Abstract
Conventional oil palm plantations, characterized by monocropping practices, are susceptible to pest infestations due to the lack of diversity in crop composition. This reliance on monoculture often necessitates heavy pesticide use, posing considerable risks to human health, the environment, and biodiversity. In contrast, regenerative agricultural approaches support ecosystem services, such as natural pest control, thereby reducing pesticide dependency and promoting biodiversity while maintaining productivity. The present study examined the composition of arthropod assemblages and understory plant species in chemical-free oil palm plantations, comparing farms with the integration of non-crop plant beds to those without intervention. We established either 10 square plant beds measuring 3 × 3 m or three rectangular beds measuring 9 × 3 m between the planting rows at each experimental plot. Using coloured pan traps and sticky traps, we assessed the relationship between understory plant species richness and arthropod assemblages, including the number of arthropod families, overall abundance, and selected trophic guilds. Our findings reveal that the integration of non-crop plant mixtures significantly enhances the number of arthropod families, as well as the abundance of predatory and phytophagous arthropods. Moreover, we observed that arthropod assemblages, most notably the number of families, overall abundance, abundance of scavengers and predators were significantly and positively correlated with the number of understory vegetation species. The study highlights the potential of establishing non-crop plant bed as a practical approach to enhancing habitat complexity for natural enemies, thereby fostering biodiversity and contribute to the resilience and functioning of agroecosystem within monoculture plantations.
Reviewing Editor:
1. Introduction
Conventional oil palm plantations are characterized by homogeneity, where a single crop is cultivated at standardized distances and ages to maximize production and productivity. While this strategy can increase yields and economic benefits, it comes at the expense of an ecological paradox (Azhar et al., Citation2015; Zemp et al., Citation2023). The expansion of monocultural crops and simplified landscapes reduces non-crop habitats, potentially exacerbating pest damage in crops (Segoli & Rosenheim, Citation2012). These landscapes often lack stand- and landscape-scale heterogeneity (Kovács-Hostyánszki et al., Citation2017; Yahya et al., Citation2023), contributing to frequent outbreaks of agricultural pests, including foliage-eating insects. Vast expanses of uniform lush vegetation of oil palm provide an ideal breeding ground and abundant food source for certain oil palm insect pests including bagworms (e.g. Metisa plana, Pteroma pendula, and Mahasena corbetti), nettle caterpillars (Darna metaleuca), and rhinoceros beetles (Oryctes rhinoceros) (Chung, Citation2012; Fauzana et al., Citation2019; Wood & Kamarudin, Citation2019). Furthermore, the absence of natural predators, which are normally present in diverse ecosystems, exacerbates the potential of pest outbreaks in oil palm monocroppings (Ashraf et al., Citation2019). A recently published study showed that stand-level habitat complexity serves important roles in attracting and supporting natural predators and provision of their services in heterogeneous oil palm cultivation (Denan et al., Citation2023; Nobilly et al., Citation2023).
To date, monocropping oil palm plantations have heavily relied on synthetic insecticides like cypermethrin and lambda-cyhalothrin to control agricultural pests (Salim et al., Citation2015; Yap, Citation2000). However, the extensive use of these pesticides in cultivated landscapes has raised significant concerns among scientific communities due to their adverse effects on human health, the environment, and biodiversity (Dhananjayan et al., Citation2020; Rani et al., Citation2021; Tohiran et al., Citation2017). Despite their short-term effectiveness in reducing pest populations, prolonged pesticide usage can lead to resistance in pests, necessitating the use of harsher chemicals and perpetuating a harmful cycle (Alyokhin & Chen, Citation2017; Houndété et al., Citation2010; Martin et al., Citation2000). Moreover, pesticides, can harm non-targeted organisms, disrupting ecosystems and impacting essential services such as pollination and natural pest control (Power, Citation2010; Sabatier et al., Citation2013; Tillman & Mulrooney, Citation2000).
In response to the ecological challenges posed by monoculture practices, recent efforts have focused on enhancing ecosystem services in agricultural landscapes. Initiatives such as the European common agricultural policy promote greening measures by establishing ecological focus areas and agricultural environmental schemes such as flower strips (Hermoso et al., Citation2022). These measures aim to provide diverse habitats, including restored grasslands and flower strips, to bolster insect biodiversity and support essential ecosystem services like pollination and pest control. Such measures have been practiced in various contexts, including grasslands (Hussain et al., Citation2023), apple orchards (Zhang et al., Citation2022), olive plantations (Karamaouna et al., Citation2019), tobacco fields (Toennisson et al., Citation2019), and others. Additionally, the importance of floral resources provided by weed communities in fields with low herbicide use is increasingly recognized for supporting arthropod predator and parasitoids (Géneau et al., Citation2012; Middleton et al., Citation2021). Therefore, strategies aimed at conserving wild plants in field margins and maintaining weed diversity in crop fields specifically in oil palm agroecosystem are advocated to promote sustainable agricultural practices.
With growing numbers of scientific literature citing the negative effects of pesticides reliance, there is an urgent need for alternative pest management strategies that reduce reliance on synthetic pesticides. Integrated pest management (IPM) principles have emerged as a viable approach for sustainable agricultural pest management in oil palm production landscapes (Caudwell, Citation2000). For instance, adopting and utilising natural predators to control agricultural pests is one of the most adopted principles of IPM. A natural biological control mechanism is established by introducing and fostering the presence of beneficial species to provide top-down control of agricultural pests (e.g. Jamian et al., Citation2017; Mulyana et al., Citation2020; Murgianto et al., Citation2022). The use of this control mechanism helps to sustain manageable pest populations and reduce the need for chemical intervention (Zainal Abidin et al., Citation2021). Through the implementation of conservation biological control, oil palm growers can achieve a more sustainable and environmentally friendly way to maintain crop productivity and yield, as well as biodiversity and ecosystem services within the cultivation landscapes.
