Abstract
Bacillus subtilis strain LPM1 was previously documented as a plant growth-promoting rhizobacterium under open field conditions. For deeper understanding, the present study was established in a shade house with three different commercial varieties of bell pepper (Prosperity RZ, Valkiria RZ, and Sven RZ) treated prior to transplanting with the B. subtilis LPM1. The trial was performed as three parallel experiments (one per variety) comparing treated (LPM1 inoculated) against untreated (control) plants, with four replicates each. At three different times i.e. 30, 45, and 60 days after inoculation (DAI) biometric parameters, including total dry biomass, foliar area, plant height, stem diameter, and total yield were measured. Physiological parameters such as photosynthetic rate (µmol CO2 m−2 s−1), stomatal conductance (mol H2O m−2 s−1), and transpiration rate (mmol H2O m−2 s−1) were also evaluated. In the vegetative stages, the rhizobacterium B. subtilis LPM1 significantly promoted some plant traits of bell pepper varieties, such as stem diameter and total dry biomass (30, 45, and 60 DAI). However, the fruit number and yield in bell pepper varieties did not show differences between treatments under shade house conditions.
1. Introduction
Bell pepper (Capsicum annuum L.), originated from Mesoamerica (Mexico and Central America) and South America, is a herbaceous annual crop with a variable height between 0.5 and more than 2 m (Barchenger & Bosland, Citation2016). Bell pepper is a major vegetable crop in Mexico; it represents 3.5% of the Gross Domestic Product, and around 30% of its production is for the international market according to the National Agriculture Department (Hahm et al., Citation2017). Therefore, bell pepper culture is one of the main horticultural activities in Mexico, occupying the third place (after tomato and cucumber) in cultivated greenhouse surface. Still, it is second in economic importance (INTAGRI, Citation2013).
Even though Mexico can provide warm conditions for better growth and improved fruit yields to bell pepper crop, it also demands crop protection from high temperatures and solar radiation (Díaz-Pérez et al., Citation2020). Horticultural crops usually employ shade nets to keep them from radiation, wind, hail, insects, and birds. Shade house conditions improve crops by reducing excessive radiation, modifying humidity and temperature, and affecting the light quality (Stamps, Citation2009). In addition, shade nets increase fruit yield and quality in bell pepper compared with open fields (Díaz-Pérez et al., Citation2020), even if at high shading levels leaf photosynthesis can be reduced in these crops (Díaz-Pérez, Citation2013; Kabir et al., Citation2020). Furthermore, soil plastic mulching and drip irrigation are other joint practices to crop shading that enhance bell pepper yields by means of increasing water and fertilizer use efficiency (Lamont, Citation2005). However, bell pepper crops are usually exposed to attacks by phytophagous insects and phytopathogens, both, in open fields, and under greenhouse and shade house covers. On the one hand, different control strategies including the spray of different types of biocides (e.g. insecticides and fungicides) are used to protect bell pepper crops against such biotic stresses. Nevertheless, using agrochemicals sometimes promotes insect and pathogen resistance, and their residual effects are detrimental to the natural environment (Tudi et al., Citation2021).
On the other hand, biological control agents represent an alternative approach to counteract the biotic stress. Among these, beneficial microorganisms such as rhizobacteria that promote growth and induce resistance in crop plants can be named. Plant growth-promoting rhizobacteria (PGPR) have been shown to be an eco-friendly biological control agent against numerous pathogens and pests. PGPRs have been widely reported to enhance plant growth by activating mechanisms such as nitrogen fixation, phosphorus solubilization, and production of several analogs of phytohormones, siderophores, HCN, and the production of hydrolytic enzymes (Kour et al., Citation2020). Therefore, PGPRs are nowadays considered bio-fertilizers and fundamental for sustainable agriculture implementation and represent an alternative success for horticultural crops. Several reports have approached bell pepper inoculation with PGPR strains. To name a couple, Hahm et al. (Citation2017) investigated three PGPR strains (Microbacterium oleivorans KNUC7586, Brevibacterium iodinum KNUC7183, and Rhizobium massiliae KNUC7586) which significantly increased the height of pepper plants (C. annuum L.) by 12.6%, 14.9%, and 15.8%, respectively, and root fresh weight by 33.2%, 50.8%, and 50.4%, respectively. Additionally, Stoll et al. (Citation2021) showed that Bacillus velezensis BBC047 improved bell pepper crop, seedlings inoculated in the nursery and plants inoculated from transplanting under greenhouse increased pepper yield by 120% and 25%, respectively.
