?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Asparagus stalks are classified as agricultural by-products owing to their antioxidant and phytochemical properties. In this study, it was used as a raw material for asparagus stalk juice, a new value-added product. Response Surface Methodology (RSM) equations were generated using the Box-Behnken design with three replications, while pasteurization temperature (65 °C, 75 °C, and 85 °C), pasteurization time (15, 20, and 25 min), and benzoic acid (0, 0.5, and 1 mg/mL) were used as the independent variables. All RSM models indicated that total viable count, CIE color, pH, total phenolic content, and DPPH scavenging activity were the main parameters affecting the physicochemical characteristics and microbiological quality of asparagus stalk juice products. The normal % probability and externally studentized residuals of all RSMs demonstrated that their predictive modelling was a promising method for quality control and assurance during asparagus stalk juice pasteurization using benzoic acid as a preservative agent. Based on the experimental data, the optimal conditions for asparagus stalk juice were obtained using 1 mg/mL benzoic acid at 75 °C for 25 min, and a pH value of approximately 3.83, which corresponded to good microbiological quality and antioxidant activity. This scientific evidence could be used as a guideline for developing quality control and quality assurance systems for asparagus stalk juice and/or vegetable juice-based products.
Introduction
In terms of health, the COVID-19 pandemic has caused widespread fear and concern among people worldwide. Anxiety about the virus has led individuals to choose healthy and well-being foods, which are believed to offer additional health benefits beyond their basic nutritional value. Previous research has reported the interrelationships among COVID-19 anxiety, health consciousness, and functional food consumption intention (Nguyen & Phan Citation2022). Globally, there is a rapid increase in both the interest and consumer demand for functionalized beverages containing nutrients capable of adding possible health benefits, such as lowering blood sugar, decreasing cholesterol levels, high antioxidant activity, ability to enhance the immune system, and help in digestion (Nazhand et al. Citation2020).
Vegetable/fruit juice-based diets have recently become popular because they are rich sources of several biologically active components that contribute to general health (Henning et al. Citation2017). Moreover, vegetable/fruit wastes have a huge economic impact on businesses in the sense that they can be developed into value-added products with natural ingredients, which are formulated to express their sustainability. Asparagus (Asparagus officinalis) is one of the most important vegetables in the asparagus family (Asparagaceae). Asparagus spears are harvested when they are still green in color with the length of 7–9 in. Purple asparagus is also available on the agricultural market. This vegetable, which is indigenous to the Mediterranean region, British Isles, Africa, Russia, and Asia, is cultivated for both medicinal and food purposes. In the United States, asparagus is harvested as a solid fresh produce (Agricultural Alternative Citation2022; Agriculture, Forestry, and Fisheries Citation2022). It produces approximately 20,000 to 25,000 acres of asparagus, with a value of $70 to $100 million. Thailand is also a major exporter, ranking seventh in the world for asparagus exports (Agricultural Alternative, Citation2022).
After the trimming process, 2–3% of the total weight of spears is commonly processed into three categories of products including fresh, frozen, and canned foods (Nindo et al. Citation2003). The residues from food processing are used as animal feeds because they are low-value materials with high cellulose content, whereas asparagus stalks are an agricultural by-product. Because of their antioxidant and phytochemical properties, asparagus by-products can be used as a rich source of new value-added products (Klunklin et al. Citation2020; Nindo et al. Citation2003; Zhao et al. Citation2011). Juice extracted from the asparagus stalk is now of interest. However, juices produced from fruits and vegetables are prone to contamination by microbial spoilage, which results in color degradation and off-flavors (Dhar et al. Citation2022; Saeeduddin et al. Citation2017; Snyder & Worobo Citation2018).
Generally, thermal preservation is the oldest and most common method of preservation, which is still used in modern juice industries, especially pasteurization techniques, to decrease microbial spoilage and food pathogens, leading to extended shelf life (Vegara et al. Citation2013). On the other hand, Rabie et al. (Citation2015) suggested that pasteurization at 90 °C for 2 min led to a significant improvement in the organoleptic characteristics of Physalis peruviana L. juice, including reduced ascorbic acid content. However, pasteurization of juice may result in nutritional loss and a change in color (Mandha et al. Citation2023). Hence, it is necessary to evaluate the physicochemical characteristics and microbial quality of the finished products prior to their launch. Therefore, there are currently a number of combined treatments for pasteurizing, such as high-pressure processing (HPP), pulsed electric field (PEF), and chemical preservatives. Chemical preservatives, especially benzoic acid, have been widely used in the food and beverage industries, and have been reported to have potential adverse effects, including metabolic acidosis, hyperactivity, convulsion, and hyperpnea in experimental humans and animals given very high doses, based on quantitative risk assessment (Kusi & Acquaah Citation2014; Leesuraplanon et al. Citation2022; Tfouni and Toledo, Citation2002). The U.S. Food and Drug Administration has recognized benzoic acid as ‘generally regarded as safe,’ (GRAS) when it is applied at certain concentrations. This ingredient could be used in foods at levels not exceeding good manufacturing practices with the current usage being a maximum level of 0.1 percent (US Food and Drug Administration, Citation2017).
As a result, consumer behaviors and the factors affecting willingness to pay for ready-to-drinks are safety and high physicochemical qualities, implying that the products are consistent with the certified quality assurance (QA) system (Li et al. Citation2019). The quality of food products is considered based on changes in physical, chemical, and microbiological parameters. Food-processing steps play an essential role in ensuring the efficacy of quality assurance systems in various food industries. Previous studies have demonstrated that mathematical models play an essential role in controlling food quality. For instance, Deepa and Jayalakshmi (Citation2022) showed that an E-nose strategy along with their mathematical model could predict the quality of meat and wine. Ajuebor et al. (Citation2022) demonstrated that the RSM model could predict the change in quality attributes of chili peppers during drying. Demirok and Yıkmış (Citation2022) also revealed that RSM based on the Box-Behnken design could be used to create predictive models for controlling the levels of bioactive compounds, amino acids, minerals, and food pathogens in tangerine juice production. Response surface methodology is an effective mathematical tool for creating predictive models where many factors and interactions affect food processing parameters along with product quality (Hayta & İşçimen Citation2017; Song et al. Citation2021). Gasaluck and Mahidsanan (Citation2018) pointed out that RSM model could be applied to guarantee the product stability of freeze-dried starter cultures and their shelf life during storage.