Integration of non-crop beneficial plants in oil palm cultivation is a promising strategy to enhance pest regulatory services by arthropod natural predators (i.e. predatory insects and parasitoids) (Calvert et al., Citation2019; Karamaouna et al., Citation2019). Agriculture systems that incorporate an array of beneficial plants are expected to sustain a healthy population of natural predators, providing varied microhabitats and resources (e.g. nectar and shelter) for targeted invertebrate species (Chen et al., Citation2019; Albrecht et al., Citation2020; Sulaiman & Talip, Citation2021). Embracing this approach will not only contribute to the suppression of phytophagous insects but also allow oil palm growers to foster a more resilient and balanced ecosystem, promoting better soil health, a lower carbon footprint, ecological balance and supporting sustainable oil palm cultivation practices, while minimising the reliance on synthetic pesticides.
The study aims to determine if the implementation of non-crop plant beds can improve the assemblages of beneficial arthropods in chemical-free oil palm plantations. We hypothesize that the number of understory plant species influenced arthropod assemblages and predicted that arthropod families and arthropod overall abundance, as well as the abundance of certain trophic guilds are higher in integrated oil palm plantations compared to non-integrated sites. During the study, we sought answers to the following questions: (1) Do arthropod assemblages, namely number of arthropod families, arthropod overall abundance, and abundance of selected trophic guilds (i.e. decomposer, parasitoid, phytophagous, pollinator, predator, and scavenger) correlate with the number of understory plant species? (2) Do arthropod assemblages differ between integrated and non-integrated oil palm plantations? The findings of this study could have wide-ranging implications for the oil palm industry, offering a potential practical conservation biological control that fosters a more harmonious relationship between productivity and sustainability. Overall, this study is not only scientifically relevant but also crucial for advancing sustainable agricultural practices and safeguarding ecological integrity within human modified production landscapes.
2. Methods
2.1. Study plots
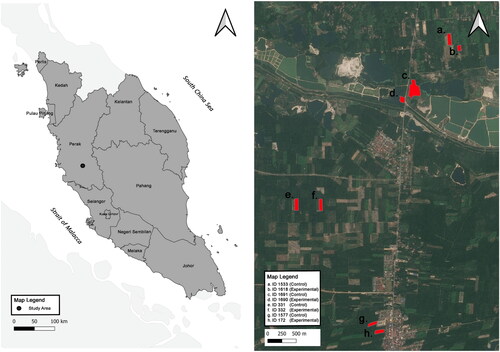
The study included surveying eight oil palm monoculture smallholdings from August to October 2022. Four experimental plots included non-crop plant beds (i.e. Neoh Ah Seng – Plot ID 332, 4°12’34.99”N, 101°7’50.00”E; Chia Voon Hong – Plot ID 172, 4°11’22.89”N, 101°8’23.69”E; Razali – Plot ID 1690, 4°13’34.83”N, 101°8’36.33”E; Mat Jailani – Plot ID 1618, 4°14’4.07”N, 101°9’8.79”E) and four witness plots did not receive treatment (i.e. Neoh Ah Seng – Plot ID 331, 4°12’34.99”N, 101°7’36.18”E; Mat Jailani – Plot ID 1533, 4°14’9.06”N, 101°9’3.32”E; Hor Kim Peow – Plot ID 1577, 4°11’27.16”N, 101° 8’19.53”E; Razali – Plot ID 1691, 4°13’40.63”N, and 101°8’43.51”E) (). The eight farms selected for this study are participants of the Wild Asia BIO program which have been adopting agrochemical-free and regenerative agriculture practices. The sampling plots were situated amidst a landscape characterised by predominantly agricultural matrices, interspersed with roads and settlements, all exhibiting a remarkable similarity. At each experimental plot, we established either 10 square plant beds measuring 3 × 3 m or three rectangular beds measuring 9 × 3 m between the planting rows. Each plant bed was planted with the following common non-crop plant species. These species include native species such as Clerodendrum paniculatum (Pagoda Flower), Melastoma malabathricum (Straits Rhododendron), Ocimum basilicum (Thai Basil), Urena lobata (Pink Burr), and/or Vitex negundo (Chinese Chaste Tree). Additionally, common naturalised plants include Senna tora (Foetid Cassia), Coleus monostachyus (Monkey’s Potato), Euphorbia hirta (Hairy Spurge), and/or Duranta erecta (Golden Dew Drop). The number of non-crop plants in each plant bed varies depending on the design. In square plant beds, there are 20 plants comprising 4 species, whereas rectangular plant beds contain 60 plants with 9 species. Additionally, all plant beds were divided into compartments for each plant category, including nectar, pollen, refuge, and extrafloral nectaries (Supplementary table S1). However, the actual number of non-crop plants varies after bed establishment, due to differences in germination success and/or plant mortality. In contrast, all understory vegetation occurred within the witness plots were naturally growing volunteer plants.
2.2. Study design
Eighty sampling points with a dimension of 3 m width × 3 m length were established throughout the study, with 10 sampling points designated for each plot. For the experimental plots, sampling points were established within the centre of the plant beds. As for the witness plots, sampling points were located between the oil palm planting rows. A sampling visit was conducted once across all study locations.
2.3. Plant survey
The plant survey involved quantifying the number of plant species within the sampling points, encompassing both woody and non-woody plants. Each identified plant was classified to the species level using published literature and potential resources (e.g. Plants of the World Online). Furthermore, the survey identified the locality status (exotic or native) and resources provided by each plant (nectar, pollen, refuge, and/or extrafloral nectaries).