Still, little is known about how PGPR can perform with shade house and mulched bell pepper cultures, although it is well documented that the benefits of shade house and mulching and PGPR treatment could result in a better growth performance for bell pepper. Here, the PGPR model of the study was Bacillus subtilis LPM1 a strain isolated from apple tree rhizosphere and characterized as PGPR with tomato plants (Chávez-Betancourt et al., Citation2006). Hence, the aim of this study was to evaluate growth promotion and yields in bell pepper varieties caused by Bacillus subtilis LPM1 inoculation under shade house conditions.
2. Materials and methods
2.1. Inoculation of bell pepper varieties (Capsicum annuum L.) with Bacillus subtilis LPM1
Bacillus subtilis strain LPM1 strain was grown routinely in liquid Luria-Bertani (LB) on a rotary shaker (150 rpm) for 12 h at 30°C. All seeds of bell pepper (Capsicum annuum L.) were germinated in peat moss until 6–7 true leaves were presented. Three commercial varieties were used: PROSPERITY RZ F1 (orange blocky sweet pepper), VALKIRIA RZ F1 (red blocky sweet pepper), and SVEN RZ F1 (yellow blocky sweet pepper) (Rijk Zwaan, the Netherlands). Before transplantation, seedlings were drench-inoculated individually with a syringe containing 10 mL of LPM1 suspension (approximately 108 CFU/mL). The control plants were non-inoculated with the LPM1 treatment.
2.2. Plant growth conditions
The experiment was carried out under shade house conditions (25% shading crystal color) in a field experimental (25° 39′ N and 101° 06′ O, 1193 masl) from March (transplanting) to October 2018 (last harvest) at the Las Encinas of Centro de Investigación en Química Aplicada (CIQA), Ramos Arizpe, Coahuila, Mexico. The culture was planted according toJeeatid et al. (Citation2017) in silver/black plastic (1.20 m wide and 0.03 mm thick film, Chesterbrook, PA, USA) mulched soil beds (0.80 m wide, 48 m long) separated 1.8 m from center to center. For each plant variety, four plots (experimental units) per treatment (LPM1 inoculated and control), each 6 m in length, were completely randomized along the 48 m mulched beds. For the growing season, bell pepper plants were fertigated through a drip irrigation system totaling 205-40-240 NPK. Drip irrigation tape was placed at the center of each bed with emitters spaced at 30 cm, and the water supply was monitored using tensiometers (Riverside, Irrometer, CA, USA).
2.3. Physiological and biometric measurements
Physiological responses were measured at 30- and 60-days after inoculation (DAI). Fully exposed leaves (fourth leaf from the top) from four randomly selected plants per experimental unit were chosen to perform leaf infrared gas exchange analysis, IRGA: CO2 fixation and H2O transpiration rates, stomatal conductance (LI-COR 64000XT LI-COR, Inc. Lincoln, NE, USA), and chlorophyll content (SPAD 502, Minolta) measurements. These were conducted under natural air temperature (around 50% RH) between 11:00 and 15:00 h, on clear days (1100 µmol photons m−2 s−1 PAR in the shade house), and IRGA set to 400 ppm reference CO2 and an airflow of 500 µmol s−1.
Biometric traits were measured at 30-, 45-, and 60-DAI. Plant size and root length were obtained with a measured tape (TRUPER, Gripper); stem diameter was measured using a Vernier (KARLEN, Digital Caliper); the dry weight of stems, roots, leaves, and flowers were measured by means of a precision balance (OAHUS), and leaf area was obtained with a LI-3100C Area Meter (LI-COR, Inc. Lincoln, NE, USA). For total plant dry biomass, a pool of leaves, stems, roots, and flowers were dried in an oven at 70°C for 72 h or until a constant weight was reached. Finally, yields from different bell pepper varieties were calculated with the fruit number and fruit weight. Fifteen harvest cuttings were attained in all three varieties, made when fruits presented physiological ripening (orange color by PSP, red-intense by VLK, and yellow-intense by SVN).
