–618. https://doi.org/10.1016/j.fsi.2015.05.010
- Dahiya, T., Gahlawat, S. K., & Sihag, R. C. (2012). Elimination of pathogenic bacterium (Micrococcus sp.) by the use of probiotics. Turkish Journal of Fisheries and Aquatic Sciences, 12(1), 185–187. https://doi.org/10.4194/1303-2712-v12_1_21
- Dahiya, T. P., Kant, R., Singh, G., & Sihag, R. C. (2012). Elimination of pathogenic bacterium, Aeromonas hydrophila by the use of probiotics. Journal of FisheriesSciences Com, 6(3), 209–214. https://doi.org/10.3153/jfscom.2012024
- Das, A., Nakro, K., Chowdhury, S., & Kamilya, D. (2013). Effects of potential probiotic Bacillus amyloliquefaciens FPTB16 on systemic and cutaneous mucosal immune responses and disease resistance of catla (Catla catla). Fish and Shellfish Immunology, 35(5), 1547–1553. https://doi.org/10.1016/j.fsi.2013.08.022
- Dawood, M. A., Magouz, F. I., Salem, M. F., & Abdel Daim, H. A. (2019). Modulation of digestive enzyme activity, blood health, oxidative responses and growthrelated gene expression in GIFT by heat-killed Lactobacillus plantarum (L-137). Aquaculture, 505, 127–136. https://doi.org/10.1016/j.aquaculture.2019.02.053
- Decamp, O., Moriarty, D. J. W., & Lavens, P. (2008). Probiotics for shrimp larviculture: Review of field data from Asia and Latin America. Aquaculture Research, 39(4), 334–338. https://doi.org/10.1111/j.1365-2109.2007.01664.x
- De Schryver, P., Defoirdt, T., Boon, N., Verstraete, W., & Bossier, P. (2012). Managing the microbiota in aquaculture systems for disease prevention and control. In B. Austin (Ed.), Infectious Disease in Aquaculture: Prevention and Control (pp. 393–418). Woodhead.
- DiCesare, A., Vignaroli, C., Luna, G. M., Pasquaroli, S., & Biavasco, F. (2012). Antibiotic resistant enterococci in seawater and sediments from a coastal fish farm. Microbial Drug Resistance, 18(5), 502–509. https://doi.org/10.1089/mdr.2011.0204
- Dimitroglou, A., Merrifield, D. L., Carnevali, O., Picchietti, S., Avella, M. A., Daniels, C. L., Güroy, D., & Davies, S. (2011). Microbial manipulations to improve fish health and production – a Mediterranean perspective. Fish & Shellfish Immunology, 30(1), 1–16. https://doi.org/10.1016/j.fsi.2010.08.009
- Dong, H. T., Senapin, S., LaFrentz, B., & Rodkhum, C. (2016). Virulence assay of rhizoid and non-rhizoid morphotypes of Flavobacterium columnare in red tilapia, Oreochromis sp., fry. Journal of Fish Diseases, 39(6), 649–655. https://doi.org/10.1111/jfd.12385
- EI-Jakee, J., Elshamy, S., Hassan, A. W., Abdelsalam, M., Younis, N., El-Hady, M. A., & Eissa, A. E. (2020). Isolation and characterization of Mycoplasmas from some moribund Egyptian fishes. Aquaculture International, 28(3), 901–912. https://doi.org/10.1007/s10499-019-00502-2
- El-Adawy, M. M., Eissa, A. E., Shaalan, M., Ahmed, A. A., Younis, N. A., Ismail, M. M., & Abdelsalam, M. (2020). Green synthesis and physical properties of Gum Arabic-silver nanoparticles and its antibacterial efficacy against fish bacterial pathogens. Aquaculture Research, 52(3), 1247–1254. https://doi.org/10.1111/are.14983
- El-Attar, A. A., & Moustafa, M. (1996). Some studies on tail and rot disease among cultured tilapia fishes. Assiut Veterinary Medical Journal, 35(70), 155–162. https://doi.org/10.21608/avmj.1996.183962
- Elgendy, M. Y., Kenawy, A. M., & El Deen, A. E. (2016). Gyrodactylus anguillae and Vibrio vulnificus infections affecting cultured eel, Anguilla anguilla. Comunicata Scientiae, 7(1), 1–11. https://doi.org/10.14295/cs.v7i1.1248
- Elgendy, M. Y., Shaalan, M., Abdelsalam, M., Eissa, A. E., El-Adawy, M. M., & Seida, A. A. (2021). Antibacterial activity of silver nanoparticles against antibiotic-resistant Aeromonas veronii infections in Nile tilapia, Oreochromis niloticus (L.), in vitro and in vivo assay. Aquaculture Research, 53(3), 901–920. https://doi.org/10.1111/are.15632
- Elgendy, M. Y., Soliman, W. S., Abbas, W. T., Ibrahim, T. B., Younes, A. M., & Omara, S. T. (2016). Investigation of some virulence determents in Aeromonas hydrophila strains obtained from different polluted aquatic environments. Jordan Journal of Biological Sciences, 7(1), 265–272.
- Falcinelli, S., Picchietti, S., Rodiles, A., Cossignani, L., Merrifield, D. L., Taddei, A. R., Maradonna, F., Olivotto, I., Gioacchini, G., & Carnevali, O. (2015). Lactobacillus rhamnosus lowers zebrafish lipid content by changing gut microbiota and host transcription of genes involved in lipid metabolism. Scientific Reports, 5(1), 9336. https://doi.org/10.1038/srep09336
- Falcinelli, S., Rodiles, A., Unniappan, S., Picchietti, S., Gioacchini, G., Merrifield, D. L., & Carnevali, O. (2016). Probiotic treatment reduces appetite and glucose level in the zebrafish model. Scientific Reports, 6(1), 18061. https://doi.org/10.1038/srep18061
- FAO. No5 Suppl. 8 Recommendations for prudent and responsible use of veterinary medicines in aquaculture. (2019). Retrieved May 2, 2022. https://www.fao.org/documents/card/en/c/ca7029en/
- FAO/WHO. (2001). Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Acid Bacteria. Report of a Joint FAO/WHO Expert Consultation. http://www.who.int/foodsafety/publications/fs_management/en/probiotics.pdf?ua=1
- Feng, J., Chang, X., Zhang, Y., Yan, X., Zhang, J., & Nie, G. (2019). Effects of Lactococcus lactis from Cyprinus carpio L. as probiotics on growth performance, innate immune response and disease resistance against Aeromonas hydrophila. Fish & Shellfish Immunology, 93, 73–81. https://doi.org/10.1016/j.fsi.2019.07.028
- Fernandez-Fernandez, R., Martini, A. C., Navarro, V. M., Castellano, J. M., Dieguez, C., Aguilar, E., Pinilla, L., & Tena-Sempere, M. (2006). Novel signals for the integration of energy balance and reproduction. Molecular and Cellular Endocrinology, 254–255, 127–132. https://doi.org/10.1016/j.mce.2006.04.026
- Figueroa, C., Bustos, P., Torrealba, D., Dixon, B., Soto, C., Conejeros, P., & Gallar, J. A. (2017). Coinfection takes its toll: Sea lice override the protective effects of vaccination against a bacterial pathogen in Atlantic salmon. Scientific Reports, 7(1), 17817. https://doi.org/10.1038/s41598-017-18180-6
- Fleming, A. (1944). The discovery of penicillin. British Medical Bulletin, 2, 4–5.