2.4. Insect sampling
At each sampling point, one yellow pan trap and one blue pan trap of the same dimensions were used to maximize arthropod sampling from different insect groups (Campbell & Hanula, Citation2007; Vrdoljak & Samways, Citation2012). Moreira et al. (Citation2016) demonstrated group-specific colour appeal which suggested that wasps were much more drawn to the yellow pan traps and bees to the blue ones. In addition, blue pan traps are also suggested to be more attractive to other insects including Lepidopteran (e.g. Hesperiidae) and Dipteran (e.g. Syrphidae) (Campbell & Hanula, Citation2007). However, many bee species are known to be attracted to the yellow colour (Leong & Thorp, Citation1999). Pan traps were laid flat at ground level and filled with 0.5 L of water. Then, detergent was added to break the surface tension of the water and salt as preservation agent. The applied ratio of water, detergent, and salt is 8:1:1. Each pan trap was active for a period of 24 hours. In addition to pan traps, one yellow sticky trap and one blue sticky trap, or glue-coated plastic boards (20 cm length × 12 cm width) were deployed at each sampling point. Yellow and blue sticky traps were utilised to target specific beneficial and pest insect groups (Atakan & Pehlivan, Citation2015). Each sticky trap was attached to a galvanized wire and/or hung on oil palm fronds and understory plants for a period of 24 hours. Captured insects were stored in specimen bottles filled with 75% alcohol solution. The specimens were identified to an arthropod family and assigned to groups based on trophic guild (, Supplementary table S2). Corresponding to the optimum bloom density of the plant beds three months after establishment, arthropod populations were sampled from August to October 2022.
Table 1. Summary statistics of explanatory variables between witness and experimental plots.
2.5. Data analysis
Spearman’s rank correlation test was used to examine the degree of association of understory vegetation species and eight predictor variables. As the response variable, the number of understory vegetation plant species was correlated individually against each predictor variable, including the number of arthropod families, overall arthropod abundance, and the abundance of selected trophic guilds (i.e. decomposer, parasitoid, phytophagous, pollinator, predator, and scavenger) (Supplementary table S1). Then, one-way ANOVA with blocking was used to compare arthropod assemblages between witness and experimental plots. There were four blocks in total; each block consisted of one witness and one experimental plot in proximity with distance less than 100 m apart. Spearman’s rack correlation and one-way ANOVA were conducted with GenStat (VSN International Ltd., Hemel Hempstead, United Kingdom).
3. Results
3.1. Arthropod general patterns
Overall, a total of 4,767 arthropods consisted of 104 arthropod families and 11 orders were recorded throughout the survey (, Supplementary table S2). Diptera made up the largest number of families recorded (25 families), followed by Hymenoptera (22 families) and Coleoptera (18 families). The arthropod orders with the lowest number of families were Blattodea, Isopoda, and Mantodea, with only one family recorded for each.
3.2. Relationships between number of plant species and predictor variables
The results showed weak positive correlations between the number of plant species with number of arthropod families and abundance of scavengers (). While, the correlation tests showed moderate positive association between the number of plant species with abundance of arthropods and predators ().
Table 2. Spearman’s rank correlation results showing the degree of association between number of plant species and predictor variables.
3.3. Effects of treatment on arthropod assemblages
Based on ANOVA, there were significant differences (p < 0.05) in number of arthropod families, as well as the abundance of phytophagous and predators between witness and experimental plots. We found the number of arthropod families was significantly greater in experimental compared to witness plots (). The abundance of phytophagous was significantly greater in experimental compared to witness. Similarly, the abundance of predators was significantly greater in experimental compared to witness plots (). There was no significant difference in the arthropod assemblages between plant bed design (square and rectangular).
Table 3. Results of one-way ANOVA comparing the insect assemblages between witness and experimental plots.
4. Discussion
The correlation analysis highlights the role of understory vegetation as key habitat features for arthropod communities in oil palm cultivation. The results showed that arthropod assemblages, most notably the overall abundance, abundance of predators, number of families, and abundance of scavengers were significantly and positively correlated with the number of understory vegetation species. This underscores the potential of plant beds to maintain diverse arthropod communities and enhance natural predation within oil palm plantations. In the case of the treatment plots, the integration of flowering plants through plant bed establishment potentially provided a supplementary floral resource for certain arthropods which are often limited within conventional oil palm plantations. For instance, native plant species such as Melastoma malabathricum have been shown to sustain diverse insect pollinators in oil palm and rubber plantations (Siregar et al., Citation2016). In addition to offering refuge, nectar, and pollen, Melastoma malabathricum is recognized for producing ant attractants (extrafloral nectaries), which are known to draw in biological control agents, such as weaver ants (Gonzálvez et al., Citation2013). Other common plant species that offer a wide range of resources for insects include Cassia tora, which provides nesting and shelter opportunities (Pokharel et al., Citation2023); Clerodendrum paniculatum, which attracts phytophagous insects, serving as food resources for arthropod predators (Mathew & Anto, Citation2007); and Mallotus barbatus and Urena lobata, which offer extra floral nectaries for insects, including predators (Kato et al., Citation2008; Saini & Raina, Citation2022) (see Supplementary table S1). Yet, other plants that provide a small range of resources, including non-flowering plants are vital for insects. A study by Huang et al. (Citation2021) revealed that plants characterized by distinctive inflorescence structures offer a conducive refuge for beneficial insects, shielding them from adverse environmental conditions. These findings align with previous study in Peninsular Malaysia, which emphasises the role of such plants in fostering favourable microclimates (Ashraf et al., Citation2019).