2.4. Data analysis
Data per variety (i.e. no comparisons between varieties were planned) were analyzed using the InfoStat program 2018 version (DiRienzo et al., Citation2018) to compare the means and significant differences using analysis of variance (ANOVA) at p ≤0.05. The graphics were obtained with GraphPad Prism version 5 for Windows, GraphPad Software, La Jolla, California, USA. Because of the above mentioned, biometric and physiological parameters were evaluated with three different sampling times, each time with four collected repetitions.
3. Results
3.1. Growth promotion in bell pepper varieties by Bacillus subtilis LPM1 under shade house conditions
Biometric variables were measured at 30, 45, and 60 DAI to compare the effects of B. subtilis LPM1 inoculation on three bell pepper varieties: Prosperity (PSP), Sven (SVN), and Valkiria (VLK). For the plant height, those treatments with LPM1 that showed significant differences were SVN at 30 DAI with p = 0.0152 *; PSP at 45 DAI with p = 0.0470 *; and SVN at 60 DAI with p = 0.0004 *** (Figure ).
Figure 1. Height of bell pepper varieties grown under shade house conditions. (PSP) Prosperity, (VLK) Valkiria, and (SVN) Sven at 30, 45, and 60 days after inoculation with Bacillus subtilis LPM1 (black bars) and uninoculated control (white bars). Level of significant differences: *, p ≤ 0.05; ***, p ≤ 0.001; one-way ANOVA.
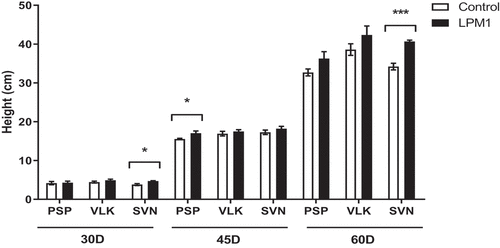
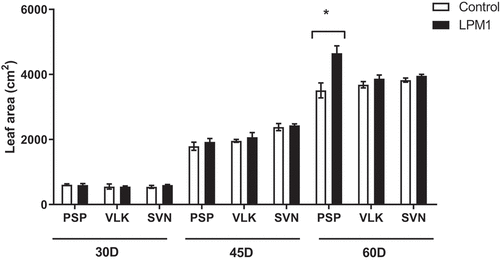
Notably, VLK treated with LPM1 showed a slight increase in plant height, but no statistical differences were detected at any time. In stem diameter, all treatments at 30 and 45 DAI did not show significant differences; although at 60 DAI those treatments with LPM1 showed significant differences in PSP with p = 0.0345 *; VLK with p = 0.0345 *; and SVN with p = 0.0365 * (Figure ). The root length of bell pepper varieties did not differ due to LPM1 inoculation at 30 and 45 DAI. However, it was significantly higher (p = 0.0422 *) for the variety SVN at 60 DAI (Figure ). Regarding the leaf area per plant (Figure ), only PSP inoculated with LPM1 presented significant differences (p = 0.0121 *) at 60 DAI, and the rest of the treatments did not show any statistical differences.
Figure 2. Stem diameter of bell pepper varieties under shade house conditions. (PSP) Prosperity, (VLK) Valkiria, and (SVN) Sven at 30, 45, and 60 days after inoculation with Bacillus subtilis LPM1 (black bars) and uninoculated control (white bars). Level of significance: *, p ≤ 0.05; one-way ANOVA.