To date, very few studies have focused on the effects of pasteurization and benzoic acid on the quality attributes of asparagus stalk juice. The objective of this study was to create a predictive mathematical model using the Box-Behnken design to control the quality of asparagus stalk juice. In addition, this study aimed to demonstrate that the predictive RSM model could be used as an alternative tool for predicting the quality of pasteurized asparagus stalk juice. Factors including color, acidity, pH, total soluble solids, DPPH radical scavenging activity, total phenolic content, and microbiological quality were also assessed.
Materials and methods
Asparagus stalk juice preparation
Discarded stalks of asparagus were obtained from an agricultural farm in the Sung Noen district, Nakhon Ratchasima province, Thailand. The stalks were cleaned with tap water and dried at 50 °C for 24 h. The dried stalks were boiled with hot water at 100 °C for 10 min. One hundred grams of boiled stalks were blended with 900 ml of water and packed to extract the juice. The prototype stalk juice was subsequently mixed with 100 g sucrose and 1.25 g salt.
Experimental design
All treatments were performed by varying the levels of benzoic acid and pasteurization factors. The samples were then cooled to ambient temperature (25–30 °C). Three variables were studied at three different levels: pasteurization temperatures (65 °C, 75 °C, and 85 °C), pasteurization times (15, 20, and 25 min), and benzoic acid concentrations based on the reference to the Codex Alimentarius Commission (Citation2021) (0, 0.5, and 1 mg/mL). Response surface methodology based on a Box-Behnken design using Design-Expert® software (version 7.0) was used. The investigated parameters included CIE color (L*, a*, and b*), pH values, soluble solids content (oBrix), titratable acidity, DPPH free radical scavenging activity, total phenolics, and total viable count. The design consisted of 15 experimental conditions, and three replications were performed for all conditions at the center point (0, 0, 0). The code values used for the experimental parameters are listed in . RSM models were created using a second-order polynomial regression model containing coefficients of linear, quadratic, and interaction effects. EquationEquation 1(1)
(1) presents the response variable (Y) as a function of three independent variables (X1, X2, and X3):
(1)
(1)
Table 1. Factors and levels applied in Box-Behnken design to produce pasteurized asparagus stalk juice.
Quality assessment of asparagus stalk juice characteristics
Analysis of total viable count
Samples were diluted with sterilized 0.85% saline, and ten-fold dilutions were made. The total viable bacterial count was determined using the standard plate count method on plate count agar (HiMedia). The samples were incubated at 37 °C for 24 h. The results were expressed as CFU/mL (FDA-BAM, Citation2001).
Analysis of color values, pH values, total soluble solids, and titratable acidity
The CIE color values of all asparagus stalk juices were analyzed using a chroma meter (CR-410, Konica Minolta, Japan) to record the L* (white-black), a* (red-green), and b* (yellow-blue). The pH of each treatment was measured using a digital pH meter (Fisher Scientific Model AB15). The soluble solids content (oBrix) of the asparagus stalk juice was measured using a refractometer. To analyze the titratable acidity of all juices, Phenolphthalein (1% w/v) was used as a color indicator. Samples were titrated using 0.1 M NaOH until the color of the solution reached a stable slightly pink color. The results were expressed as a percentage of citric acid (Mahidsanan and Koonsirijareanpukdee, Citation2021).
Analysis of total phenolic content
The total phenolic content (TPC) was measured using the Folin-Ciocalteu method (Velioglu et al. Citation1998), with some modifications. A 20 µL sample was mixed with 1000 µL of Folin-Ciocalteu reagent (1:10) and 2.0 µL sodium carbonate (Na2CO3) solution (20% w/v). After 5 min of reaction in the absence of light at room temperature, absorbance was measured at 765 nm. Gallic acid was used as a standard, and the results were expressed as mg of GAE equivalent/mL of sample (Mahidsanan and Koonsirijareanpukdee, Citation2021).
Analysis of DPPH radical scavenging activity
Each sample (1 mL) was mixed with 3 mL of 0.2 mmol/L DPPH ethanol solution. The absorbance of the reaction mixture was measured at 517 nm after reaction in the dark at room temperature for 30 min. The blank control consisted of 95% ethanol and DPPH ethanol solution. The DPPH free radical scavenging rate (%) of each sample was calculated following EquationEquation 2(2)
(2) : where A0 is the absorbance of the blank and As is the absorbance of the samples (Mahidsanan and Koonsirijareanpukdee, Citation2021).
(2)
(2)
Statistical analysis
Regression analyses were performed using pasteurization temperature, pasteurization time, and benzoic acid concentration as independent variables, while total viable count, L*, a*, b*, pH values, titratable acidity, total soluble solids content, total phenolic content, and DPPH radical scavenging activity were dependent variables. The significance of all terms in the regression equation was analyzed using analysis of variance (ANOVA) at the 95% confidence interval to examine their effect on characteristics of asparagus stalk juice.