Our study demonstrates that the integration of non-crop plant mixtures with varying patch dimensions (square, 3 × 3 m or rectangular, 9 × 3 m) significantly enhances arthropod diversity within oil palm plantations. Experimental plots exhibited a pronounced richness in number of arthropod families compared to the witness plots. Our results suggest that even small patches of diverse flora strategically placed within oil palm plantations can contribute positively to arthropod biodiversity. According to our findings, enhancing the diversity of understory vegetation through the inclusion of non-crop plant beds within oil palm plantations results in a 15% increase in arthropod family richness. Indeed, integration of non-crop and/or small crop plants is beneficial for biodiversity in homogenous oil palm plantations and has shown to improve biodiversity by improving habitat structural complexity, increasing variation of microhabitats and resources (Jamian et al., Citation2017, Ashraf et al., Citation2018). Maintaining diverse plant communities within oil palm cultivation could have huge positive impacts on above- and below-ground biodiversity (Ashton-Butt et al., Citation2018; Luke et al., Citation2019). However, this emphasizes the necessity for a well-thought-out spatial arrangement that incorporates designated plant beds within oil palm plots. This arrangement proves particularly advantageous considering the limited flight range (ranging from 80 to 400 m) of beneficial insects. The results were consistent with those related to other plants (such as Turnera subulata, Senna cobanensis, and Antigonon leptopus), which are introduced into traditional oil plantations in Peninsular Malaysia as integrated pest management programs (Jamian et al., Citation2017).
Understory diversification has the potential not only to attract but also to sustain existing biodiversity populations (Aratrakorn et al., Citation2006; Luke et al., Citation2019; Nobilly et al., Citation2022), particularly focusing on beneficial species that play pivotal roles in providing a wide array of ecosystem services. Previous studies showed that establishment of wild and/or introduced flowering plants has proven to be an effective strategy for enhancing critical ecosystem services such as pollination, natural predation, and nutrient cycling across diverse crop systems (Calvert et al., Citation2019; Carvalheiro et al., Citation2012; Karamaouna et al., Citation2019; Kleijn et al., Citation2011; Zhang et al., Citation2022). In our case, non-crop plant beds improve the persistence of natural predators, thus improving the provision of natural predation in oil palm cultivation. Certain predatory insects (e.g. Reduviidae and Carabidae) have been reported to thrive in oil palm plantations integrated with non-crop plants (Jamian et al., Citation2017), as well as small crop plants (Ashraf et al., Citation2018). Albrecht et al. (Citation2020) synthesized evidence indicating that flower strips can significantly contribute to conservation biological control, accounting for up to 16% of pest control services within neighbouring crops. The provision of diverse resources by plants, including shelter, nectar, alternative prey/hosts, and pollen, substantially contributes to the survival and dynamics of insect populations, amplifying their functional significance, especially in predation, within adjacent crops (Peñalver-Cruz et al., Citation2020). In our field experiment, non-crop flowering plants with open floral structure such as Clerodendrum paniculatum, Ocimum basilicum, and Vitex negundo favor natural predators within oil palm plantations. Flowering plants with open floral structures offer higher glucose and fructose levels (Campbell et al., Citation2012). This suggests that the floral structure of plant species has a great influence on the assemblage of natural enemies. Flowering plants can influence predatory insect richness and abundance through plant volatiles (Dötterl & Vereecken, Citation2010), visual cues (Begum et al., Citation2004), and floral resources (Tscharntke et al., Citation2005). Thus, intercropping or maintaining plants with open floral structures can be considered as a well-practiced habitat management measure that plays a positive role in biological pest control.
Our data showed that phytophagous arthropods tend to be more persistence within experimental plots. Such findings may indicate that greater attention needs to be given with regards to non-crop plant species selection. Certain plant species are known to attract phytophagous arthropods (Palmer & Pullen, Citation2001). Yet, phytophagous insects attracted to plant beds also serve as food resources or hosts for predatory and parasitoid insects (Campbell et al., Citation2017; Palmer & Pullen, Citation2001). Based on our field observations, non-crop plant species Urena lobata, have been observed to sustain phytophagous insect populations that attract biological control agents in oil palm landscapes. However, the specific interactions between non-crop plants and phytophagous insects within oil palm cultivation prompt further investigation.
5. Conclusion
Adoption of regenerative agricultural practices that enhances arthropod diversity should be promoted within conventional monoculture plantations. Our findings highlight the potential application of non-crop plant beds as a feasible option to increase habitat complexity that nurtures biodiversity and contributes to ecosystem resilience and functioning within monoculture plantations. However, a well-thought-out spatial arrangement that incorporates designated plant beds within oil palm plots is crucial. Furthermore, our findings underscore the crucial role of non-crop plants in making a substantial contribution to local biodiversity. More research is needed on the interactions between non-crop plants and arthropod communities, focusing on their long-term effects on ecosystem services and feasibility in large-scale agriculture. The present study offers a crucial resource for oil palm farmers, estate managers and policymakers aiming to promote nature-based solutions applying ecological engineering and conservation biological control for insect pest control in palm oil production and the conservation of local biodiversity within oil palm production landscapes.
Supplemental Material
Download MS Word (24.2 KB)Acknowledgement
We would like to thank Sustainable Agriculture Network (SAN), in collaboration with CABI, Dr. Paul Jepson, Srum Agroecologia, UPM, and Wild Asia, for pivotal role in designing the plant beds, determining composition, and organizing spatial arrangement. We also thank Wild Asia for establishing and maintaining the plant beds on the four experimental plots and for recruiting oil palm farmers from the Wild Asia Group Scheme. This study was made possible through the funding provided by FERRERO TRADING LUX S.A.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are presented within the article and its supplementary materials. Raw data supporting these findings can be obtained from the corresponding author upon reasonable request.