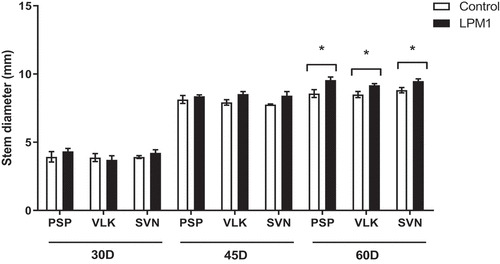
Figure 3. Root length of bell pepper varieties under shade house conditions. (PSP) Prosperity, (VLK) Valkiria, and (SVN) Sven at 30, 45, and 60 days after inoculation with Bacillus subtilis LPM1 (black bars) and uninoculated control (white bars). Level of significance: *, p ≤ 0.05; one-way ANOVA.
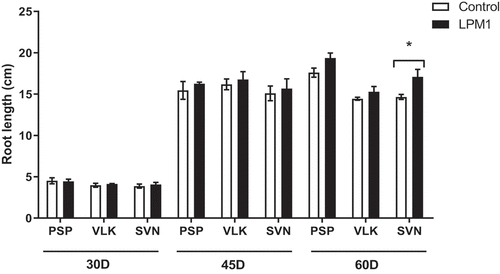
Figure 4. Leaf area of bell pepper varieties under shade house conditions. (PSP) Prosperity, (VLK) Valkiria, and (SVN) Sven at 30, 45, and 60 days after inoculation with Bacillus subtilis LPM1 (black bars) and uninoculated control (white bars). Level of significance: *, p ≤ 0.05; one-way ANOVA.
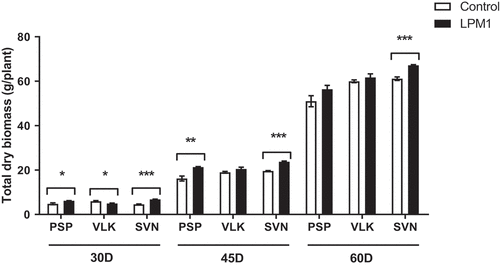
In addition, when the total dry biomass was evaluated per treatment, several differences were detected at different sampling times (Figure ). At the 30 DAI, all bell pepper varieties inoculated with LPM1 presented significant differences in PSP (p = 0.0226 *), VLK (p = 0.0315 *), and SVN (p < 0.0001 ***) between inoculated and uninoculated controls. Plants evaluated at 45 DAI only showed high differences with LPM1 treatment in PSP (p = 0.0039 **), and SVN (p < 0.0001 ***). However, such a difference was only observed (p = 0.0004 ***) in SVN plants evaluated at 60 DAI.
Figure 5. Total dry biomass of bell pepper varieties under shade house conditions. (PSP) Prosperity, (VLK) Valkiria, and (SVN) Sven at 30, 45, and 60 days after inoculation with Bacillus subtilis LPM1 (black bars) and uninoculated control (white bars). Level of significance: *, p ≤ 0.05; **, p ≤ 0.01, ***, p ≤ 0.001. (One-way ANOVA, Bonferroni posttest at p ≤ 0.05).
3.2. Eco-physiological variables: gas exchange, stomatal conductance, and chlorophyll content
Several measurements were performed among LPM1-inoculated and uninoculated bell pepper plants aiming to evaluate eco-physiological responses, namely: CO2 assimilation rates, stomatal conductance, and transpiration rate. For the photosynthesis rates (Figure ), LPM1-inoculated SVN plants assessed with 30 DAI showed significant differences (p = 0.0040 **) compared to control plants.
Figure 6. Photosynthesis rate of bell pepper varieties under shade house conditions. (PSP) Prosperity, (VLK) Valkiria, and (SVN) Sven at 30 and 60 days after inoculation with Bacillus subtilis LPM1 (black bars) and uninoculated control (white bars). Level of significance: **, p ≤ 0.01, one-way ANOVA.