- Gatesoupe, F. J. (1999). The use of probiotics in aquaculture. Aquaculture, 180(1–2), 147–165. https://doi.org/10.1016/S0044-8486(99)00187-8
- Geovanny, D. G. R., Balcázar, J. L., & Ma, S. (2007). 838 Probiotics as control agents in aquaculture. Journal of Ocean University of China, 6(1), 76–79. https://doi.org/10.1007/s11802-007-0076-8
- German, D. P., Horn, M. H., & Gawlicka, A. (2004). Digestive enzyme activities in herbivorous and carnivorous prickleback fishes (Teleostei: Stichaeidae): Ontogenetic, dietary, and phylogenetic effects. Physiological and Biochemical Zoology: PBZ, 77(5), 789–804. https://doi.org/10.1086/422228
- Ghori, I., Tubassam, M., Ahmad, T., Zuberi, A., & Imran, M. (2022). Gut microbiome modulation mediated by probiotics: Positive impact on growth and health status of Labeo rohita. Frontiers in Physiology, 13, 949559. https://doi.org/10.3389/fphys.2022.949559
- Giannenas, I., Karamaligas, I., Margaroni, M., Pappas, I., Mayer, E., Encarnação, P., & Karagouni, E. (2015). Effect of dietary incorporation of a multi-strain probiotic on growth performance and health status in rainbow trout (Oncorhynchus mykiss). Fish Physiology and Biochemistry, 41(1), 119–128. https://doi.org/10.1007/s10695-014-0010-0
- Gioacchini, G., Giorgini, E., Olivotto, I., Maradonna, F., Merrifield, D. L., & Carnevali, O. (2014). The Influence of Probiotics on Zebrafish Danio Rerio Innate Immunity and Hepatic Stress. Zebrafish, 11(2), 98–106. https://doi.org/10.1089/zeb.2013.0932
- Gioacchini, G., Giorgini, E., Vaccari, L., & Carnevali, O. (2014). Can probiotics affect reproductive processes of aquatic animals? In D. Merrifield & E. Ringø (Eds.), Aquaculture Nutrition: Gut Health, Probiotics and Prebiotics (pp. 328–346). John Wiley & Sons Ltd. https://doi.org/10.1002/9781118897263.ch12
- Gioacchini, G., Maradonna, F., Lombardo, F., Bizzaro, D., Olivotto, I., & Carnevali, O. (2010). Increase of fecundity by probiotic administration in zebrafish (Danio rerio). Reproduction, 140(6), 953–959. https://doi.org/10.1530/REP-10-0145
- Gomez, D., Sunyer, J. O., & Salinas, I. (2013). The mucosal immune system of fish: The evolution of tolerating commensals while fighting pathogens. Fish & Shellfish Immunology, 35(6), 1729–1739. https://doi.org/10.1016/j.fsi.2013.09.032
- Gorgoglione, B., Bailey, C., & Fast, M. D. (2020). Co-infections and multiple stressors in fish. Bull Eur Assoc Fish Pathol, 40(1), 4–19. https://eafp.org/download/2020-volume40/issue_1/40-1-04-gorgoglione.pdf
- Gorissen, M., Bernier, N. J., Nabuurs, S. B., Flik, G., & Huising, M. O. Two divergent leptin paralogues in zebrafish (Danio rerio) that originate early in teleostean evolution. (2009). The Journal of Endocrinology, 201(3), 329–339. 1677/JOE-09-0034. https://doi.org/10.1677/JOE-09-0034
- Gram, L., & Melchiorsen, J. (1996). Interaction between fish spoilage bacteria Pseudomonas sp. and Shewanella putrefaciens in fish extracts and on fish tissue. Journal of Applied Bacteriology, 80(6), 589–595. https://doi.org/10.1111/j.1365-2672.1996.tb03262.x
- Gram, L., Melchiorsen, J., Spanggaard, B., Huber, I., & Nielsen, T. F. (1999). Inhibition of Vibrio anguillarum by Pseudomonas fluorescens AH2, a possible probiotic treatment of fish. Applied and Environmental Microbiology, 65(3), 969–973. https://doi.org/10.1128/AEM.65.3.969-973.1999
- Guardiola, F. A., Bahi, A., Bakhrouf, A., & MÁ, E. (2017). Effects of dietary supplementation with fenugreek seeds, alone or in combination with probiotics, on gilthead seabream(Sparus aurata L.) skinmucosal immunity. Fish & Shellfish Immunology, 65, 169–178. https://doi.org/10.1016/j.fsi.2017.04.014
- Gueimonde, M., Jalonen, L., He, F., Hiramatsu, M., & Salminen, S. (2006). Adhesion and competitive inhibition and displacement of human enteropathogens by selected lactobacilli. Food Research International, 39(4), 467–471. https://doi.org/10.1016/j.foodres.2005.10.003
- Guo, X., Chen, D.-D., Peng, K.-S., Cui, Z.-W., Zhang, X.-J., Li, S., & Zhang, Y. A. (2016). Identification and characterisation of Bacillus subtilis from grass carp (Ctenopharynodon idellus) for use as probiotic additives in aquatic feed. Fish & Shellfish Immunology, 52, 74–84. https://doi.org/10.1016/j.fsi.2016.03.017
- Haenen, O. L. M., Dong, H. T., Hoai, T. D., Crumlish, M., Karunasagar, I., Barkham, T., Chen, S. L., Zadoks, R., Kiermeier, A., Wang, B., Gamarro, E. G., Takeuchi, M., Azmai, M. N. A., Fouz, B., Pakingking, R., Wei, Z. W., & Bondad‐Reantaso, M. G. (2023). Bacterial diseases of tilapia, their zoonotic potential and risk of antimicrobial resistance. Reviews in Aquaculture, 15(S1), 154–185. https://doi.org/10.1111/raq.12743
- Hai, N. V. (2015). The use of probiotics in aquaculture. Journal of Applied Microbiology, 119(4), 917–935. https://doi.org/10.1111/jam.12886
- Hai, V. N., & Fotedar, R. (2009). Comparison of the effects of the prebiotics (Bio-Mos® and β-1,3-D-glucan) and the customised probiotics (Pseudomonas synxantha and P. aeruginosa) on the culture of juvenile western king prawns (Penaeus latisulcatus Kishinouye, 1896). Aquaculture, 289(3–4), 310–316. https://doi.org/10.1016/j.aquaculture.2009.02.001
- Hamdan, A. M., El-Sayed, A. F. M., & Mahmoud, M. M. (2016). Effects of a novel marine probiotic, Lactobacillus plantarum AH 78, on growth performance and immune response of Nile tilapia (Oreochromis niloticus). Journal of Applied Microbiology, 120(4), 1061–1073. https://doi.org/10.1111/jam.13081
- Han, B., Long, W.-Q., He, J.-Y., Liu, Y.-J., Si, Y.-Q., & Tian, L.-X. (2015). Effects of dietary Bacillus licheniformis on growth performance, immunological parameters, intestinal morphology and resistance of juvenile Nile tilapia (Oreochromis niloticus) to challenge infections. Fish & Shellfish Immunology, 46(2), 225–231. https://doi.org/10.1016/j.fsi.2015.06.018
- Hasan, K. N., & Banerjee, G. (2020). Recent studies on probiotics as beneficial mediator in aquaculture: Review. The Journal of Basic and Applied Zoology, 81(1), 53. https://doi.org/10.1186/s41936-020-00190-y
- Hellio, C., Bado-Nilles, A., Gagnaire, B., Renault, T., & Thomas-Guyon, H. (2007). Demonstration of a true phenoloxidase activity and activation of a ProPO cascade in pacific oyster, Crassostrea gigas (Thunberg) in vitro. Fish & Shellfish Immunology, 22(4), 433–440. https://doi.org/10.1016/j.fsi.2006.06.014
- Hlordzi, V., Kuebutornye, F. K. A., Afriyie, G., Abarike, E. D., Lu, Y., Chi, S., & Anokyewaa, M. A. (2020). The use of Bacillus species in maintenance of water quality in aquaculture: A review. Aquaculture Reports, 18, 100503. https://doi.org/10.1016/j.aqrep.2020.100503
- Hoseinifar, S. H., Sun, Y.-Z., Wang, A., & Zhou, Z. (2018). Probiotics as means of diseases control in aquaculture, a review of current knowledge and future perspectives. Frontiers in Microbiology, 9, 2429. https://doi.org/10.3389/fmicb.2018.02429
- Huang, J. C. (2008). The role of peroxisome proliferator-activated receptors in the development and physiology of gametes and preimplantation embryos. PPAR Research, 2008, 1–7. https://doi.org/10.1155/2008/732303
- Huang, Y., Zhang, L., Tiu, L., & Wang, H. H. (2015). Characterization of antibiotic resistance in commensal bacteria from an aquaculture ecosystem. Front Microbiology, 6, 914. https://doi.org/10.3389/fmicb.2015.00914
- Hura, M. U. D., Zafar, T., Borana, K., Prasad, J. R., & Iqbal, J. (2018). Effect of commercial probiotic Bacillus megaterium on water quality in composite culture of major carps. The International Journal of Agricultural Science, 8, 268–273.