Additional information
Notes on contributors
Ahmad R. Norhisham
A. R. Norhisham, an entomologist, focuses on understanding insect population dynamics under different landscape management approaches for biological conservation. His research also explores how biodiversity conservation can improve pest management strategies.
Muhammad Syafiq Yahya
M. S. Yahya is a tropical ecologist who is keen to understand the impacts of agricultural expansion and intensification on biodiversity.
Sharifah Nur Atikah
S. N. Atikah, a tropical ecologist, explores the effects of urbanization and forest fragmentation on tropical avian biodiversity. Her research also focuses on weed identification and ecology.
Syari Jamian
J. Syari is an entomologist specializing in biocontrol approaches under agricultural landscapes, focusing on sustainable pest management strategies and ecosystem health.
Oliver Bach
O. Bach is a biologist at Sustainable Agriculture Network (SAN) working to transform agriculture for the benefit of people and nature through practical approaches, focusing on nature-based solutions and participatory management for resilient communities and sustainable landscapes.
Mona McCord
Mona McCord is an agronomist dedicated to transforming agriculture to secure sustainable communities through a global network.
John Howes
J. Howes is an ecologist specializing in investigating environmentally sustainable practices within agricultural production landscapes.
Badrul Azhar
B. Azhar is a conservation scientist with primary research interest in biodiversity patterns and processes within human-modified landscapes.
References
- Albrecht, M., Kleijn, D., Williams, N. M., Tschumi, M., Blaauw, B. R., Bommarco, R., Campbell, A. J., Dainese, M., Drummond, F. A., Entling, M. H., Ganser, D., Arjen de Groot, G., Goulson, D., Grab, H., Hamilton, H., Herzog, F., Isaacs, R., Jacot, K., Jeanneret, P., … Sutter, L. (2020). The effectiveness of flower strips and hedgerows on pest control, pollination services and crop yield: a quantitative synthesis. Ecology Letters, 23(10), 1–11. https://doi.org/10.1111/ele.13576
- Alyokhin, A., & Chen, Y. H. (2017). Adaptation to toxic hosts as a factor in the evolution of insecticide resistance. Current Opinion in Insect Science, 21, 33–38. https://doi.org/10.1016/j.cois.2017.04.006
- Aratrakorn, S., Thunhikorn, S., & Donald, P. F. (2006). Changes in bird communities following conversion of lowland forest to oil palm and rubber plantations in southern Thailand. Bird Conservation International, 16(01), 71–82. https://doi.org/10.1017/S0959270906000062
- Ashraf, M., Sanusi, R., Zulkifli, R., Tohiran, K. A., Moslim, R., Ashton-Butt, A., & Azhar, B. (2019). Alley-cropping system increases vegetation heterogeneity and moderates extreme microclimates in oil palm plantations. Agricultural and Forest Meteorology, 276–277, 107632. https://doi.org/10.1016/j.agrformet.2019.107632
- Ashraf, M., Zulkifli, R., Sanusi, R., Tohiran, K. A., Terhem, R., Moslim, R., Norhisham, A. R., Ashton-Butt, A., & Azhar, B. (2018). Alley-cropping system can boost arthropod biodiversity and ecosystem functions in oil palm plantations. Agriculture, Ecosystems and Environment, 260, 19–26. https://doi.org/10.1016/j.agee.2018.03.017
- Ashton-Butt, Adham, Aryawan, Anak A. K., Hood, Amelia S. C., Naim, Mohammad, Purnomo, Dedi, Wahyuningsih, Resti, Willcock, Simon, Poppy, Guy M., Caliman, Jean-Pierre, Turner, Edgar C., Foster, William A., Peh, Kelvin S.-H., Snaddon, Jake L., Suhardi, S. (2018). Understory vegetation in oil palm plantations benefits soil biodiversity and decomposition rates. Frontiers in Forests and Global Change 1, 10. https://doi.org/10.3389/ffgc.2018.00010
- Atakan, E., & Pehlivan, S. (2015). Attractiveness of various colored sticky traps to some pollinating insects in apple. Turkish Journal of Zoology, 39(3), 474–481. https://doi.org/10.3906/zoo-1403-62
- Azhar, B., Saadun, N., Puan, C. L., Kamarudin, N., Aziz, N., Nurhidayu, S., & Fischer, J. (2015). Promoting landscape heterogeneity to improve the biodiversity benefits of certified palm oil production: Evidence from Peninsular Malaysia. Global Ecology and Conservation, 3, 553–561. https://doi.org/10.1016/j.gecco.2015.02.009
- Begum, M., Gurr, G. M., Wratten, S. D., & Nicol, H. I. (2004). Flower color affects tri-trophic-level biocontrol interactions. Biological Control, 30(3), 584–590. https://doi.org/10.1016/j.biocontrol.2004.03.005
- Calvert, F., Hollingsworth, R. G., Wall, M., & Follett, P. A. (2019). Survey of flowering plants in Hawaii as potential banker plants of anthocorid predators for thrips control. Journal of Asia-Pacific Entomology, 22(3), 638–644. https://doi.org/10.1016/j.aspen.2019.05.001
- Campbell, A. J., Biesmeijer, J. C., Varma, V., & Wäckers, F. L. (2012). Realising multiple ecosystem services based on the response of three beneficial insect groups to floral traits and trait diversity. Basic and Applied Ecology, 13(4), 363–370. https://doi.org/10.1016/j.baae.2012.04.003
- Campbell, J. W., & Hanula, J. L. (2007). Efficiency of Malaise traps and colored pan traps for collecting flower visiting insects from three forested ecosystems. Journal of Insect Conservation, 11(4), 399–408. https://doi.org/10.1007/s1084-006-9055-4
- Campbell, A. J., Wilby, A., Sutton, P., & Wäckers, F. (2017). Getting more power from your flowers: Multi-functional flower strips enhance pollinators and pest control agents in apple orchards. Insects, 8(3), 101. https://doi.org/10.3390/insects8030101
- Carvalheiro, L. G., Seymour, C. L., Nicolson, S. W., & Veldtman, R. (2012). Creating patches of native flowers facilitates crop pollination in large agricultural fields: Mango as a case study. Journal of Applied Ecology, 49(6), 1373–1383. https://doi.org/10.1111/j.1365-664.2012.02217.x
- Caudwell, R. W. (2000). The successful development and implementation of an integrated pest management system for oil palm in Papua New Guinea. Integrated Pest Management Reviews, 5(4), 297–301. https://doi.org/10.1023/A:1012915132646
- Chen, L. L., Yuan, P., You, M. S., Pozsgai, G., Ma, X., Zhu, H., & Yang, G. (2019). Cover crops enhance natural enemies while help suppressing pests in a tea plantation. Annals of the Entomological Society of America, 112(4), 348–355. https://doi.org/10.1093/aesa/say050
- Chung, G. F. (2012). Effect of pests and diseases on oil palm yield. In Palm oil (pp. 163–210). AOCS Press.