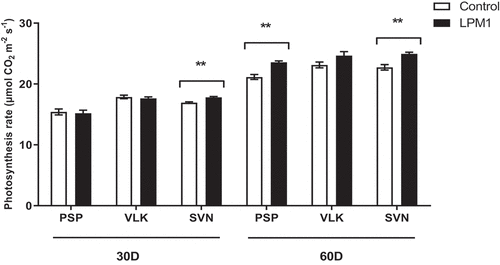
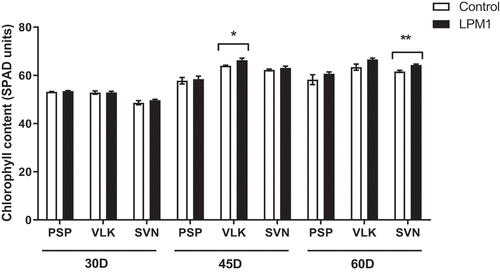
Later, 60 DAI significant differences were detected in PSP and SVN LPM1-treated, with p = 0.002 ** and p = 0.0051 **, respectively. Regarding stomatal conductance (Figure ), the measurements showed that 30 DAI only SVN-LPM1 treatment had significant differences (p = 0.0314 *) compared to control, similarly at 60 DAI, PSP-LPM1 displayed a statistical difference (p = 0.0268 *) against the uninoculated control. Finally, the transpiration rate only showed significant differences (p = 0.0458 *) between PSP-LPM1 and PSP-control (Figure ). Concerning the chlorophyll content, which was also measured under the same conditions and times (Figure ), only VLK-LPM1 and SVN-LPM1 plants at 45 DAI resulted in a significant difference (p = 0.0352 * and p = 0.0035 **, respectively) when contrasted against uninoculated control plants.
Figure 7. Stomatal conductance of bell pepper varieties under shade house conditions. (PSP) Prosperity, (VLK) Valkiria, and (SVN) Sven at 30 and 60 days after inoculation with Bacillus subtilis LPM1 (black bars) and uninoculated control (white bars). Level of significance: *, p ≤ 0.05; one-way ANOVA.
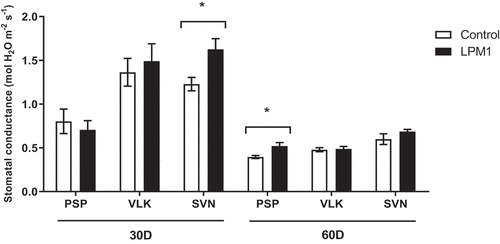
Figure 8. Transpiration of bell pepper varieties under shade house conditions. (PSP) Prosperity, (VLK) Valkiria, and (SVN) Sven at 30 and 60 days after inoculation with Bacillus subtilis LPM1 (black bars) and uninoculated control (white bars). Level of significance: *, p ≤ 0.05; one-way ANOVA.
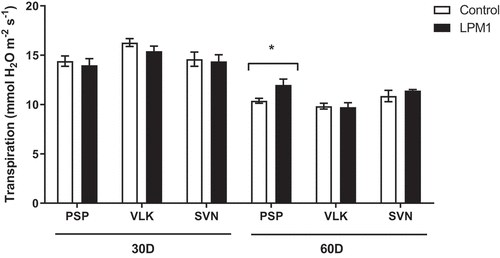
Figure 9. Chlorophyll content of bell pepper varieties under shade house conditions. (PSP) Prosperity, (VLK) Valkiria, and (SVN) Sven at 30, 45, and 60 days after inoculation with Bacillus subtilis LPM1 (black bars) and uninoculated control (white bars). Level of significance: *, p ≤ 0.05; **, p ≤ 0.01; one-way ANOVA.
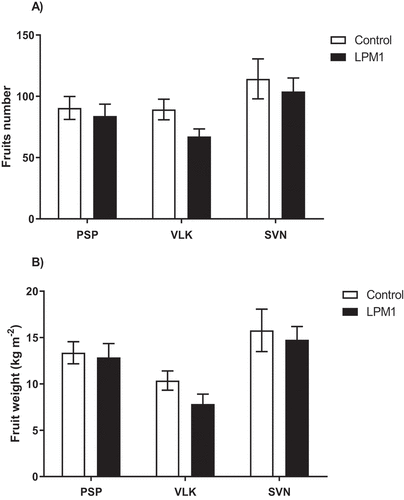
3.3. Yields of bell pepper varieties with B. subtilis LPM1 inoculation
Yields of the three tested varieties were evaluated in terms of fruit number and fruit weight (Figure ); however, none of them showed any statistical differences either for fruit number or for fruit weight per square meter.
