Abstract
This research aims to investigate the effects of vermicompost on the growth of wheat plants (Triticum aestivum L.), soil enzyme activities, and nutrient uptake, while also monitoring plant development through thermal imaging. The study was conducted on wheat seeds sown in soil enriched with various rates of vermicompost (Control: 0, T1: 2, T2: 4, T3: 6, and T4: 8 Mg ha−1) and continued under greenhouse conditions for 120 days. The results showed that increasing doses of vermicompost application significantly increased the activities of soil enzymes such as β-glucosidase, urease, and catalase, and promoted the dry weight development of the wheat plant (P < 0.05). Additionally, thermal imaging analysis revealed that vermicompost application significantly reduced the leaf temperatures of wheat plants, significantly promoting the growth of the plant’s upper parts (P < 0.05). While vermicompost significantly increased the concentration of essential nutrients such as nitrogen (N) and phosphorus (P) in plants (P < 0.05), its effect on macro elements (K, Mg, Ca) was not statistically significant (P > 0.05). However, a significant increase in the concentration of micronutrients such as iron (Fe), zinc (Zn), copper (Cu), and manganese (Mn) was observed in the plants (P < 0.05). The findings of this study suggest the use of vermicompost application along with thermal imaging techniques to optimize soil enzymes, plant dry matter quantity, macro and micronutrients, and overall plant growth in areas with similar soil properties.
Reviewing Editor:
SubjectS:
1. Introduction
The deficiency of soil organic matter (SOM) makes the use of organic fertilizers extremely important. Specifically, organic fertilizers enhance the organic matter levels in soils and support plant growth to a degree comparable to chemical fertilizers, increasing their appeal. Vermicompost, in particular, stands out as an organic fertilizer that can both raise soil organic matter levels and significantly enhance plant growth.Vermicompost, an organic amendment produced through the bio-fermentation processes facilitated by earthworm digestive enzymes, enhances soil fertility and health through several mechanisms (Ebrahimi et al., Citation2021a; Citation2021b; Serri et al., Citation2021; Zhang et al., Citation2020). The surge in organic farming has underscored the preference for organic over synthetic fertilizers, given the rapid decomposition of soil organic matter through natural processes, which diminishes soil fertility. Containing 90–95% organic matter, vermicompost serves as an excellent soil improver, enhancing various plant growth metrics by increasing the availability of essential nutrients like nitrogen (N), phosphorus (P), potassium (K), and zinc (Zn) (K. Sharma & Garg, Citation2018; Liu et al., Citation2019; Rupasinghe & Leelamanie, Citation2020). Research has demonstrated its efficacy in boosting germination rates, yield, plant height, stem girth, leaf size, dry weight, foliar count, and grain weight, alongside reducing nitrate accumulation in crops and improving fruit quality through elevated levels of vitamin C, carbohydrates, and proteins (Jami et al., Citation2020; Piñero et al., Citation2020). Thermal imaging technology facilitates the monitoring of plant health throughout their growth cycle, offering a means to detect and address issues promptly (Ma et al., Citation2024). The benefits thermal imaging presents to growers encompass early detection of plant stress, enhancement of irrigation strategies, effective disease and pest management, productivity assessment, and the expedited facilitation of decisions based on empirical data (Ali et al., Citation2019; Grant et al., Citation2007).
Soil health is intricately linked to its biological activity, with soil enzymes playing a pivotal role in maintaining biogeochemical cycles by facilitating organic matter decomposition and nutrient cycling (Adetunji et al., Citation2017; Jing et al., Citation2019). Among these, β-glucosidase catalyzes the breakdown of glucosides, contributing to soil fertility and the carbon cycle, and serves as a crucial indicator of soil health (Cañizares et al., Citation2011; Partey et al., Citation2019). Similarly, urease activity, prevalent across various biological entities, is integral to the nitrogen cycle, facilitating nitrogen mineralization and correlating positively with soil nitrification processes (Cordero et al., Citation2019; Tao et al., Citation2018). Dehydrogenase activity, indicative of soil microbial vitality, responds rapidly to environmental changes, making it a sensitive gauge for soil health (Chen et al., Citation2017; Dick & Burns, Citation2002).
This study aims to investigate the impact of vermicompost application on specific soil enzyme activities and the growth of wheat (Triticum aestivum L.) plants, highlighting its potential as a sustainable agricultural practice.
2. Materials and methods
2.1. Pot experiment design
The vermicompost material used as an organic fertilizer in the experiment was obtained from Ekosolfarm company, and the characteristics of the material are provided in .
Table 1. Chemical properties of vermicompost used in the experiment.
The experimental design incorporated a pot experiment with a total of five treatments to assess the effects of vermicompost on plant growth and soil characteristics. The control group consisted solely of soil, without any vermicompost. The vermicompost treatments were systematically varied: T1 received 2 Mg ha−1 (equivalent to 4 g per pot), T2 was treated with 4 Mg ha−1 (8 g per pot), T3 had 6 Mg ha−1 (12 g per pot), and T4 received the highest concentration of 8 Mg ha−1 (16 g per pot) of vermicompost. The specified quantities of vermicompost for each treatment were evenly mixed with the soil in a plastic container before being transferred into pots. Each pot, designed to hold a final soil volume of 5 kg, measured 21.6 cm in diameter by 20 cm in height. The soil utilized in this study was predominantly clayey, with a composition of 26% sand, 58% clay, and 16% silt, characterized by its high alkalinity and lime content but poor in organic matter content. The properties of the soil used in the experiment are detailed further in .
Table 2. Some physical and chemical properties of the soil used in the experiment.