- Denan, N., Norhisham, A. R., Sanusi, R., Stone, J., & Azhar, B. (2023). Stand-level habitat characteristics and edge habitats drive biological pest control services in the understory of oil palm plantations. Biological Control, 183, 105261. https://doi.org/10.1016/j.biocontrol.2023.105261
- Dhananjayan, V., Jayakumar, S., & Ravichandran, B. (2020). Conventional methods of pesticide application in agricultural field and fate of the pesticides in the environment and human health. Controlled Release of Pesticides for Sustainable Agriculture. 1–39.
- Dötterl, S., & Vereecken, N. J. (2010). The chemical ecology and evolution of bee-flower interactions: A review and perspectives. Canadian Journal of Zoology, 88(7), 668–697. https://doi.org/10.1139/Z10-031
- Fauzana, H., Sutikno, A., & Salbiah, D. (2019). Population fluctuations Oryctes rhinoceros L. beetle in plant oil palm (Elaeis guineensis Jacq.) given mulching oil palm empty bunch. CROPSAVER - Journal of Plant Protection, 1(1), 42–47. https://doi.org/10.24198/cs.v1i1.16998
- Géneau, C. E., Wäckers, F. L., Luka, H., Daniel, C., & Balmer, O. (2012). Selective flowers to enhance biological control of cabbage pests by parasitoids. Basic and Applied Ecology, 13(1), 85–93. https://doi.org/10.1016/j.baae.2011.10.005
- Gonzálvez, F. G., Santamaría, L., Corlett, R. T., & Rodríguez Gironés, M. A. (2013). Flowers attract weaver ants that deter less effective pollinators. Journal of Ecology, 101(1), 78–85. https://doi.org/10.1111/1365-2745.12006
- Hermoso, V., Carvalho, S. B., Giakoumi, S., Goldsborough, D., Katsanevakis, S., Leontiou, S., Markantonatou, V., Rumes, B., Vogiatzakis, I. N., & Yates, K. L. (2022). The EU Biodiversity Strategy for 2030: Opportunities and challenges on the path towards biodiversity recovery. Environmental Science & Policy, 127(June 2021), 263–271. https://doi.org/10.1016/j.envsci.2021.10.028
- Houndété, T. A., Kétoh, G. K., Hema, O. S., Brévault, T., Glitho, I. A., & Martin, T. (2010). Insecticide resistance in field populations of Bemisia tabaci (Hemiptera: Aleyrodidae) in West Africa. Pest Management Science, 66(11), 1181–1185. https://doi.org/10.1002/ps.2008
- Huang, J. C. C., Hsieh, Y. C., Lu, S. S., Yeh, W. C., Liang, J. Y., Lin, C. J., & Tung, G. S. (2021). Flower-visiting insects of genus Melastoma (Myrtales: Melastomataceae) at the Fushan Botanical Garden, Taiwan. Biodiversity Data Journal, 9, e60315. https://doi.org/10.3897/BDJ.9.e60315
- Hussain, R. I., Walcher, R., Vogel, N., Krautzer, B., Rasran, L., & Frank, T. (2023). Effectiveness of flowers strips on insect’s restoration in intensive grassland. Agriculture, Ecosystems and Environment, 348(October 2022), 108436. https://doi.org/10.1016/j.agee.2023.108436
- Jamian, S., Norhisham, A., Ghazali, A., Zakaria, A., & Azhar, B. (2017). Impacts of 2 species of predatory Reduviidae on bagworms in oil palm plantations. Insect Science, 24(2), 285–294. https://doi.org/10.1111/1744-7917.12309
- Karamaouna, F., Kati, V., Volakakis, N., Varikou, K., Garantonakis, N., Economou, L., Birouraki, A., Markellou, E., Liberopoulou, S., & Edwards, M. (2019). Ground cover management with mixtures of flowering plants to enhance insect pollinators and natural enemies of pests in olive groves. Agriculture, Ecosystems and Environment, 274(June 2018), 76–89. https://doi.org/10.1016/j.agee.2019.01.004
- Kato, M., Kosaka, Y., Kawakita, A., Okuyama, Y., Kobayashi, C., Phimminith, T., & Thongphan, D. (2008). Plant-pollinator interactions in tropical monsoon forests in Southeast Asia. American Journal of Botany, 95(11), 1375–1394. https://doi.org/10.3732/ajb.0800114
- Kleijn, D., Rundlöf, M., Scheper, J., Smith, H. G., & Tscharntke, T. (2011). Does conservation on farmland contribute to halting the biodiversity decline? Trends in Ecology & Evolution, 26(9), 474–481. https://doi.org/10.1016/j.tree.2011.05.009
- Kovács-Hostyánszki, A., Espíndola, A., Vanbergen, A. J., Settele, J., Kremen, C., & Dicks, L. V. (2017). Ecological intensification to mitigate impacts of conventional intensive land use on pollinators and pollination. Ecology Letters, 20(5), 673–689. https://doi.org/10.1111/ele.12762
- Leong, J. M., & Thorp, R. W. (1999). Colour-coded sampling: the pan trap colour preferences of oligolectic and nonoligolectic bees associated with a vernal pool plant. Ecological Entomology, 24(3), 329–335. https://doi.org/10.1046/j.1365-2311.1999.00196.x