Results and discussions
Response surface analysis of total viable count in asparagus stalk juice
As shown in and , the level of benzoic acid had a significant impact on the total viable count in pasteurized asparagus stalk juices (P < 0.05). The experimental data for the response variable were fitted to a linear model () according to EquationEquation 3(3)
(3) . The total viable count decreased with an increase in benzoic acid levels, especially in all combination treatments with the addition of 1.00 mg/mL benzoic acid, which revealed a total viable bacterial count of less than 25 CFU/mL. According to fruit juice and nectar standards, the maximum total bacterial count allowed by the FDA in ready-to-drink juice is 25 CFU/mL, (Ahmed et al. Citation2019). Interestingly, the microbial count could be effectively decreased by using 1 mg/mL benzoic acid at 85 °C for 20 min (0 CFU/mL). Similarly, Nwachukwu et al. (Citation2015) found that when the concentration of benzoic acid was increased from 250 mg/L to 1000 mg/L, and the growth rates of Bacillus, Staphylococcus, Psuedomonas, Lactobacillus and Gluconobacter species decreased. This could imply that the concentration of benzoic acid is the most important factor in controlling juice quality, especially regarding microbiological safety. This phenomenon may be attributed to the fact that benzoic acid, with a pKa of roughly 4.2, acts as a preservative agent with anti-microbial activity (Ben Braïek & Smaoui Citation2021; McNamee et al. Citation2010; Romli et al. Citation2023). These properties can be changed to undissociated forms, resulting in penetration and inactivation of microbial cells by decreasing their internal pH values or interfering with metabolic activities (Theron & Lues Citation2010). Similar findings were reported by Lamo et al. (Citation2019), who found that the combination of induction pasteurization with sodium benzoate (0–1 mg/mL) preserved the quality of guava juice. Heat pasteurization is generally used to promote the microbial quality of juice products. Moreover, Kabuo et al. (Citation2015) found that combining pasteurization with benzoic acid resulted in minimal microbial growth in the 3rd week, suggesting that the combination treatment prolonged the shelf life of juice better than the addition of a preservative agent. Likewise, Geraldi et al. (Citation2021) reported that thermal pasteurization was effective in ensuring microbiological stability, shelf life, and functional and nutritional values of Jabuticaba juices during storage at 4 °C.
(3)
(3)
Figure 1. Response surface plot of total viable count as a function of temperature and time for asparagus stalk juice.
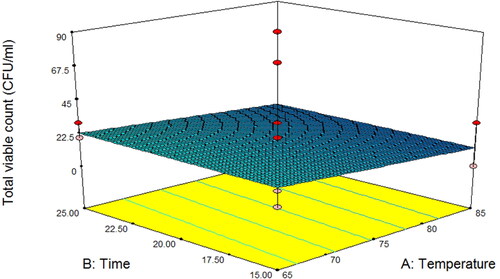
Table 3. F values and their statistical significance for different response variables of asparagus stalk juice.
Response surface analysis of color values, pH values, titratable acidity, and total soluble solids in asparagus stalk juice
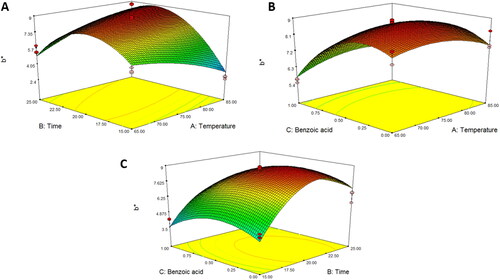
shows that L* and a* values were affected by the pasteurization temperature and benzoic acid concentration, whereas b* values were affected by both pasteurization time and benzoic acid concentration (P < 0.001). All color parameters were fitted to quadratic models (). The predictive model equations for L*, a*, and b* are shown in EquationEquations 4(4)
(4) , Equation5
(5)
(5) , and Equation6
(6)
(6) , respectively. The results revealed that increasing the pasteurization temperature and benzoic acid concentration resulted in a gradual decrease in the L* value. An increase in temperature can induce both enzymatic and non-enzymatic browning reactions, resulting in the decomposition of ascorbic acid and caramelization of sugar, thereby reducing the luminosity of food products (do Amaral Souza et al. Citation2019). Moreover, it is noteworthy that there was an increase in the color a* value when the pasteurization time and benzoic acid concentration increased. A positive a* value corresponds to a red hue, whereas a negative a* value indicates a green hue. Thus, a slight increase in a* could imply that an increase in pasteurization time and benzoic acid concentration caused the color of the juice to change from green to a red and/or brown hue. In contrast, the b* value was found to have decreased, indicating that the juice color was close to blue. These phenomena are similar to those of Rabie et al. (Citation2015), who found that a higher thermal pasteurization time decreased the b* value in ground cherry juice, resulting in a close-to-dark and/or brown color. Therefore, thermal pasteurization with benzoic acid increased the redness (a*) values, which was due to the degradation of pigment caused by heat, changing the color to reddish-brown (Kathiravan et al. Citation2014). Similarly, Chandran et al. (Citation2012) found that the pigment in beetroot puree changed to brown during heat treatment.
Figure 2. Response surface plot of L* as a function of (A) temperature and time, (B) temperature and benzoic acid, and (C) time and benzoic acid for asparagus stalk juice.
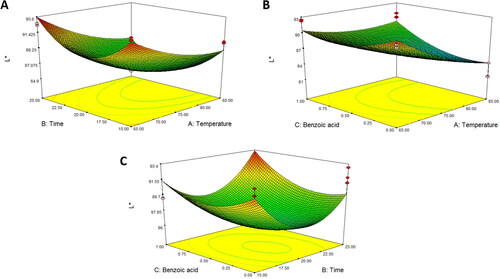
Figure 3. Response surface plot of a* as a function of (A) temperature and time, (B) temperature and benzoic acid, and (C) time and benzoic acid for asparagus stalk juice.