- Irianto, A., & Austin, B. (2002a). Probiotics in aquaculture. Journal of Fish Diseases, 25(11), 633–642. https://doi.org/10.1046/j.1365-2761.2002.00422.x
- Irianto, A., & Austin, B. (2002b). Probiotics in aquaculture. Journal of Fish Diseases, 25, 633–642. https://doi.org/10.1046/j.1365-2761.2002.00422.x/
- Irianto, A., & Austin, B. (2003). Use of dead probiotic cells to control furunculosis in rainbow trout. Oncorhynchus Mykiss (Walbaum) Journal of Fish Diseases, 26(1), 59–62. https://doi.org/10.1046/j.1365-2761.2003.00414.x
- Jakhar, V., Sihag, R. C., & Gahlawat, S. K. (2013a). Effect of probiotics on growth and survival of giant freshwater prawn (Macrobrachium rosenbergii de Man). Research on Crops, 14(4), 1264–1268.
- Jakhar, V., Sihag, R. C., & Gahlawat, S. K. (2013b). Survey and characterization of diseases in giant freshwater prawn (Macrobrachium rosenbergii de Man) in Haryana. Indian Journal of Animal Research, 47(6), 479–485.
- Jang, W. J., Lee, J. M., Hasan, M. T., Lee, B.-J., Lim, S. G., & Kong, I.-S. (2019). Effects of probiotic supplementation of a plant-based protein diet on intestinal microbial diversity, digestive enzyme activity, intestinal structure, and immunity in olive flounder (Paralichthys olivaceus). Fish & Shellfish Immunology, 92, 719–727. https://doi.org/10.1016/j.fsi.2019.06.056
- Kim, D. H., & Austin, B. (2006). Innate immune responses in rainbow trout (Oncorhynchus mykiss, Walbaum) induced by probiotics. Fish & Shellfish Immunology, 21(5), 513–524. https://doi.org/10.1016/j.fsi.2006.02.007
- Kim, S., Covington, A., & Pamer, E. G. (2017). The intestinal microbiota: Antibiotics, colonization resistance, and enteric pathogens. Immunological Reviews, 279, 90–105. https://doi.org/10.1111/imr.12563
- Kotob, M. H., Menanteau Ledouble, S., Kumar, G., Abdelzaher, M., & El-Matbouli, M. (2016). The impact of coinfections on fish: A review. Veterinary Research, 47(1), 98. https://doi.org/10.1186/s13567-016-0383-4
- Krogdahl, Å., Hemre, G. I., & Mommsen, T. (2005). Carbohydrates in fish nutrition: Digestion and absorption in postlarval stages. Aquaculture Nutrition, 11(2), 103–122. https://doi.org/10.1111/j.1365-2095.2004.00327.x
- Kuebutornye, F. K. A., Abarike, E. D., & Lu, Y. (2019). A review on the application of Bacillus as probiotics in aquaculture. Fish & Shellfish Immunology, 87, 820–828. https://doi.org/10.1016/j.fsi.2019.02.010
- Kumar, R., Mukherjee, S. C., Ranjan, R., & Nayak, S. K. (2008). Enhanced innate immune parameters in Labeo rohita (Ham.) following oral administration of Bacillus subtilis. Fish & Shellfish Immunology, 24(2), 168–172. https://doi.org/10.1016/j.fsi.2007.10.008
- Lara-Flores, M., Olvera-Novoa, M. A., Guzmán-Méndez, B. E., & López-Madrid, W. (2003). Use of the bacteria Streptococcus faecium and Lactobacillus acidophilus, and the yeast Saccharomyces cerevisiae as growth promoters in Nile tilapia (Oreochromis niloticus). Aquaculture, 216(1–4), 193–201. https://doi.org/10.1016/S0044-8486(02)00277-6
- Lategan, M. J., Torpy, F. R., & Gibson, L. F. (2004). Control of saprolegniosis in the eel Anguilla australis Richardson, by Aeromonas media strain A199. Aquaculture, 240(1–4), 19–27. https://doi.org/10.1016/j.aquaculture.2004.04.009
- Lawley, T. D., & Walker, A. W. (2013). Intestinal colonisation resistance. Immunology, 138(1), 1–11. https://doi.org/10.1111/j.1365-2567.2012.03616.x
- Lazado, C. C., Caipang, C. M. A., & Estante, E. G. (2015). Prospects of host-associated microorganisms in fish and penaeids as probiotics with immunomodulatory functions. Fish & Shellfish Immunology, 45(1), 2–12. https://doi.org/10.1016/j.fsi.2015.02.023
- Letchumanan, V., Chan, K.-G., & Lee, L.-H. (2015). An insight of traditional plasmid curing in Vibrio species. Front Microbiology, 6, 735. https://doi.org/10.3389/fmicb.2015.00735
- Levavi-Sivan, B., Bogerd, J., Mañanós, E. L., Gómez, A., & Lareyre, J. J. (2010). Perspectives on fish gonadotropins and their receptors. General & Comparative Endocrinology, 165(3), 412–437. https://doi.org/10.1016/j.ygcen.2009.07.019
- Lieke, T., Meinelt, T., Hoseinifar, S. H., Pan, B., Straus, D. L., & Steinberg, C. E. W. (2019). Sustainable aquaculture requires environmental‐friendly treatment strategies for fish diseases. Reviews in Aquaculture, 12(2), 943–965. https://doi.org/10.1111/raq.12365
- Lin, S., Mao, S., Guan, Y., Luo, L., Luo, L., & Pan, Y. (2012). Effects of dietary chitosan oligosaccharides and Bacillus coagulans on the growth, innate immunity and resistance of koi (Cyprinus carpio koi). Aquaculture, 342, 36–41. https://doi.org/10.1016/j.aquaculture.2012.02.009
- Li, J., Tan, B., & Mai, K. (2009). Dietary probiotic Bacillus OJ and isomaltooligosaccharides influence the intestine microbial populations, immune responses and resistance to white spot syndrome virus in shrimp (Litopenaeus vannamei). Aquaculture, 291, 35–40. https://doi.org/10.1016/j.aquaculture.2009.03.005
- Liu, H., Wang, S., Cai, Y., Guo, X., Cao, Z., Zhang, Y., Liu, S., Yuan, W., Zhu, W., Zheng, Y., Xie, Z., Guo, W., & Zhou, Y. (2017). Dietary administration of Bacillus subtilis HAINUP40 enhances growth, digestive enzyme activities, innate immune responses and disease resistance of tilapia, Oreochromis niloticus. Fish & Shellfish Immunology, 60, 326–333. https://doi.org/10.1016/j.fsi.2016.12.003