- Luke, Sarah H., Purnomo, Dedi, Advento, Andreas Dwi, Aryawan, Anak Agung Ketut, Naim, Mohammad, Pikstein, Rachel N., Ps, Sudharto, Rambe, T. Dzulfikar S., Caliman, Jean-Pierre, Snaddon, Jake L., Foster, William A., Turner, Edgar C., Soeprapto. (2019). Effects of understory vegetation management on plant communities in oil palm plantations in Sumatra, Indonesia. Frontiers in Forests and Global Change 2, 33. https://doi.org/10.3389/ffgc.2019.00033
- Martin, T., Ochou, G. O., Hala-N’Klo, F., Vassal, J.-M., & Vaissayre, M. (2000). Pyrethroid resistance in the cotton bollworm, Helicoverpa armigera (Hübner), in West Africa. Pest Management Science, 56(6), 549–554. https://doi.org/10.1002/(SICI)1526-4998(200006)56:6<549::AID-PS160>3.0.CO;2-Y
- Mathew, G., & Anto, M. (2007). In situ conservation of butterflies through establishment of butterfly gardens: A case study at Peechi, Kerala, India. Current Science, 93(3), 337–347.
- Middleton, E. G., Macrae, I. V., & Philips, C. R. (2021). Floral plantings in large-scale commercial agroecosystems support both pollinators and arthropod predators. Insects, 12(2), 91. https://doi.org/10.3390/insects12020091
- Moreira, E. F., Santos, R. L. D. S., Penna, U. L., Angel-Coca, C., de Oliveira, F. F., & Viana, B. F. (2016). Are pan traps colors complementary to sample community of potential pollinator insects? Journal of Insect Conservation, 20(4), 583–596. https://doi.org/10.1007/s10841-016-9890-x
- Mulyana, A. N., Priyambodo, S., Triwidodo, H., Hendarjanti, H., & Sahari, B. (2020). An assessment of the reproduction, predation, and nesting behavior of Sulawesi Masked-owl (Tyto rosenbergii) in oil palm plantation: A case study of West and Central Sulawesi, Indonesia. Biodiversitas Journal of Biological Diversity, 21(12) https://doi.org/10.13057/biodiv/d211226
- Murgianto, F., Putra, S. K., & Ardiyanto, A. (2022 Role of The Barn Owl Tyto alba javanica as a Biological Agent for Rat Pest Control in The Oil Palm Plantation of Bumitama Agri Ltd [Paper presentation]. In IOP Conference Series: Earth and Environmental Science (Vol. 985, No. 1, p. 012048). IOP Publishing. https://doi.org/10.1088/1755-1315/985/1/012048
- Nobilly, F., Atikah, S. N., Yahya, M. S., Jusoh, S., Cun, G. S., Norhisham, A. R., … Azhar, B. (2022). Rotational cattle grazing improves understory vegetation biodiversity and structural complexity in oil palm plantations (Vol. 22, No. 1, pp. 13–26). John Wiley & Sons Australia, Ltd. https://doi.org/10.1111/wbm.12246
- Nobilly, F., Atikah, S. N., Yahya, M. S., Jusoh, S., Maxwell, T. M. R., Norhisham, A. R., Tohiran, K. A., Zulkifli, R., & Azhar, B. (2023). Do silvopastoral management practices affect biological pest control in oil palm plantations? BioControl, 68(4), 411–424. https://doi.org/10.1007/s10526-023-10196-4
- Palmer, W. A., & Pullen, K. R. (2001). The phytophagous arthropods associated with Senna obtusifolia (Caesalpiniaceae) in Mexico and Honduras and their prospects for utilization for biological control. Biological Control, 20(1), 76–83. https://doi.org/10.1006/bcon.2000.0879
- Peñalver-Cruz, A., Alvarez, D. & Lavandero, B. (2020). Do hedgerows influence the natural biological control of woolly apple aphids in orchards?. Journal of Pest Science, 93, 219–234. https://doi.org/10.1007/s10340-019-01153-1
- Pokharel, S. S., Yu, H., Fang, W., Parajulee, M. N., & Chen, F. (2023). Intercropping cover crops for a vital ecosystem service: A review of the biocontrol of insect pests in tea agroecosystems. Plants (Basel, Switzerland), 12(12), 2361. https://doi.org/10.3390/plants12122361
- Power, A. G. (2010). Ecosystem services and agriculture: Tradeoffs and synergies. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 365(1554), 2959–2971. https://doi.org/10.1098/rstb.2010.0143
- Rani, L., Thapa, K., Kanojia, N., Sharma, N., Singh, S., Grewal, A. S., Srivastav, A. L., & Kaushal, J. (2021). An extensive review on the consequences of chemical pesticides on human health and environment. Journal of Cleaner Production, 283, 124657. https://doi.org/10.1016/j.jclepro.2020.124657
- Sabatier, R., Meyer, K., Wiegand, K., & Clough, Y. (2013). Non-linear effects of pesticide application on biodiversity-driven ecosystem services and disservices in a cacao agroecosystem: A modeling study. Basic and Applied Ecology, 14(2), 115–125. https://doi.org/10.1016/j.baae.2012.12.006
- Saini, S., & Raina, M. (2022). Spider Nectivory from Extranuptial Nectaries of Urena lobata L.: A Case from Indian Subcontinent. National Academy Science Letters, 45(6), 531–535. https://doi.org/10.1007/s40009-022-01176-w
- Salim, H., Rawi, C. S. M., Ahmad, A. H., & Al-Shami, S. A. (2015). Efficacy of insecticide and bioinsecticide ground sprays to control Metisa plana Walker (Lepidoptera: Psychidae) in oil palm plantations, Malaysia. Tropical Life Sciences Research, 26(2), 73–83.