4. Discussion
In this study, we report the effects of B. subtilis strain LPM1 on bell pepper varieties (SVN, PSP, and VLK) at different vegetative stages in which growth promotion was evident but not enough to positively impact crop yield. Limited studies are available on PGPRs interacting with crop plants and exerting some benefits with plastic mulches under shade house culture. Plastic mulches and shade net for bell pepper improvement resulted in a better performance with B. subtilis LPM1 treatment.
Canul-Tun et al. () performed a study to evaluate the positive effects on bell pepper plants with four plastic mulches (black, aluminum, silver, and white) under shade house conditions. They concluded that bell pepper plants with soil plastic mulches under shade house conditions resulted in an increased leaf area, shoot dry weight, and early and total yield compared to control (bare soil), resulting in a 16.2% higher total yield in bell pepper plants grown with black plastic films. For pepper crops, positive shade house effects have been reported when a reduction of sunlight intensity by 30% to 47% occurs, obtaining high yields, and increased fruit numbers compared to those grown under full light (Jeeatid et al., Citation2017). It was also reported that when hot peppers (C. chinense) cultivars were grown with 50% and 70% shade of full light intensity, the cultivars showed the highest capsaicinoid production according to shade level (Jeeatid et al., Citation2017). However, it has been shown that shading is increased for bell pepper growth, leaf photosynthesis, electron transport, and transpiration are decreased (Kabir et al., Citation2023).
While in the present study, the drench inoculation of the bell pepper varieties with the B. subtilis LPM1 showed growth promotion, this promotion was not steady along the crop cycle. Although not for bell pepper but for sugarcane, Morgado-González et al. (Citation2015) also reported evidence of variable increases produced in plant traits by numerous PGPR, providing a glance to the fact that specific PGPR-plant genotype relations vary their effect according to crop development. The promotion results here obtained were similar to those of Stoll et al. (Citation2021), who reported that PGPR Bacillus velezensis strain BBC047 improved the growth of the bell pepper crop at different phenological stages under greenhouse conditions. Angulo-Castro et al. (Citation2018) evaluated the same parameters in bell pepper and jalapeño inoculated with Pseudomonas tolaasii and Bacillus pumilus beneficial bacteria; both strains showed benefits compared to control plants (non-inoculated) grown in a climatic chamber. The total dry biomass is indicative of growth promotion in bell pepper varieties. For this trait, only SVN with LPM1 treatment showed significant increases along the three sampling dates (30, 45, and 60 DAI; Figure ); meanwhile, PSP and VLK significantly showed more biomass in early dates (PSP at 30 and 45 DAI, and VLK at 30 DAI). These results presented in bell pepper dry biomass were similar to Han et al. (Citation2021), which confirmed that bell pepper previously inoculated by GPR Pseudomonas sp. strain SCPG-7 augmented the dry weight by around 31.2% compared to the non-inoculated control.
Furthermore, in our study, the results for physiological parameters evaluated presented different behaviors between treatments. For example, the chlorophyll content for VLK at 45 DAI and SVN at 60 DAI inoculated with LPM1 resulted in a significant increase in SPAD units. Xie et al. (Citation2009) reported that Arabidopsis thaliana Col-0 exposed to volatile emissions from the PGPR strain B. subtilis strain GB03 showed an augment for chlorophyll content and photosynthesis efficiency under in vitro conditions. Only SVN at 30 and 60 DAI inoculated with LPM1 showed significant differences regarding the photosynthetic rate. PSP at 60 DAI also showed differences, indicating that LPM1 treatment could promote growth by accelerating the photosynthetic machinery. The results of stomatal conductance and transpiration showed a similar behavior between treatments, and only at 60 DAI showed differences by LMP1 inoculated bell pepper varieties.
The application of PGPRs in bell pepper crops using plastic mulches under shade house conditions is little reported. Here, our study confirmed that the beneficial rhizobacteria B. subtilis LPM1 increased the growth promotion at the vegetative and productive stages in bell pepper varieties. The results presented here could represent a successful strategy for horticultural crops and contribute to sustainable agriculture by optimizing pesticide use and fertilizer applications. Future studies regarding plant resistance and plant–LPM1 interactions will be evaluated against phytophagous pests and phytopathogenic microorganisms.