- Marshall, B. M., & Levy, S. B. (2011). Food animals and antimicrobials: impacts on human health. Clinical microbiology reviews, 24, 718–733. https://doi.org/10.1128/CMR.00002-11
- Martı́nez-Cruz, P., Ibáñez, A. L., Monroy-Hermosillo, O. A., & Ramı́rez-Saad, H. C. (2012). Use of probiotics in aquaculture. ISRN Microbiology, 916845, 1–13. https://doi.org/10.5402/2012/916845
- Mechaly, A. S., Viñas, J., & Piferrer, F. (2013). The kisspeptin system genes in teleost fish, their structure and regulation, with particular attention to the situation in Pleuronectiformes. General & Comparative Endocrinology, 188, 258–268. https://doi.org/10.1016/j.ygcen.2013.04.010
- Medina, M., Sotil, G., Flores, V., Fernández, C., & Sandoval, N. (2020). In vitro assessment of some probiotic properties and inhibitory activity against Yersinia ruckeri of bacteria isolated from rainbow trout Oncorhynchus mykiss (Walbaum). Aquaculture Reports, 18, 100447. https://doi.org/10.1016/j.aqrep.2020.100447
- Merrifield, D. L., Dimitroglou, A., Foey, A., Davies, S. J., Baker, R. T., Bøgwald, J., Castex, M., & Ringø, E. (2010). The current status and future focus of probiotic and prebiotic applications for salmonids. Aquaculture, 302(1–2), 1–18. https://doi.org/10.1016/j.aquaculture.2010.02.007
- Michael, E. T., Amos, S. O., & Hussaini, L. T. (2014). A review on probiotics application in aquaculture. Fisheries and Aquaculture Journal, 5(4), 4. https://doi.org/10.4172/2150-3508.10000111
- Migliarini, B., Piccinetti, C. C., Martella, A., Maradonna, F., Gioacchini, G., & Carnevali, O. (2011). Perspectives on endocrine disruptor effects on metabolic sensors. General & Comparative Endocrinology, 170(3), 416–423. https://doi.org/10.1016/j.ygcen.2010.11.025
- Milner, E., Stevens, B., An, M., Lam, V., Ainsworth, M., Dihle, P., Stearns, J., Dombrowski, A., Rego, D., & Segars, K. (2021). Utilising Probiotics for the Prevention and Treatment of Gastrointestinal Diseases. Frontiers in Microbiology, 12, 689958. https://doi.org/10.3389/fmicb.2021.689958
- Mohammadian, T., Nasirpour, M., Tabandeh, M. R., & Mesbah, M. (2019). Synbiotic effects of b-glucan, mannan oligosaccharide and Lactobacillus casei on growth performance, intestine enzymes activities, immune-hematological parameters and immune-related gene expression in common carp, Cyprinus carpio: An experimental infection with Aeromonas hydrophila. Aquaculture, 511, 634197. https://doi.org/10.1016/j.aquaculture.2019.06.005
- Moustafa, M., Eissa, A. E., Laila, A. M., Gaafar, A. Y., Abumourad, I. M. K., & Elgendy, M. Y. (2015). Investigations into the potential causes of mass kills in mari-cultured gilthead sea bream (Sparus aurata) at Northern Egypt. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 6(1), 466–477.
- Mukai, T., Asasaka, T., Sato, E., Mori, K., Matsumoto, M., & Ohori, H. (2002). Inhibition of binding of Helicobacter pylori to the glycolipid receptors by probiotic Lactobacillus reuteri. FEMS Immunology and Medical Microbiology, 35(2), 105–110. https://doi.org/10.1111/j.1574-695X.2002.tb00541.x
- Muthukumar, P., & Kandeepan, C. (2015). Isolation, identification and characterisation of probiotic organisms from intestine of fresh water fishes. International Journal of Current Microbiology and Applied Sciences, 4(3), 607–616.
- Nath, S., Matozzo, V., Bhandari, D., & Faggio, C. (2019). Growth and liver histology of Channa punctatus exposed to a common biofertiliser. Natural Product Research, 33(11), 1591–1598. https://doi.org/10.1080/14786419.2018.1428586
- Navarrete, P., & Tovar-Ramı́rez, D. (2014). Use of yeasts as probiotics in fish aquaculture. In M. Hernandez-Vergara & C. Perez-Castro (Eds.), Sustainable Aquaculture Techniques 1 (pp. 135–172). IntechOpen. https://doi.org/10.5772/57196
- NavinChandran, M., Iyapparaj, P., Moovendhan, S., Ramasubburayan, R., Prakash, S., Immanuel, G., & Palavesam, A. (2014). Influence of probiotic bacterium Bacillus cereus isolated from the gut of wild shrimp Penaeus monodon in turn as a potent growth promoter and immune enhancer in P. monodon. Fish and Shellfish Immunology, 36(1), 38–45. https://doi.org/10.1016/j.fsi.2013.10.004
- Nayak, S. K. (2010). Probiotics and immunity: Fish perspectives. Fish & Shellfish Immunology, 29(1), 2–14. https://doi.org/10.1016/j.fsi.2010.02.017
- Nayak, S. K., Swain, P., & Mukherjee, S. C. (2007). Effect of dietary supplementation of probiotic and vitamin C on the immune response of Indian major carp. Fish & Shellfish Immunology, 23(4), 892–896. https://doi.org/10.1016/j.fsi.2007.02.008
- Newaj-Fyzul, A., Adesiyun, A. A., Mutani, A., Ramsubhag, A., Brunt, J., & Austin, B. (2007). Bacillus subtilis AB1 controls Aeromonas infection in rainbow trout (Oncorhynchus mykiss, Walbaum). Journal of Applied Microbiology, 103(5), 1699–1706. https://doi.org/10.1111/j.1365-2672.2007.03402.x
- Newaj-Fyzul, A., Al-Harbi, A. H., & Austin, B. (2014). Review: Developments in the use of probiotics for disease control in aquaculture. Aquaculture, 431, 1–11.