- Segoli, M., & Rosenheim, J. A. (2012). Should increasing the field size of monocultural crops be expected to exacerbate pest damage? Agriculture, Ecosystems and Environment, 150, 38–44. https://doi.org/10.1016/j.agee.2012.01.010
- Siregar, E. H., Atmowidi, T., & Kahono, S. (2016). Diversity and abundance of insect pollinators in different agricultural lands in Jambi, Sumatera. HAYATI Journal of Biosciences, 23(1), 13–17. https://doi.org/10.1016/j.hjb.2015.11.002
- Sulaiman, M. N., & Talip, M. S. A. (2021). Sustainable control of bagworm (Lepidoptera: Psychidae) in oil palm plantation: a review paper. International Journal of Agriculture, 11(1988), 47–55.
- Tillman, P. G., & Mulrooney, J. E. (2000). Effect of selected insecticides on the natural enemies Coleomegilla maculata and Hippodamia convergens (Coleoptera: Coccinellidae), Geocoris punctipes (Hemiptera: Lygaeidae), and Bracon mellitor, Cardiochiles nigriceps, and Cotesia marginiventris (Hymenoptera: Braconidae) in cotton. Journal of Economic Entomology, 93(6), 1638–1643. https://doi.org/10.1603/0022-0493-93.6.1638
- Toennisson, T. A., Klein, J. T., & Burrack, H. (2019). Measuring the effect of non-crop flowering plants on natural enemies in organic tobacco. Biological Control, 137(July), 104023. https://doi.org/10.1016/j.biocontrol.2019.104023
- Tohiran, K. A., Nobilly, F., Zulkifli, R., Maxwell, T., Moslim, R., & Azhar, B. (2017). Targeted cattle grazing as an alternative to herbicides for controlling weeds in bird-friendly oil palm plantations. Agronomy for Sustainable Development, 37(6), 1–11. https://doi.org/10.1007/s13593-017-0471-5
- Tscharntke, T., Klein, A. M., Kruess, A., Steffan-Dewenter, I., & Thies, C. (2005). Landscape perspectives on agricultural intensification and biodiversity-ecosystem service management. Ecology Letters, 8(8), 857–874. https://doi.org/10.1111/j.1461-0248.2005.00782.x
- Vrdoljak, S. M., & Samways, M. J. (2012). Optimising coloured pan traps to survey flower visiting insects. Journal of Insect Conservation, 16(3), 345–354. https://doi.org/10.1007/s10841-011-9420-9
- Wood, B. J., & Kamarudin, N. (2019). A review of developments in integrated pest management (IPM) of bagworm (Lepidoptera: Psychidae) infestation in oil palms in Malaysia. Journal of Oil Palm Research, 31(4), 529–539. https://doi.org/10.21894/jopr.2019.0047
- Yahya, M. S., Atikah, S. N., Mukri, I., Oon, A., Hawa, A., Sanusi, R., Norhisham, A. R., Lechner, A. M., & Azhar, B. (2023). Potential of agroforestry orchards as a conservation set-aside initiative in industrial rubber tree and oil palm plantations for avian biodiversity. Biodiversity and Conservation, 32(6), 2101–2125. https://doi.org/10.1007/s10531-023-02594-y
- Yap, T. H. (2000). The intelligent management of Lepidoptera leaf eaters in mature oil palm by trunk injection (a review of principles). Planter, Kuala Lumpur, 76(887), 99–107.
- Zainal Abidin, C. M. R., Mohd Noor, H., Hamid, N. H., Ravindran, S., Puan, C. L., Kasim, A., & Salim, H. (2021). Comparison of effectiveness of introduced barn owls, Tyto javanica javanica, and rodenticide treatments on rat control in oil palm plantations. Journal of Pest Science, 95(2), 1009–1022. https://doi.org/10.1007/s10340-021-01423-x
- Zemp, D. C., Guerrero-Ramirez, N., Brambach, F., Darras, K., Grass, I., Potapov, A., Röll, A., Arimond, I., Ballauff, J., Behling, H., Berkelmann, D., Biagioni, S., Buchori, D., Craven, D., Daniel, R., Gailing, O., Ellsäßer, F., Fardiansah, R., Hennings, N., … Kreft, H. (2023). Tree islands enhance biodiversity and functioning in oil palm landscapes. Nature, 618(7964), 316–321. https://doi.org/10.1038/s41586-023-06086-5
- Zhang, X., Ouyang, F., Su, J., Li, Z., Yuan, Y., Sun, Y., Sarkar, S. C., Xiao, Y., & Ge, F. (2022). Intercropping flowering plants facilitate conservation, movement and biocontrol performance of predators in insecticide-free apple orchard. Agriculture, Ecosystems and Environment, 340(April), 108157. https://doi.org/10.1016/j.agee.2022.108157