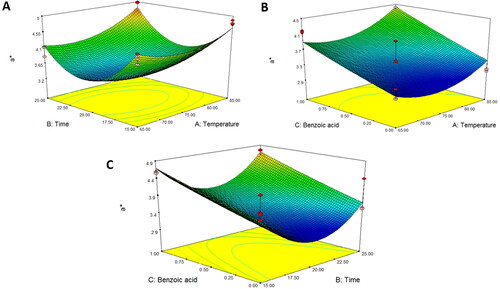
Figure 4. Response surface plot of b* as a function of (A) temperature and time, (B) temperature and benzoic acid, and (C) time and benzoic acid for asparagus stalk juice.
presents that the pH values were affected by the pasteurization temperature and benzoic acid concentration (P < 0.001), which were fitted to the quadratic models () and presented in EquationEquation 7(7)
(7) . The results showed that an increase in both the pasteurization temperature (85 °C), in parallel and benzoic acid concentration was the most effective in preventing microbial growth. Therefore, asparagus stalk juice was incubated with 1 mg/mL benzoic acid at 85 °C for 20 min and a pH value of approximately 3.65. These combined factors decreased the total viable cell count (). Likewise, benzoic acid was effective in reducing pathogens inoculated on cherry tomatoes and in preventing cross-contamination. Microbial inactivation increases with a decrease in pH value (Chen, et al. Citation2022). In fact, an increase in benzoic acid level enhances a bacteriostatic antiseptic that is only active under acidic food and drink conditions, resulting in the free undissociated benzoic acid form, which has preservative properties (Jorge, Citation2003). Acid action is exerted through membrane disruption, alteration of intracellular pH homeostasis, and enzyme inhibition, including the enzymes involved in oxidative phosphorylation and loss of energy generation (Iammarino et al. Citation2011). Temperature was also considered to improve the effectiveness of benzoic acid during processing. The average total soluble solids content and titratable acidity percentage of all treatments were approximately 10.051 and 0.038, respectively, because the predictive equation could not be developed. These results are similar to those reported by do Amaral Souza et al. (Citation2019) and Negri Rodríguez et al. (Citation2021), who observed that conventional pasteurization did not affect the total soluble solids of fruit and vegetable juices. Saeeduddin et al. (Citation2015) also revealed that commercial pasteurization processing conditions had no influence on the titratable acidity and obrix of pear juice. According to reports by Bendaali et al. (Citation2022), Krishnan Kesavan et al. (Citation2023), and Mahidsanan and Koonsirijareanpukdee (Citation2021), color and pH values could be used as significant parameters to monitor changes in the quality of juice under various food processing factors.
(4)
(4)
(5)
(5)
(6)
(6)
(7)
(7)
Figure 5. Response surface plot of pH as a function of (A) temperature and time, (B) temperature and benzoic acid, and (C) time and benzoic acid for asparagus stalk juice.
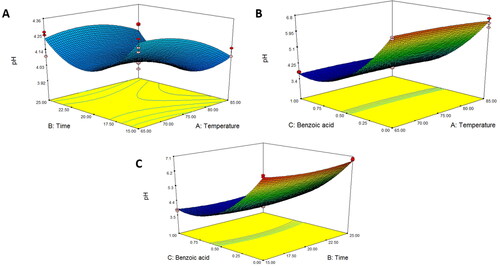
Table 2. Experimental results of quality parameters of asparagus stalk juice.
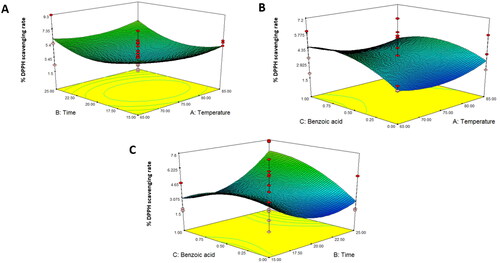
Response surface analysis of total phenolic content and DPPH scavenging activity in asparagus stalk juice
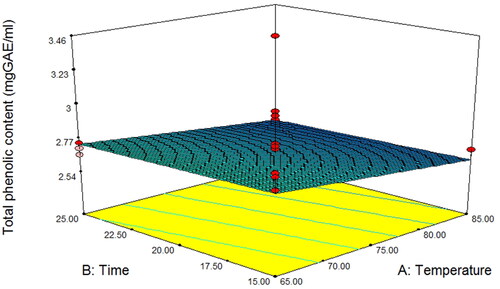
Phenolic compounds are the main contributors to antioxidant activity in many fruit and vegetable juices (Basumatary et al. Citation2020; Bhagat & Chakraborty Citation2022; Peng et al. Citation2017). These are the natural bioactive compounds found in asparagus, which give it a high nutritional quality that plays an important role in maintaining human health, especially in eliciting beneficial functions such as antioxidants (Iwassa et al. Citation2019). Therefore, maintaining this quality may be a concern because of its sensitivity to juice processing. Phenolic content of asparagus stalk juice () were affected by both temperature and time (P < 0.05), and fitted to a linear model () according to EquationEquation 8(8)
(8) . shows that the two combinations with the highest level of antioxidant activity were 75 °C for 25 min, 1 mg/mL benzoic acid, and 65 °C for 15 min, 0.5 mg/mL benzoic acid. The results indicated that the total phenolic content decreased with an increase in pasteurization temperature and time. Asparagus is mainly composed of flavonoids with high levels of quercetin glycoside, isoquercitrin, and phenolic acids such as p-coumaric, caffeic, chlorogenic acid, and ferulic acids (Solana et al. Citation2015). Our findings were in accordance with the research of Spanos and Wrolstad (Citation1992), who reported that thermal pasteurization at 80 °C for 15 min decreased the total phenols in apple juice by up to 50%. Gardner et al. (Citation2000) reported a decrease in the phenolic content of thermally pasteurized apple juice. Asami et al. (Citation2003) found that the total phenolic content of peach samples reduced after pasteurization. According to Aydogan-coskun et al. (Citation2020), pasteurization significantly decreased the caffeic acid content in astragalus honey, whereas other detected phenolics showed no significant difference after heat treatment. Oliveira et al. (Citation2012) also identified phenolic compounds in peach samples and revealed that protocatechuic acid was reduced by pasteurization. In addition, Aguilar-Rosas et al. (Citation2007) suggested that the loss of phenolic concentration impairs juice sensorial characteristics. Although our investigation found a reduction in total phenolics in asparagus stalk juice after mild heat treatment, a previous study demonstrated that pasteurization might retain phenolics in fruit- and vegetable-based products (Rashmi & Negi Citation2020).