- Newaj-Fyzul, A., & Austin, B. (2015). Probiotics, immunostimulants, plant products and oral vaccines, and their role as feed supplements in the control of bacterial fish diseases. Journal of Fish Diseases, 38(11), 937–955. https://doi.org/10.1111/jfd.12313
- Newaj-Fyzul, A., Mutani, A., Ramsubhag, A., & Adesiyun, A. (2008). Prevalence of bacterial pathogens and their anti-microbial resistance in tilapia and their pond water in Trinidad. Zoonoses and Public Health, 55(4), 206–213. https://doi.org/10.1111/j.1863-2378.2007.01098.x
- Nguyen, T. L., Chun, W.-K., Kim, A., Kim, N., Roh, H. J., Lee, Y., Yi, M., Kim, S., Park, C.-I., & Kim, D.-H. (2018). Dietary probiotic effect of Lactococcus lactis WFLU12 on low-molecular-weight metabolites and growth of olive flounder (Paralichythys olivaceus). Frontiers in Microbiology, 9, 2059. https://doi.org/10.3389/fmicb.2018.02059
- Nguyen, T. L., Park, C.-I., & Kim, D.-H. (2017). Improved growth rate and disease resistance in olive flounder, Paralichthys olivaceus, by probiotic Lactococcus lactis WFLU12 isolated from wild marine fish. Aquaculture, 471, 113–120. https://doi.org/10.1016/j.aquaculture.2017.01.008
- Nicholson, P., Fathi, M. A., Fischer, A., Mohan, C., Schieck, E., Mishra, N., Heinimann, A., Frey, J., Wieland, B., & Jores, J. (2017). Detection of tilapia lake virus in Egyptian fish farms experiencing high mortalities in 2015. Journal of Fish Diseases, 40(12), 1925–1928. https://doi.org/10.1111/jfd.12650
- Nicholson, P., Mon-On, N., Jaemwimol, P., Tattiyapong, P., & Surachetpong, W. (2020). Coinfection of tilapia lake virus and Aeromonas hydrophila synergistically increased mortality and worsened the disease severity in tilapia (Oreochromis spp.). Aquaculture, 520, 734746. https://doi.org/10.1016/j.aquaculture.2019.734746
- Nikoskelainen, S., Ouwehand, A., Salminen, S., & Bylund, G. (2001). Protection of rainbow trout (Oncorhynchus mykiss) from furunculosis by Lactobacillus rhamnosus. Aquaculture, 198(3–4), 229–236. https://doi.org/10.1016/S0044-8486(01)00593-2
- Ohga, H., Adachi, H., Matsumori, K., Kodama, R., Nyuji, M., Selvaraj, S., Kato, K., Yamamoto, S., Yamaguchi, A., & Matsuyama, M. (2015). mRNA levels of kisspeptins, kisspeptin receptors, and GnRH1 in the brain of chubmackerel during puberty. Comparative Biochemistry & Physiology Part A, Molecular & Integrative Physiology, 179, 104–112. 09.012. https://doi.org/10.1016/j.cbpa.2014.09.012
- Palermo, F. A., Mosconi, G., Avella, M. A., Carnevali, O., Verdenelli, M. C., Cecchini, C., & Polzonetti-Magni, A. M. (2011). Modulation of cortisol levels, endocannabinoid receptor 1A, proopiomelanocortin and thyroid hormone receptor alpha mRNA expressions by probiotics during sole (Solea solea) larval development. General & Comparative Endocrinology, 171(3), 293–300. https://doi.org/10.1016/j.ygcen.2011.02.009
- Pandiyan, P., Balaraman, D., Thirunavukkarasu, R., George, E. G. J., Subaramaniyan, K., Manikkam, S., & Sadayappan, B. (2013). Probiotics in aquaculture. Drug Invention Today, 5(1), 55–59. https://doi.org/10.1016/j.dit.2013.03.003
- Panigrahi, A., & Azad, I. S. (2007). Microbial intervention for better fish health in aquaculture: The Indian scenario. Fish Physiology and Biochemistry, 33(4), 429–440. https://doi.org/10.1007/s10695-007-9160-7
- Papadopoulou, O., Argyri, A., Varzakis, E. E., Tassou, C., & Chorianopoulos, N. (2018). Greek functional feta cheese: Enhancing quality and safety using a Lactobacillus plantarum strain with probiotic potential. Food Microbiology, 74, 21–33. https://doi.org/10.1016/j.fm.2018.02.005
- Papadopoulou, O. S., Doulgeraki, A., Panagou, E., & Argyri, A. A. (2023). Editorial: Recent advances and future perspective in probiotics isolated from fermented foods: From quality assessment to novel products. Frontiers in Microbiology, 14, 1150175. https://doi.org/10.3389/fmicb.2023.1150175
- Pattukumar, V., Kanmani, P., Kumar, R. S., Yuvaraj, N., Paari, A., & Arul, V. (2014). Enhancement of innate immune system, survival and yield in Penaeus monodon reared in ponds using Streptococcus phocae PI80. Aquaculture Nutrition, 20(5), 505–513. https://doi.org/10.1111/anu.12103
- Pereira, A. M. P. T., Silva, L. J. G., Meisel, L. M., & Pena, A. (2015). Fluoroquinolones and Tetracycline Antibiotics in a Portuguese Aquaculture System and Aquatic Surroundings: Occurrence and Environmental Impact. Journal of Toxicology and Environmental Health. Part A, 78(15), 959–975. https://doi.org/10.1080/15287394.2015.1036185
- Pfundt, B., Sauerwein, H., & Mielenz, M. (2009). Leptin mRNA and Protein Immunoreactivity in Adipose Tissue and Liver of Rainbow Trout (Oncorhynchus mykiss) and Immunohistochemical Localization in Liver. Anatomia, histologia, embryologia, 38(6), 406–410. https://doi.org/10.1111/j.1439-0264.2009.00951.x
- Pham, D. K., Chu, J., Do, N. T., Brose, F., Degand, G., Delahaut, P., & Wertheim, H. F. L. (2015). Monitoring Antibiotic Use and Residue in Freshwater Aquaculture for Domestic Use in Vietnam. EcoHealth, 12(3), 480–489. https://doi.org/10.1007/s10393-014-1006-z
- Picchietti, S., Fausto, A. M., Randelli, E., Carnevali, O., Taddei, A. R., Buonocore, F., Scapigliati, G., & Abelli, L. (2009). Early treatment with Lactobacillus delbrueckii strain induces an increase in intestinal T-cells and granulocytes and modulates immune-related genes of larval Dicentrarchus labrax (L.). Fish & Shellfish Immunology, 26(3), 368–376. https://doi.org/10.1016/j.fsi.2008.10.008
- Picchietti, S., Guerra, L., Selleri, L., Buonocore, F., Abelli, L., Scapigliati, G., Mazzini, M., & Fausto, A. M. (2008). Compartmentalisation of T cells expressing CD8α and TCR β in developing thymus of sea bass Dicentrarchus labrax (L.). Developmental and Comparative Immunology, 32(2), 92–99. https://doi.org/10.1016/j.dci.2007.04.002
- Picchietti, S., Mazzini, M., Taddei, A. R., RENNA, R., FAUSTO, A., MULERO, V., CARNEVALI, O., CRESCI, A., & ABELLI, L. (2007). Effects of administration of probiotic strains on GALT of larval gilthead seabream: Immunohistochemical and ultrastructural studies. Fish and Shellfish Immunology, 22(1–2), 57–67. https://doi.org/10.1016/j.fsi.2006.03.009
- Piccinetti, C. C., Migliarini, B., Petrosino, S., DiMarzo, V., & Carnevali, O. (2010). Anandamide and AM251, via water, modulate food intake at central and peripheral level in fish. General & Comparative Endocrinology, 166(2), 259–267. https://doi.org/10.1016/j.ygcen.2009.09.017
- Poulsen, L., Siersbæk, M., & Mandrup, S. (2012). Ppars: Fatty acid sensors controlling metabolism. Semin. Seminars in Cell & Developmental Biology, 23(6), 631–639. https://doi.org/10.1016/j.semcdb.2012.01.003
- Qin, C., Xu, L., Yang, Y., He, S., Dai, Y., Zhao, H., & Zhou, Z. (2014). Comparison of fecundity and offspring immunity in zebrafish fed Lactobacillus rhamnosus CICC 6141 and Lactobacillus casei BL23. Reproduction, 147(1), 53–64. https://doi.org/10.1530/REP-13-0141
- Rahiman, K. M. M., Jesmi, Y., Thomas, A. P., & Hatha, A. A. M. (2010). Probiotic effect of Bacillus NL110 and Vibrio NE17 on the survival, growth performance and immune response of Macrobrachium rosenbergii (de Man). Aquaculture Research, 41(9), e120–e134. https://doi.org/10.1111/j.1365-2109.2009.02473.x