Author contribution statement
CERS, JVL, and ABP performed shade house experiments and photosynthesis measurements; CERS collected the data. ACF and OM analyzed and interpreted data. AFO and JHVS planned the work and wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgments
We thank the late Dr. Luis Ibarra Jiménez (Centro de Investigación en Química Aplicada) for their support during experimental performance. We also thank Dr. Alberto Flores Olivas (Departamento de Parasitología, Universidad Autónoma Agraria Antonio Narro, Saltillo, Mexico) for providing the Bacillus subtilis strain LPM1. This work was partially funded by the Consejo Nacional de Ciencia y Tecnología (CONACyT) (Cátedras-CONACyT No. 1333 to JHVS).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data obtained during the bioassays are available from the corresponding author upon reasonable request.
Additional information
Notes on contributors
Antonio Cárdenas-Flores
Adolfo Baylón-Palomino and Jairo Vázquez-Lee are technicians in Plasticulture at the Departamento de Biociencias y Agrotecnología, CIQA.
Carlos Eric Ruíz-Salas
Antonio Cárdenas-Flores and Oussama Mounzer are full researchers at the Departamento de Biociencias y Agrotecnología, CIQA.
Adolfo Baylón-Palomino
Carlos Eric Ruíz-Salas, former postgraduate student at Centro de Investigación en Química Aplicada (CIQA).
Alberto Flores-Olivas
Alberto Flores-Olivas is a full researcher at the Departamento de Parasitología at Universidad Autónoma Agraria Antonio Narro.
José Humberto Valenzuela-Soto
José Humberto Valenzuela-Soto is a researcher at the CONAHCYT-CIQA at Departamento de Biociencias y Agrotecnología.
References
- Angulo-Castro, A., Ferrera-Cerrato, R., Alarcón, A., Almaraz-Suárez, J. J., Delgadillo-Martínez, J., Jiménez-Fernández, M., & García-Barradas, O. (2018). Crecimiento y eficiencia fotoquímica del fotosistema II en plántulas de 2 variedades de Capsicum annuum L. inoculadas con rizobacterias u hongos micorrícicos arbusculares. Revista Argentina de microbiologia, 50(2), 178–10. https://doi.org/10.1016/j.ram.2017.03.011
- Barchenger, D. W., & Bosland, P. W. (2016). Exogenous applications of capsaicin inhibits seed germination of Capsicum annuum. Scientia horticulturae, 203, 29–31. https://doi.org/10.1016/j.scienta.2016.03.009
- Chávez-Betancourt, C., Olalde Portugal, V., Sánchez-Arizpe, A., Padrón-Corral, E., & Flores-Olivas, A. (2006). Uso de rizobacterias para el control de hongos fitopatógenos y promoción de crecimiento en plantas. Rev Agrar Nueva Epoca, 3, 24–31. http://repositorio.uaaan.mx:8080/xmlui/handle/123456789/3641
- Díaz-Pérez, J. C. (2013). Bell pepper (Capsicum annuum L.) crop as affected by shade level: Microenvironment, plant growth, leaf gas exchange, and leaf mineral nutrient concentration. HortScience, 48(2), 175–182. https://doi.org/10.21273/HORTSCI.48.2.175
- Díaz-Pérez, J. C., John, S., Kabir, K., Alvarado-Chávez, M. Y., Cutiño-Jiménez, A., Bautista, A. M., Gunawan, J., & Nambeesan, G. (2020). Bell pepper (Capsicum annuum L.) under colored shade nets: Fruit yield, postharvest transpiration, color, and chemical composition. HortScience, 55(2), 181–187. https://doi.org/10.21273/HORTSCI14464-19
- DiRienzo, J. A., Casanoves, F., Balzarini, M. G., Gonzalez, L., Tablada, M., & Robledo, C. W. (2018). InfoStat versión 2018. Centro de Transferencia InfoStat, FCA, Universidad Nacional de Córdoba. http://www.infostat.com.ar