Figure 6. Response surface plot of total phenolic content as a function of temperature and time for asparagus stalk juice.
As illustrated in , the percentage of DPPH scavenging activity was fitted to a quadratic model according to the predictive model calculated using EquationEquation 9(9)
(9) . However, temperature, time, and benzoic acid concentration had no effect on DPPH levels in asparagus stalk juice. Al-Juhaimi et al. (Citation2018) and Nayak et al. (Citation2015) demonstrated that conventional thermal processing does not affect the antioxidant activity of foods. Mandha et al. (Citation2023) also reported that pasteurization did not affect the antioxidant capacity of mango juice products, using both ABTS and DPPH analyses.
(8)
(8)
(9)
(9)
Analysis of goodness of fit of statistical RSM model equations and their residues
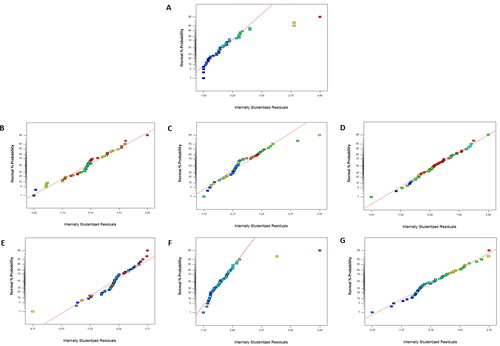
The model constructed in this study () was evaluated using both the predicted R2 and adjusted R2 values for all predictive model equations, based on the theory of Frost (Citation2013). The predicted R2 of the total viable count model (0.0322) was in reasonable agreement with the adjusted R2 of 0.0498, indicating that the change in microbiological quality could be explained by the linear model. The pH value and all color parameters, including L*, a*, and b*, were well fitted to quadratic models, while the predicted R2 values were in reasonable agreement with their adjusted R2. Similarly, the predicted R2 of the total phenolic value was in reasonable agreement with its adjusted R2, indicating that the change in total phenolic content could be explained by the linear model. However, an anomaly was found in the model for DPPH scavenging activity, because its predicted R2 was not in reasonable agreement with the adjusted R2. This finding was in accordance with Gong et al. (Citation2022), who reported a similar phenomenon for the change in polysaccharides, flavonoids, and polyphenols during Noni liquor processing. The analysis of the normal % probability plot of residuals for all models, including total viable count, L*, a*, b*, pH, total phenolic content, and % DPPH scavenging activity are shown in , respectively. Most of the residuals followed a normal distribution and were concentrated in a straight line. Hence, the errors in the proposed RSM predictive models are identical and normally distributed. Consequently, predictive regression equations were well fitted for each response expressed as a function of the Box-Behnken design experimental results.
Conclusions
In the present study, the statistical and RSM graphical analyses demonstrated that the total viable count, CIE color (L*, a*, b*), pH values, total phenolic content, and percentage of DPPH scavenging rate significantly affected the physicochemical and microbiological quality of asparagus stalk juice. The application of RSM and its predictive modelling could be a promising tool for achieving an effective method for quality control and assurance during asparagus stalk juice pasteurization with benzoic acid preservation. The findings obtained herein are believed to be important for further developing asparagus stalk juice and/or vegetable juice-based products with highly effective quality control during pasteurization and storage.
Authors’ contribution
Conceptualization, sample collection, preparation and design of research work (all the authors); execution of laboratory experiments and data collection (TM); Analysis of data and interpretation (all the authors); supervision of the work (TM); preparation of the manuscript (all the authors).
Acknowledgements
The authors would like to acknowledge Rajamangala University of Technology Isan and Burapha University, Chanthaburi Campus for providing valuable laboratory rooms and facilities, and Miss Martha Maloi Eromine for helping with English proofreading. We would also like to thank Mr. Sirisopon Prapaspong for helping some measurement.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that supports the findings of the study are available within the manuscript.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
Notes on contributors
Priyada Sittisart
Priyada Sittisart is a Lecture in Department of Agricultural Technology, Faculty of Science and Arts, Burapha University, Chanthaburi Campus, Chanthaburi, Thailand. She obtained her PhD degree (Food Technology) from Suranaree University of Technology in 2020. She has expertise in Food Microbiology, Food Fermentation, and the Application of natural antimicrobial to eliminate and/or control hazardous pathogens in food processing, including food safety assessment.
Thitikorn Mahidsanan
Thitikorn Mahidsanan is an Assistant Professor in Department of Food Science and Technology, Faculty of Agricultural Innovation and Technology, Rajamangala University of Technology Isan, Nakhon Ratchasima, Thailand. He obtained his PhD degree (Food Technology) from Suranaree University of Technology in 2017. He has expertise in Food Microbiology and Food Fermentation.
References
- Agricultural alternative. (2022). Asparagus production. https://extension.psu.edu/asparagus-production
- Agriculture, forestry, and fisheries. (2022). Asparagus. https://www.dalrrd.gov.za/Portals/0/Brochures%20and%20Production%20guidelines/Production%20guidelines%20Asparagus.pdf Assessed on 2 October 2022.