- Rahimnejad, S., Guardiola, F. A., Leclercq, E., Ángeles Esteban, M., Castex, M., Sotoudeh, E., & Lee, S.-M. (2018). Effects of dietary supplementation with Pediococcus acidilactici MA18/5M, galactooligosaccharide and their synbiotic on growth, innate immunity and disease resistance of rockfish (Sebastes schlegeli). Aquaculture, 482, 36–44. https://doi.org/10.1016/j.aquaculture.2017.09.020
- Ray AK. (2017). The advancement of probiotics research and its application in fish farming industries. Research in Veterinary Science, 115, 66–77. https://doi.org/10.1016/j.rvsc.2017.01.016
- Ray, A., Ghosh, K., & Ringø, E. (2012). Enzyme-producing bacteria isolated from fish gut: A review. Aquaculture Nutrition, 18(5), 465–492. https://doi.org/10.1111/j.1365-2095.2012.00943.x
- Rengpipat, S., Rukpratanporn, S., Piyatiratitivorakul, S., & Menasaveta, P. (2000). Immunity enhancement in black tiger shrimp (Penaeus monodon) by a probiont bacterium (Bacillus S11). Aquaculture, 191(4), 271–288. https://doi.org/10.1016/S0044-8486(00)00440-3
- Ringø, E. (1999). Does Carnobacterium divergens isolated from Atlantic salmon, Salmo salar L., colonize the gut of early developing turbot, Scophthalmus maximus L., larvae? Aquaculture Research, 30(3), 229–232. https://doi.org/10.1046/j.1365-2109.1999.00269.x
- Ringø, E., Birkbeck, T. H., Munro, P. D., Vadstein, O., & Hjelmeland, K. (1996). The effect of early exposure to Vibrio Pelagius on the aerobic bacterial flora of turbot, Scophthalmus maximus (L.) larvae. The Journal of Applied Bacteriology, 81(2), 207–211. https://doi.org/10.1111/j.1365-2672.1996.tb04502.x
- Ringø, E., Doan, H. V., Lee, S., & Song, S. K. (2020). Lactic acid bacteria in shellfish: Possibilities and challenges. Reviews in Fisheries Science & Aquaculture, 28(2), 139–169. https://doi.org/10.1080/23308249.2019.1683151
- Ringø, E., & Gatesoupe, F. J. (1998). Lactic acid bacteria in fish: A review. Aquaculture, 160(3–4), 177–203. https://doi.org/10.1016/S0044-8486(97)00299-8
- Ringø, E., Hoseinifar, S. H., Ghosh, K., Doan, H., Van Beck, B. R., & Song, S. K. (2018). Lactic acid bacteria in finfish—an update. Frontiers in Microbiology, 9, 1818. https://doi.org/10.3389/fmicb.2018.01818
- Ringø, E., Li, X., Doan, H., & Ghosh, K. (2022). Interesting Probiotic Bacteria Other Than the More Widely Used Lactic Acid Bacteria and Bacilli in Finfish. Frontiers in Marine Science, 9, 848037. https://doi.org/10.3389/fmars.2022.848037
- Ringø, E., & Vadstein, O. (1998). Colonisation of Vibrio Pelagius and Aeromonas caviae in early developing turbot (Scophthalmus maximus L.) larvae. Journal of Applied Microbiology, 84(2), 227–233. https://doi.org/10.1046/j.1365-2672.1998.00333.x
- Robertson, P. A. W., O’Dowd, C., Burrells, C., Williams, P., & Austin, B. (2000). Use of Carnobacterium sp. as a probiotic for Atlantic salmon (Salmo salar L.) and rainbow trout (Oncorhynchus mykiss Walbaum). Aquaculture, 185(3–4), 235–243. https://doi.org/10.1016/S0044-8486(99)00349-X
- Sakai, M. (1999). Current research status of fish immunostimulants. Aquaculture, 172(1–2), 63–92. https://doi.org/10.1016/S0044-8486(98)00436-0
- Sakai, M., Yoshida, T., Atsuta, S., & Kobayashi, M. (1995). Enhancement of resistance to vibriosis in rainbow trout, Oncorhynchus mykiss (Walbaum), by oral administration of Clostridium butyricumbacterin. Journal of Fish Diseases, 18(2), 187–190. https://doi.org/10.1111/j.1365-2761.1995.tb00276.x
- Salinas, I., Abelli, L., Bertoni, F., Picchietti, S., Roque, A., Furones, D., Cuesta, A., Meseguer, J., & Esteban, M. Á. (2008). Monospecies and multispecies probiotic formulations produce different systemic and local immunostimulatory effects in the gilthead seabream (Sparus aurata L.). Fish and Shellfish Immunology, 25(1–2), 114–123. https://doi.org/10.1016/j.fsi.2008.03.011
- Salmerón, C., Johansson, M., Angotzi, A. R., Rønnestad, I., Jönsson, E., Björnsson, B. T., Gutiérrez, J., Navarro, I., & Capilla, E. (2015). Effects of nutritional status on plasma leptin levels and in vitro regulation of adipocyte leptin expression and secretion in rainbow trout. General & Comparative Endocrinology, 210, 114–123. https://doi.org/10.1016/j.ygcen.2014.10.016
- Sanchez-Garrido, M. A., & Tena-Sempere, M. (2013). Metabolic control of puberty: Roles of leptin and kisspeptins. Hormones and Behavior, 64(2), 187–194. https://doi.org/10.1016/j.yhbeh.2013.01.014
- Sewaka, M., Trullas, C., Chotiko, A., Rodkhum, C., Chansue, N., Boonanuntanasarn, S., & Pirarat, N. (2019). Efficacy of synbiotic Jerusalem artichoke and Lactobacillus rhamnosus GG-supplemented diets on growth performance, serum biochemical parameters, intestinal morphology, immune parameters and protection against Aeromonas veronii in juvenile red tilapia (Oreochromis spp.). Fish & Shellfish Immunology, 86, 260–268. https://doi.org/10.1016/j.fsi.2018.11.026
- Sharifuzzaman, S. M., Abbass, A., Tinsley, J. W., & Austin, B. (2011). Subcellular components of probiotics Kocuria SM1 and Rhodococcus SM2 induce protective immunity in rainbow trout (Oncorhynchus mykiss,Walbaum) against Vibrio anguillarum. Fish and Shellfish Immunology, 30(1), 347–353. https://doi.org/10.1016/j.fsi.2010.11.005
- Sharifuzzaman, S., Al-Harbi, A., & Austin, B. (2014). Characteristics of growth, digestive system functionality, and stress factors of rainbow trout fed probiotics Kocuria SM1 and Rhodococcus SM2. Aquaculture, 418, 55–61. https://doi.org/10.1016/j.aquaculture.2013.10.006
- Sharifuzzaman, S., & Austin, B. (2017). Probiotics for disease control in aquaculture. In Diagnosis and Control of Diseases of Fish and Shellfish (pp. 189–222). John Wiley & Sons Inc. https://doi.org/10.1002/9781119152125.ch8
- Sharma, P., Sihag, R. C., & Gahlawat, S. K. (2013). Relative Efficacy of Two Probiotics in Controlling the Epizootic Ulcerative Syndrome Disease in Mrigal (Cirrhinus mrigala Ham.). Journal of Fisheries and Aquatic Science, 8(2), 305–322. https://doi.org/10.3923/jfas.2013.305.322
- Shefat, S. H. T. (2018). Probiotic strains used in aquaculture. International Research Journal of Pharmacy, 7, 43–55. https://doi.org/10.14303/irjm.2018.023
- Sica, M. G., Brugnoni, L. I., Marucci, P. L., & Cubitto, M. A. (2012). Characterisation of probiotic properties of lactic acid bacteria isolated from an estuarine environment for application in rainbow trout (Oncorhynchus mykiss, Walbaum) farming. Antonie Van Leeuwenhoek, 101(4), 869–879. https://doi.org/10.1007/s10482-012-9703-5
- Sihag, R. C., & Sharma, P. (2012). Probiotics: The new ecofriendly alternative measures of disease control for sustainable aquaculture. Journal of Fisheries and Aquatic Science, 7(2), 72–103. https://doi.org/10.3923/jfas.2012.72.103
- Simo´n, R., Docando, F., Nuñez-Ortiz, N., Tafalla, C., & D´ıaz-Rosales, P. (2021). Mechanisms Used by Probiotics to Confer Pathogen Resistance to Teleost Fish. Frontiers in Immunology, 12, 653025. https://doi.org/10.3389/fimmu.2021.653025
- Singh, M. P., Pathak, D., Sharma, G. K., & Sharma, C. S. (2011). Peroxisome proliferator-activated receptors (PPARS): A target with a broad therapeutic potential for human diseases: An overview. Pharmacology, 2, 58–89.