- Hahm, M. S., Son, J., Hwang, Y. J., Kwon, D. K., & Ghim, S. Y. (2017). Alleviation of salt stress in pepper (Capsicum annuum L.) plants by plant-growth promoting rhizobacteria.J. Microbiol Biotechnol, 27(10), 1790–1797. https://doi.org/10.4014/jmb.1609.09042
- Han, Y., Liu, S., Chen, F., Deng, X., Miao, Z., Wu, Z., & Ye, B. C. (2021). Characteristics of plant growth–promoting rhizobacteria SCPG-7 and its effect on the growth of Capsicum annuum L. Environmental Science & Pollution Research, 28(9), 11323–11332. https://doi.org/10.1007/s11356-020-11388-6
- INTAGRI. (2013). Aspectos clave para la producción exitosa de pimiento en invernadero. Serie Horticultura Protegida Núm. Artículos Técnicos de INTAGRI. México. https://www.intagri.com/articulos/horticultura-protegida/aspectos-claves-produccion-pimiento-invernadero
- Jeeatid, N., Techawongstien, S., Suriharn, B., Bosland, P. W., & Techawongstien, S. (2017). Light intensity affects capsaicinoid accumulation in hot pepper (Capsicum chinense Jacq.) cultivars. Hortic Environ Biote, 58(2), 103–110. https://doi.org/10.1007/s13580-017-0165-6
- Kabir, M. Y., Díaz-Pérez, J. C., & Nambeesan, S. U. (2020). Effect of shade levels on plant growth, physiology, and fruit yield in bell pepper (Capsicum annuum L.). Acta horticulturae, 1268(1268), 311–318. https://doi.org/10.17660/ActaHortic.2020.1268.42
- Kabir, M. Y., Nambeesan, S. U., & Díaz-Pérez, J. C. (2023). Carbon dioxide and light curves and leaf gas exchange responses to shade levels in bell pepper (Capsicum annuum. Plant Science, 326, 111532. https://doi.org/10.1016/j.plantsci.2022.111532
- Kour, D., Rana, K. L., Yadav, A. N., Yadav, N., Kumar, M., Kumar, V., Vyas, P., Dhaliwal, H. S., & Saxena, A. K. (2020). Microbial biofertilizers: Biosources and eco-friendly technologies for agricultural and environmental sustainability. Biocatalysis and Agricultural Biotechnology, 23, 101487. https://doi.org/10.1016/j.bcab.2019.101487
- Lamont, J. W. (2005). Plastics: Modifying the microclimate for the production of vegetable crops. HortTechnology, 15(3), 477–481. https://doi.org/10.21273/HORTTECH.15.3.0477
- Morgado-González, A., Espinosa-Victoria, D., & Gómez-Merino, F. C. (2015). Efficiency of plant growth promoting rhizobacteria (PGPR) in sugarcane. Terra Lat, 33(4), 321–330.
- Stamps, R. H. (2009). Use of colored shade netting in horticulture. HortScience, 44(2), 239–241. https://doi.org/10.21273/HORTSCI.44.2.239
- Stoll, A., Salvatierra-Martínez, R., González, M., Cisternas, J., Rodriguez, A., Vega-Gálvez, A., & Bravo, J. (2021). Importance of crop phenological stages for the efficient use of PGPR inoculants. Scientific Reports, 11(1), 19548. https://doi.org/10.1038/s41598-021-98914-9
- Tudi, M., Daniel Ruan, H., Wang, L., Lyu, J., Sadler, R., Connell, D., Chu, C., & Phung, D. T. (2021). Agriculture development, pesticide application and its impact on the environment. International Journal of Environmental Research and Public Health, 18(3), 1112. https://doi.org/10.3390/ijerph18031112
- Xie, X., Zhang, H., & Pare, P. (2009). Sustained growth promotion in Arabidopsis with long-term exposure to the beneficial soil bacterium Bacillus subtilis (GB03). Plant Signaling & Behavior, 4(10), 948–953. https://doi.org/10.4161/psb.4.10.9709