- Aguilar-Rosas, S. F., Ballinas-Casarrubias, M. L., Nevarez-Moorillon, G. V., Martin-Belloso, O., & Ortega-Rivas, E. (2007). Thermal and pulsed electric fields pasteurization of apple juice: Effects on physicochemical properties and flavour compounds. Journal of Food Engineering, 83(1), 41–46. https://doi.org/10.1016/j.jfoodeng.2006.12.011
- Ahmed, T., Das, K. K., & Uddin, M. A. (2019). The Microbiological quality of commercial fruit juices-current perspectives. Bangladesh Journal of Microbiology, 35(2), 128–133. https://doi.org/10.3329/bjm.v35i2.42643
- Ajuebor, F., Aworanti, O. A., Agbede, O. O., Agarry, S. E., Afolabi, T. J., & Ogunleye, O. O. (2022). Drying process optimization and modelling the drying kinetics and quality attributes of dried chili pepper (Capsicum frutescens L.). Trends in Sciences, 19(17), 5752–5752. https://doi.org/10.48048/tis.2022.5752
- Al-Juhaimi, F., Ghafoor, K., Özcan, M. M., Jahurul, M. H. A., Babiker, E. E., Jinap, S., Sahena, F., Sharifudin, M. S., & Zaidul, I. S. M. (2018). Effect of various food processing and handling methods on preservation of natural antioxidants in fruits and vegetables. Journal of Food Science and Technology, 55(10), 3872–3880. https://doi.org/10.1007/s13197-018-3370-0
- Asami, D. K., Hong, Y. J., Barrett, D. M., & Mitchell, A. E. (2003). Processing‐induced changes in total phenolics and procyanidins in clingstone peaches. Journal of the Science of Food and Agriculture, 83(1), 56–63. https://doi.org/10.1002/jsfa.1275
- Aydogan-Coskun, B., Coklar, H., & Akbulut, M. (2020). Effect of heat treatment for liquefaction and pasteurization on antioxidant activity and phenolic compounds of Astragalus and sunflower-cornflower honeys. Food Science and Technology, 40(3), 629–634. https://doi.org/10.1590/fst.15519
- Basumatary, B., Nayak, P. K., Chandrasekar, C. M., Nath, A., Nayak, M., & Kesavan, R. K. (2020). Impact of thermo sonication and pasteurization on the physicochemical, microbiological and anti-oxidant properties of pomelo (Citrus maxima) juice. International Journal of Fruit Science, 20(sup3), S2056–S2073. https://doi.org/10.1080/15538362.2020.1848751
- Ben Braïek, O., & Smaoui, S. (2021). Chemistry, safety, and challenges of the use of organic acids and their derivative salts in meat preservation. Journal of Food Quality, 2021, 1–20. https://doi.org/10.1155/2021/6653190
- Bendaali, Y., Vaquero, C., González, C., & Morata, A. (2022). Elaboration of an organic beverage based on grape juice with positive nutritional properties. Food Science & Nutrition, 10(6), 1768–1779. https://doi.org/10.1002/fsn3.2795
- Bhagat, B., & Chakraborty, S. (2022). Potential of pulsed light treatment to pasteurize pomegranate juice: Microbial safety, enzyme inactivation, and phytochemical retention. LWT, 159, 113215. https://doi.org/10.1016/j.lwt.2022.113215
- Chandran, J., Nisha, P., Rekha, S., Singhal., & Anirudha, B. (2012). Degradation of colour in beetroot (Beta vulgaris L.): a kinetics study. Journal of Food Science and Technology, 51(10), 2678–2684. https://doi.org/10.1007/s13197-012-0741-9
- Chen, H., Brashears, M. M., & Zhong, Q. (2022). Sodium benzoate and sodium bisulfate as preservatives in apple juice and alternative sanitizers for washing cherry tomatoes. International Journal of Food Microbiology, 372, 109697. https://doi.org/10.1016/j.ijfoodmicro.2022.109697
- Codex Alimentarius Commission. (2021). Food additive group details. https://www.fao.org/gsfaonline/groups/details.html?id=162 Assessed on 15 October 2022.
- Deepa, S. N., & Jayalakshmi, N. Y. (2022). An intelligent neural network algorithm for uncertainty handling in sensor failure scenario of food quality assurance model. Computer Assisted Methods in Engineering and Science, 29(1–2), 105–123.
- Demirok, N. T., & Yıkmış, S. (2022). Combined effect of ultrasound and microwave power in tangerine juice processing: Bioactive compounds, amino acids, minerals, and pathogens. Processes, 10(10), 2100. https://doi.org/10.3390/pr10102100
- Dhar, R., Basak, S., & Chakraborty, S. (2022). Pasteurization of fruit juices by pulsed light treatment: A review on the microbial safety, enzymatic stability, and kinetic approach to process design. Comprehensive Reviews in Food Science and Food Safety, 21(1), 499–540. https://doi.org/10.1111/1541-4337.12864
- do Amaral Souza, F. d C., Gomes Sanders Moura, L., de Oliveira Bezerra, K., Paiva Lopes Aguiar, J., Moreira Mar, J., Sanches, E. A., dos Santos, F. F., Bakry, A. M., Nicolau Paulino, B., & Campelo, P. H. (2019). Thermosonication applied on camu–camu nectars processing: Effect on bioactive compounds and quality parameters. Food and Bioproducts Processing, 116, 212–218. https://doi.org/10.1016/j.fbp.2019.06.003
- FDA-BAM. (2001). In FDA’s bacteriological analytical manual. Retrieved October 18, 2023, from https://www.fda.gov/food/laboratory-methods-food/bacteriological-analytical-manual-bam
- Frost, J. (2013). Multiple regression analysis: Use adjusted R-squared and predicted R-squared to include the correct number of variables. The Minitab Blog.