- Soltani, M., Abdy, E., Alishahi, M., Mirghaed, A. T., & Hosseini-Shekarabi, P. (2017). Growth performance, immune-physiological variables and disease resistance of common carp (Cyprinus carpio) orally subjected to different concentrations of Lactobacillus plantarum. Aquaculture International, 25(5), 1913–1933. https://doi.org/10.1007/s10499-017-0164-8
- Soomro, A. H., Masud, T., & Anwaar, K. (2001). Role of lactic acid bacteria (LAB) in food preservation and human health – a review. Pakistan Journal of Nutrition, 1(1), 20–24. https://doi.org/10.3923/pjn.2002.20.24
- Standen, B. T., Rawling, M. D., & Davies, S. J. (2013). Probiotic Pediococcus acidilactici moderates both localised intestinal- and peripheral-immunity in tilapia (Oreochromis niloticus). Fish and Shellfish Immunology, 35(4), 1097–1104. https://doi.org/10.1016/j.fsi.2013.07.018
- Stienstra, R., Netea-Maier, R. T., Riksen, N. P., Joosten, L. A. B., & Netea, M. G. (2017). Specific and Complex Reprogramming of Cellular Metabolism in Myeloid Cells during Innate Immune Responses. Cell Metabolism, 26(1), 142–156. https://doi.org/10.1016/j.cmet.2017.06.001
- Strøm, E., & Ringø, E. (1993). Changes in the bacterial composition of early developing cod, Gadus morhua (L.) larvae following inoculation of Lactobacillus plantarum into the water. In B. T. Walther & H. J. Fyhn (Eds.), Physiology and Biochemical Aspects of Fish Development (pp. 226–228). University of Bergen.
- Sugita, H., Hirose, Y., Matsuo, N., & Deguchi, Y. (1998). Production of the antibacterial substance by Bacillus sp. strain NM12, an intestinal bacterium of Japanese coastal fish. Aquaculture, 165(3–4), 269–280. https://doi.org/10.1016/S0044-8486(98)00267-1
- Sugita, H., Miyajima, C., & Deguchi, Y. (1991). The vitamin B12-producing ability of the intestinal microflora of freshwater fish. Aquaculture, 92, 267–276. https://doi.org/10.1016/0044-8486(91)90028-6
- Sugita, H., Takahashi, J., & Deguchi, Y. (1992). Production and consumption of biotin by the intestinal microflora of cultured freshwater fishes. Bioscience, Biotechnology, and Biochemistry, 56(10), 1678–1679. https://doi.org/10.1271/bbb.56.1678
- Sun, Y., He, M., Cao, Z., Xie, Z., Liu, C., Wang, S., Guo, W., Zhang, X., & Zhou, Y. (2018). Effects of dietary administration of Lactococcus lactis HNL12 on growth, innate immune response, and disease resistance of humpback grouper (Cromileptes altivelis). Fish & Shellfish Immunology, 82, 296–303. https://doi.org/10.1016/j.fsi.2018.08.039
- Talpur, A. D., Munir, M. B., Mary, A., & Hashim, R. (2014). Dietary probiotics and prebiotics improved food acceptability, growth performance, haematology and immunological parameters and disease resistance against Aeromonas hydrophila in snakehead (Channa striata) fingerlings. Aquaculture, 426–427, 14–20. https://doi.org/10.1016/j.aquaculture.2014.01.013
- Tan, L.-H., Chan, K.-G., Lee, L.-H., & Goh, B.-H. (2016). Streptomyces Bacteria as Potential Probiotics in Aquaculture. Frontiers in Microbiology, 7, 79. https://doi.org/10.3389/fmicb.2016.00079
- Tang, H., Liu, Y., Luo, D., Ogawa, S., Yin, Y., Li, S., Zhang, Y., Hu, W., Parhar, I. S., Lin, H., Liu, X., & Cheng, C. H. (2015). The kiss/kissr systems are dispensable for zebrafish reproduction: Evidence from gene knockout studies. Endocrinology, 156(2), 589–599. https://doi.org/10.1210/en.2014-1204
- Tapia-Paniagua, T. S., Diaz-Rosales, P., Garcia de la Banda, I., Lobo, C., Clavijo, E., Balebona, M. C., & Morinigo, M. A. (2014). Modulation of certain liver fatty acids in Solea senegalensis is influenced by the dietary administration of probiotic microorganisms. Aquaculture, 424, 234–238. https://doi.org/10.1016/j.aquaculture.2014.01.003
- Tapia-Paniagua, S., Dı́az-Rosales, P., Leóon-Rubio, J., de La, B. I., Lobo, C., Alarcón, F., Chabrillo´n, M., Rosas Ledesma, P., Varela, J. L., Ruiz-Jarabo, I., Arijo, S., Esteban, M. A., Martı´nez-Manzanares, E., Mancera, J. M., Balebona, M. C., & Morin˜igo, M. A. (2012). Use of the probiotic Shewanella putrefaciens Pdp11 on the culture of Senegalese sole (Solea senegalensis, Kaup 1858) and gilthead seabream (Sparus aurata L.). Aquaculture International, 20(6), 1025–1039. https://doi.org/10.1007/s10499-012-9509-5
- Tarkhani, R., Imani, A., Hoseinifar, S. H., Moghanlou, K. S., & Manaffar, R. (2020). The effects of host-associated Enterococcus faecium CGMCC1.2136 on serum immune parameters, digestive enzymes activity and growth performance of the Caspian roach (Rutilus rutilus caspicus) fingerlings. Aquaculture, 519, 734741. https://doi.org/10.1016/j.aquaculture.2019.734741
- Tena-Sempere, M. (2007). Roles of ghrelin and leptin in the control of reproductive function. Neuroendocrinology, 86(3), 229–241. https://doi.org/10.1159/000108410
- Thankappan, B., Ramesh, D., Ramkumar, S., Natarajaseenivasan, K., & Anbarasu, K. (2015). Characterization of Bacillus spp. From the Gastrointestinal Tract of Labeo rohita—Towards to Identify Novel Probiotics Against Fish Pathogens. Applied Biochemistry and Biotechnology, 175(1), 340–353. https://doi.org/10.1007/s12010-014-1270-y
- Thy, H. T. T., Tri, N. N., Quy, O. M., Fotedar, R., Kannika, K., Unajak, S., & Areechon, N. (2017). Effects of the dietary supplementation of mixed probiotic spores of Bacillus amyloliquefaciens 54A, and Bacillus pumilus 47B on growth, innate immunity and stress responses of striped catfish (Pangasianodon hypophthalmus). Fish & Shellfish Immunology, 60, 391–399. https://doi.org/10.1016/j.fsi.2016.11.016
- Tinoco, A. B., Näslund, J., Delgado, M. J., de Pedro, N., Johnsson, J. I., & Jönsson, E. (2014). Ghrelin increases food intake, swimming activity and growth in juvenile brown trout (Salmo trutta). Physiology & Behavior, 124, 15–22. https://doi.org/10.1016/j.physbeh.2013.10.034
- Tomova, A., Ivanova, L., Buschmann, A. H., Rioseco, M. L., Kalsi, R. K., Godfrey, H. P., & Cabello, F. C. (2015). Antimicrobial resistance genes in marine bacteria and human uropathogenicEscherichia colifrom a region of intensive aquaculture. Environmental microbiology reports, 7(5), 803–809. https://doi.org/10.1111/1758-2229.12327
- Toranzo, A. E., Magariños, B., & Romalde, J. L. (2005). A review of the main bacterial fish diseases in mariculture systems. Aquaculture, 246(1–4), 37–61. https://doi.org/10.1016/j.aquaculture.2005.01.002
- Unniappan, S. Ghrelin: An emerging player in the regulation of reproduction in non-mammalian vertebrates. (2010). General & Comparative Endocrinology, 167(3), 340–343. Review. https://doi.org/10.1016/j.ygcen.2009.12.003
- Van Doan, H., Hoseinifar, S. H., Dawood, M. A. O., Chitmanat, C., & Tayyamath, K. (2017). Effects of Cordyceps militaris spent mushroom substrate and Lactobacillus plantarum on mucosal, serum immunology and growth performance of Nile tilapia (Oreochromis niloticus). Fish & Shellfish Immunology, 70, 87–94. https://doi.org/10.1016/j.fsi.2017.09.002
- Van Doan, H., Hoseinifar, S. H., Ringø, E., Angeles Esteban, M., Dadar, M., Dawood, M. A. O., & Faggio, C. (2019). Host-associated probiotics: A key factor in sustainable aquaculture. Reviews in Fisheries Science & Aquaculture, 28(1), 16–42. https://doi.org/10.1080/23308249.2019.1643288
- Van Doan, H., Hoseinifar, S. H., Tapingkae, W., Seel-Audom, M., Jaturasitha, S., Dawood, M. A., Wongmaneeprateep, S., Thu, T. T. N., & MÁ, E. (2020). Boosted growth performance, mucosal and serum immunity, and disease resistance Nile tilapia (Oreochromis niloticus) fingerlings using corncob-derived xylooligosaccharide and Lactobacillus plantarum CR1T5. Probiotics and Antimicrobial Proteins, 12(2), 400–411. https://doi.org/10.1007/s12602-019-09554-5
- Verschuere, L., Rombaut, G., Sorgeloos, P., & Verstraete, W. (2000). Probiotic bacteria as biological control agents in aquaculture. Microbiology and Molecular Biology Reviews: MMBR, 64(4), 655–671. https://doi.org/10.1128/mmbr.64.4.655-671.2000
- Vine, N. G., Leukes, W. D., & Kaiser, H. (2004). In vitro growth characteristics of five candidate aquaculture probiotics and two fish pathogens grown in fish intestinal mucus. FEMS Microbiology Letters, 231(1), 145–152. https://doi.org/10.1016/S0378-1097(03)00954-6
- Vine, N. G., Leukes, W. D., & Kaiser, H. (2006). Probiotics in marine larviculture. FEMS Microbiology Reviews, 30(3), 404–427. https://doi.org/10.1111/j.1574-6976.2006.00017.x
- Vine, N. G., Leukes, W. D., Kaiser, H., Daya, S., Baxter, J., & Hecht, T. (2004). Competition for attachment of aquaculture candidate probiotic and pathogenic bacteria on fish intestinal mucus. Journal of Fish Diseases, 27(6), 319–326. https://doi.org/10.1111/j.1365-2761.2004.00542.x
- Wang, Y. B., & Gu, Q. (2010). Effect of probiotics on white shrimp (Penaeus vannamei) growth performance and immune response. Marine Biology Research, 6(3), 327–332. https://doi.org/10.1080/17451000903300893
- Wang, X., Li, H., Zhang, X., Li, Y., Ji, W., & Xu, H. (2000). Microbial flora in the digestive tract of adult penaeid shrimp (Penaeus chinensis). The Journal of Ocean University of China, 30(3), 493–498.
- Wang, X., Sun, Y., Wang, L., Li, X., Qu, K., & Xu, Y. (2017). Synbiotic dietary supplement affects growth, immune responses and intestinal microbiota of Apostichopus japonicus. Fish & Shellfish Immunology, 68, 232–242. https://doi.org/10.1016/j.fsi.2017.07.027
- Wanka, K. M., Damerau, T., Costas, B., Krueger, A., Schulz, C., & Wuertz, S. (2018). Isolation and characterisation of native probiotics for fish farming. BMC Microbiology, 18(1), 119. https://doi.org/10.1186/s12866-018-1260-2
- Wei, L. S., Goh, K. W., Abdul Hamid, N. K., Abdul Kari, Z., Wee, W., & Van Doan, H. (2022). A mini-review on co-supplementation of probiotics and medicinal herbs: Application in aquaculture. Frontiers in Veterinary Science, 9, 869564. https://doi.org/10.3389/fvets.2022.869564
- WHO. Global action plan on antimicrobial resistance. 2015. Retrieved May 2, 2023. https://www.who.int/publications/i/item/9789241509763
- Won, E. T., & Borski, R. J. (2013). Endocrine regulation of compensatory growth in fish. Frontiers in Endocrinology, 4(4), 74. https://doi.org/10.3389/fendo.2013.00074
- Wuertz, S., Schroeder, A., & Wanka, K. M. (2021). Probiotics in Fish Nutrition—Long-Standing Household Remedy or Native Nutraceuticals? Water, 13(10), 1348. https://doi.org/10.3390/w13101348
- Xia, Y., Lu, M., Chen, G., Cao, J., Gao, F., Wang, M., Liu, Z., Zhang, D., Zhu, H., & Yi, M. (2018). Effects of dietary Lactobacillus rhamnosus JCM1136 and Lactococcus lactis subsp. Lactis JCM5805 on the growth, intestinal microbiota, morphology, immune response and disease resistance of juvenile Nile tilapia, Oreochromis niloticus. Fish & Shellfish Immunology, 76, 368–379. https://doi.org/10.1016/j.fsi.2018.03.020
- Xie, Z., Guo, W., & Zhou, Y. (2017). Dietary administration of Bacillus subtilis HAINUP40 enhances growth, digestive enzyme activities, innate immune responses and disease resistance of tilapia, Oreochromis niloticus. Fish & Shellfish Immunology, 60, 326–333. https://doi.org/10.1016/j.fsi.2016.12.003/
- Xiong, W., Sun, Y., Zhang, T., Ding, X., Li, Y., Wang, M., & Zeng, Z. (2015). Antibiotics, Antibiotic Resistance Genes, and Bacterial Community Composition in Fresh Water Aquaculture Environment in China. Microbial ecology, 70(2), 425–432. https://doi.org/10.1007/s00248-015-0583-x
- Yu, L., Zhai, Q., Zhu, J., Zhang, C., Li, T., Liu, X., Zhao, J., Zhang, H., Tian, F., & bChen, W. (2017). Dietary Lactobacillus plantarum supplementation enhances growth performance and alleviates aluminum toxicity in tilapia. Ecotoxicology & Environmental Safety, 143, 307–314. https://doi.org/10.1016/j.ecoenv.2017.05.023
- Zaher, H. A., Nofal, M. I., Hendam, B. M., Elshaer, M. M., Alothaim, A. S., & Eraqi, M. M. (2021). Prevalence and antibiogram of Vibrio parahaemolyticus and Aeromonas hydrophila in the flesh of Nile tilapia, with special reference to their virulence genes detected using multiplex PCR technique. Antibiotics (Basel), 10(6), 654. https://doi.org/10.3390/antibiotics10060654
- Zhai, Q., Wang, H., Tian, F., Zhao, J., Zhang, H., & Chen, W. (2017). Dietary Lactobacillus plantarum supplementation decreases tissue lead accumulation and alleviates lead toxicity in Nile tilapia (Oreochromis niloticus). Aquaculture Research, 48(9), 5094–5103. https://doi.org/10.1111/are.13326
- Zhang, C.-N., Zhang, J.-L., Guan, W.-C., Zhang, X.-F., Guan, S.-H., Zeng, Q.-H., Cheng, G. F., & Cui, W. (2017). Effects of Lactobacillus delbrueckii on immune response, disease resistance against Aeromonas hydrophila, antioxidant capability and growth performance of Cyprinus carpio Huanghe var. Fish & Shellfish Immunology, 68, 84–91. https://doi.org/10.1016/j.fsi.2017.07.012
- Zohar, Y., Muñoz-Cueto, J. A., Elizur, A., & Kah, O. Neuroendocrinology of reproduction in teleost fish. (2009). General & Comparative Endocrinology, 165(3), 438–455. Review. https://doi.org/10.1016/j.ygcen.2009.04.017
- Zorriehzahra, M. J., Delshad, S. T., Adel, M., Tiwari, R., Karthik, K., Dhama, K., & Lazado, C. C. (2016). Probiotics as beneficial microbes in aquaculture: An update on their multiple modes of action: A review. The Veterinary Quarterly, 36(4), 228–241. https://doi.org/10.1080/01652176.2016.1172132