- Gardner, P. T., White, T. A., McPhail, D. B., & Duthie, G. G. (2000). The relative contributions of vitamin C, carotenoids and phenolics to the antioxidant potential of fruit juices. Food Chemistry, 68(4), 471–474. https://doi.org/10.1016/S0308-8146(99)00225-3
- Gasaluck, P., & Mahidsanan, T. (2018). The consequences of implicit factors as cross-protective stresses on freeze-dried Bacillus subtilis SB-MYP-1 with soybean flour during storage. LWT, 90, 475–482. https://doi.org/10.1016/j.lwt.2017.12.068
- Geraldi, M. V., Betim Cazarin, C. B., Dias-Audibert, F. L., Pereira, G. A., Carvalho, G. G., Kabuki, D. Y., Catharino, R. R., Pastore, G. M., Behrens, J. H., Cristianini, M., & Maróstica Júnior, M. R. (2021). Influence of high isostatic pressure and thermal pasteurization on chemical composition, color, antioxidant properties and sensory evaluation of jabuticaba juice. LWT, 139, 110548. https://doi.org/10.1016/j.lwt.2020.110548
- Gong, S., Yang, F., Wang, Q., & Wu, T. (2022). Processing of noni liquor based on response surface methodology. PeerJ, 10, e13817. https://doi.org/10.7717/peerj.13817
- Hayta, M., & İşçimen, E. M. (2017). Optimization of ultrasound-assisted antioxidant compounds extraction from germinated chickpea using response surface methodology. LWT, 77, 208–216. https://doi.org/10.1016/j.lwt.2016.11.037
- Henning, S. M., Yang, J., Shao, P., Lee, R.-P., Huang, J., Ly, A., Hsu, M., Lu, Q.-Y., Thames, G., Heber, D., & Li, Z. (2017). Health benefit of vegetable/fruit juice-based diet: Role of microbiome. Scientific Reports, 7(1), 2167. https://doi.org/10.1038/s41598-017-02200-6
- Iammarino, M., Di Taranto, A., Palermo, C., & Muscarella, M. (2011). Survey of benzoic acid in cheeses: contribution to the estimation of an admissible maximum limit. Food Addit. Contam. Part B, 4, 231–237.
- Iwassa, I. J., dos Santos Ribeiro, M. A., Meurer, E. C., Cardozo‐Filho, L., Bolanho, B. C., & da Silva, C. (2019). Effect of subcritical water processing on the extraction of compounds, composition, and functional properties of asparagus by‐product. Journal of Food Process Engineering, 42(4), e13060. https://doi.org/10.1111/jfpe.13060
- Jorge, K. (2003). Soft drink chemical composition. Encyclopedia of food sciences and nutrition (2nd ed., pp. 5346–5352). Academic Press.
- Kabuo, N. O., Omeire, G. C., & Ibeabuchi, J. C. (2015). Extraction and preservation of cashew juice using sorbic and benzoic acids. American Journal of Food Technology, 3(2), 4.
- Kathiravan, T., Nadanasabapathi, S., & Kumar, R. (2014). Standardization of process condition in batch thermal pasteurization and its effect on antioxidant, pigment and microbial inactivation of Ready to Drink (RTD) beetroot (Beta vulgaris L.) juice. International Food Research Journal, 21(4), 1305–1312.
- Klunklin, W., Jantanasakulwong, K., Phimolsiripol, Y., Leksawasdi, N., Seesuriyachan, P., Chaiyaso, T., Insomphun, C., Phongthai, S., Jantrawut, P., Sommano, S. R., Punyodom, W., Reungsang, A., Ngo, T. M. P., & Rachtanapun, P. (2020). Synthesis, characterization, and application of carboxymethyl cellulose from asparagus stalk end. Polymers, 13(1), 81. https://doi.org/10.3390/polym13010081
- Krishnan Kesavan, R., Begum, S., Das, P., & Nayak, P. K. (2023). Hurdle effect of thermosonication and non‐thermal processing on the quality characteristics of fruit juices: An overview. Journal of Food Process Engineering, 46(6), e14310. https://doi.org/10.1111/jfpe.14310
- Kusi, J. K., & Acquaah, S. O, Department of Chemistry, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana. (2014). Levels of benzoic acid in soft drinks and fruit juices in Ghana. IOSR Journal of Environmental Science, Toxicology and Food Technology, 8(12), 36–39. https://doi.org/10.9790/2402-081233639
- Lamo, C., Shahi, N. C., Singh, A., & Singh, A. K. (2019). Pasteurization of guava juice using induction pasteurizer and optimization of process parameters. LWT, 112, 108253. https://doi.org/10.1016/j.lwt.2019.108253
- Leesuraplanon, C., Jayasena, V., & Karnpanit, W. (2022). Risk assessment of exposure to benzoic acid and benzene from consumption of functional drinks. International Journal of Food Science & Technology, 57(10), 6805–6812. https://doi.org/10.1111/ijfs.16029
- Li, J., Cheng, H., Liao, X., Liu, D., Xiang, Q., Wang, J., Chen, S., Ye, X., & Ding, T. (2019). Inactivation of Bacillus subtilis and quality assurance in Chinese bayberry (Myrica rubra) juice with ultrasound and mild heat. LWT, 108, 113–119. https://doi.org/10.1016/j.lwt.2019.03.061
- Mahidsanan, T., & Koonsirijareanpukdee, P. (2021). Assessment of quality models of Lingzhi stalk juice pasteurization. Burapha Science Journal, 3, 1502–1515.
- Mandha, J., Shumoy, H., Matemu, A. O., & Raes, K. (2023). Characterization of fruit juices and effect of pasteurization and storage conditions on their microbial, physicochemical, and nutritional quality. Food Bioscience, 51, 102335. https://doi.org/10.1016/j.fbio.2022.102335
- McNamee, C., Noci, F., Cronin, D. A., Lyng, J. G., Morgan, D. J., & Scannell, A. G. M. (2010). PEF based hurdle strategy to control Pichia fermentans, Listeria innocua and Escherichia coli k12 in orange juice. International Journal of Food Microbiology, 138(1-2), 13–18. https://doi.org/10.1016/j.ijfoodmicro.2009.12.001
- Nayak, B., Liu, R. H., & Tang, J. (2015). Effect of processing on phenolic antioxidants of fruits, vegetables, and grains—a review. Critical Reviews in Food Science and Nutrition, 55(7), 887–919. https://doi.org/10.1080/10408398.2011.654142
- Nazhand, A., Souto, E. B., Lucarini, M., Souto, S. B., Durazzo, A., & Santini, A. (2020). Ready to use therapeutical beverages: Focus on functional beverages containing probiotics, prebiotics and synbiotics. Beverages, 6(2), 26. https://doi.org/10.3390/beverages6020026
- Negri Rodríguez, L. M., Arias, R., Soteras, T., Sancho, A., Pesquero, N., Rossetti, L., Tacca, H., Aimaretti, N., Rojas Cervantes, M. L., & Szerman, N. (2021). Comparison of the quality attributes of carrot juice pasteurized by ohmic heating and conventional heat treatment. LWT, 145, 111255. https://doi.org/10.1016/j.lwt.2021.111255
- Nguyen, T. T., & Phan, H. T. T. (2022). Impact of COVID-19 anxiety on functional foods consuming intention: role of electronic word of mouth. Heliyon, 8(11), e11344. https://doi.org/10.1016/j.heliyon.2022.e11344
- Nindo, C., Sun, T., Wang, S. W., Tang, J., & Powers, J. R. (2003). Evaluation of drying technologies for retention of physical quality and antioxidants in asparagus (Asparagus officinalis, L.). LWT, 36(5), 507–516.
- Nwachukwu, I. N., Onyeneto, T. C., & Nwogwugwu, N. U. (2015). The effect of pH and chemical preservatives on the growth of bacterial isolates from commercial samples of fruit juices sold in South.
- Oliveira, A., Pintado, M., & Almeida, D. P. (2012). Phytochemical composition and antioxidant activity of peach as affected by pasteurization and storage duration. LWT, 49(2), 202–207.
- Peng, J., Tang, J., Barrett, D. M., Sablani, S. S., Anderson, N., & Powers, J. R. (2017). Thermal pasteurization of ready-to-eat foods and vegetables: Critical factors for process design and effects on quality. Critical Reviews in Food Science and Nutrition, 57(14), 2970–2995. https://doi.org/10.1080/10408398.2015.1082126
- Rabie, M. A., Soliman, A. Z., Diaconeasa, Z. S., & Constantin, B. (2015). Effect of pasteurization and shelf life on the physicochemical properties of Physalis (Physalis peruviana L.) Juice. Journal of Food Processing and Preservation, 39(6), 1051–1060. https://doi.org/10.1111/jfpp.12320
- Rashmi, H. B., & Negi, P. S. (2020). Phenolic acids from vegetables: A review on processing stability and health benefits. Food Research International, 136, 109298. https://doi.org/10.1016/j.foodres.2020.109298
- Romli, N. F. A., Sukor, R., Rukayadi, Y., & Mohsin, A. Z. (2023). The efficacy of sodium benzoate and potassium sorbate in inhibiting the growth of food fungi and bacteria. Songklanakarin Journal of Science & Technology, 45(1), 138–145.
- Saeeduddin, M., Abid, M., Jabbar, S., Wu, T., Hashim, M. M., Awad, F. N., … Zeng, X. (2015). Quality assessment of pear juice under ultrasound and commercial pasteurization processing conditions. LWT, 64(1), 452–458.
- Saeeduddin, M., Abid, M., Jabbar, S., Wu, T., Yuan, Q., Riaz, A., Hu, B., Zhou, L., & Zeng, X. (2017). Nutritional, microbial and physicochemical changes in pear juice under ultrasound and commercial pasteurization during storage. Journal of Food Processing and Preservation, 41(6), e13237. https://doi.org/10.1111/jfpp.13237
- Snyder, A. B., & Worobo, R. W. (2018). The incidence and impact of microbial spoilage in the production of fruit and vegetable juices as reported by juice manufacturers. Food Control, 85, 144–150. https://doi.org/10.1016/j.foodcont.2017.09.025
- Solana, M., Boschiero, I., Dall’Acqua, S., & Bertucco, A. (2015). A comparison between supercritical fluid and pressurized liquid extraction methods for obtaining phenolic compounds from Asparagus officinalis L. The Journal of Supercritical Fluids, 100, 201–208. https://doi.org/10.1016/j.supflu.2015.02.014
- Song, H., Moon, E. W., & Ha, J. H. (2021). Application of response surface methodology based on a box-behnken design to determine optimal parameters to produce brined cabbage used in Kimchi. Foods (Basel, Switzerland), 10(8), 1935. https://doi.org/10.3390/foods10081935
- Spanos, G. A., & Wrolstad, R. E. (1992). Phenolics of apple, pear, and white grape juices and their changes with processing and storage. A review. Journal of Agricultural and Food Chemistry, 40(9), 1478–1487. https://doi.org/10.1021/jf00021a002
- Tfouni, S. A. V., & Toledo, M. C. F. (2002). Determination of benzoic and sorbic acids in Brazilian food. Food Control, 13(2), 117–123. https://doi.org/10.1016/S0956-7135(01)00084-6
- Theron, M. M., & Lues, J. R. (2010). Organic acids and food preservation. CRC press.
- US Food and Drug Administration. (2017). CFR-code of federal regulations title 21. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1021&SearchTerm=benzoic%20acid
- Vegara, S., Martí, N., Mena, P., Saura, D., & Valero, M. (2013). Effect of pasteurization process and storage on the color and shelf life of pomegranate juice. LWT, 54(2), 592–596.
- Velioglu, Y., Mazza, G., Gao, L., & Oomah, B. D. (1998). Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. Journal of agricultural and food chemistry, 46(10), 4113–4117.
- Zhao, Q., Kennedy, J. F., Wang, X., Yuan, X., Zhao, B., Peng, Y., & Huang, Y. (2011). Optimization of ultrasonic circulating extraction of polysaccharides from Asparagus officinalis using response surface methodology. International Journal of Biological Macromolecules, 49(2), 181–187. https://doi.org/10.1016/j.ijbiomac.2011.04.012