For each experimental condition, we conducted three replicates (n = 3). The experiment was conducted in the research greenhouse of the Faculty of Agriculture at Harran University (37° 10’ 14.65'’ N, 49° 0’ 9.98'’ E). The experiment began on November 20, 2022, with the sowing of wheat (Triticum aestivum L.) seeds under greenhouse conditions and concluded on March 20, 2023. The arrangement of pots within the greenhouse adhered to a randomized design protocol, involving a total of 15 pots across 5 treatment levels with 3 replicates each.
2.2. Seed cultivation to pots
Following the sowing of 20 wheat seeds on the upper layer of each pot’s soil, these seeds were then lightly covered with an additional layer of soil. The wheat variety selected for this experiment was Ceyhan-99, a type of bread wheat widely grown in the local area. To facilitate more accurate observation of growth patterns during the germination stage, the number of seedlings in each pot was thinned to ensure a consistent count of 10 plants per pot. This approach was adopted to optimize conditions for monitoring plant development and health. The wheat seeds were obtained from the Ceylanpınar Agricultural Enterprise Directorate (TİGEM).
2.3. Soil sampling and chemical analyses
Before the analysis for available plant nutrients and soil properties, soil samples were air-dried, crushed using a wooden mallet, sifted through a 4 mm sieve, and prepared for examination. Soil salinity and pH were measured in a soil-water mixture using specific ratios (1:5 w:v for salinity and 1:2.5 w:v for pH), following the methods described by Zelazny et al. (Citation1986). Organic matter content was identified through wet digestion with K2Cr2O7, in accordance with (Nelson & Sommers, Citation1996). DTPA solution, as outlined by Lindsay and Norvell (Citation1978) was used to extract micronutrients (Fe, Zn, Cu, Mn) and replaceable cations (K, Ca, Mg) using a 1 N ammonium acetate solution (pH 7.0) at a 1:4 w:v ratio, following (Helmke & Sparks, Citation1996). The solutions were then filtered and analyzed for elemental content using an ICP-OES instrument. Phosphorus availability was determined using a sodium bicarbonate solution (1:20 w:v, pH 8.4) and measured with a UV-VIS spectrophotometer (Sparks, Citation1996). Total nitrogen analysis was conducted by combining samples with a Kjeldahl tablet, adding H2SO4, and processing through combustion and distillation, according to Bremner (Citation1982).
2.4. Plant sampling and analysis
After harvest, the plant samples were initially washed three times in distilled water and then blotted to remove excess moisture. They were subsequently placed in paper bags and dried at 65 °C in an oven for 48 hours. Following the drying process, the samples were pulverized using a blender. The analysis began with the addition of 5 ml of HNO3, followed by 4 ml of HClO4. This mixture was then heated to 200 °C for three hours on a hot plate (Jones & Case, Citation1990). The determination of total elemental concentrations in the plant samples, following this wet decomposition process, was carried out using an ICP-OES instrument.
2.5. Soil enzyme assays
Dehydrogenase activity was determined by incubating soil samples with 2,3,5-triphenyltetrazolium chloride (TTC) at 25 °C for 24 hours. The resultant triphenylformazan (TPF) was quantified at 485 nm using a UV-VIS spectrophotometer (Optima SP-3000), adhering to the protocol established by Tabatabai (Citation1982). Urease activity, denoted by EC 3.5.1.5, was meticulously assessed through the incubation of samples with urea at an optimal temperature of 37 °C for a duration of one hour. Subsequent to this incubation, the reaction’s product, dissolved ammonium, was visualized by the introduction of a buffer solution (pH 6.7), leading to a measurable color change. This colorimetric change was precisely quantified at 578 nm using the designated spectrophotometer, strictly adhering to the established methodologies by Tabatabai and Bremner (Citation1972). The assessment of β-Glucosidase activity, identified by the enzyme commission number EC 3.2.1.21, entailed incubating the samples with p-nitrophenyl-β-D-glucopyranoside at a constant temperature of 37 °C for a period of one hour. This process facilitated the liberation of free p-nitrophenols, the concentration of which was accurately determined by measuring the absorbance at 400 nm. This quantification adhered to the rigorous methodology established by Tabatabai (Citation1982). Catalase activity, classified under EC 1.11.1.6, was meticulously evaluated by combining soil samples with a phosphate buffer (pH 7) and a 3% hydrogen peroxide solution. The reaction’s efficacy was determined by measuring the volume of oxygen released at a controlled temperature of 20 °C, over a duration of five minutes. This measurement protocol adheres to the methodology outlined by Beck (Citation1971).
2.6. Thermal image processing and analysis
Utilizing an infrared-enabled thermal imaging camera (FLIR T540), thermal imaging tasks were executed. This camera is equipped with a 464 x 348 pixel detector and has a thermal sensitivity of 0.03 °C (<30 °C, 30 mK), allowing it to detect even very slight temperature differences. The FLIR T540 features a user-friendly interface and various measurement tools, as well as manual and automatic focusing capabilities. The equipment, mounted on a tripod, was pre-configured for emissivity before starting the capture of extensive thermal images that covered the entire plant. The thermal imaging process was conducted during the stem extension phase (stage 8) of wheat according to the Feekes growth scale. Following the aggregation of thermal images, the FLIR Tools software was applied for an in-depth analysis, which facilitated the extraction of temperature readings. Based on this analysis, average temperatures were calculated. To ensure a comprehensive representation of the plant, eight measurements were strategically taken, with two readings from each side of the plant, selected from various angles.
2.7. Statistical analyses
The analysis commenced with descriptive statistics and variance analysis on the dataset to assess the impacts of applying vermicompost. For parameters demonstrating significant differences as per the variance analysis (P < 0.05), Duncan’s test, a notable method among post-hoc multiple comparison tests, was utilized. To identify the relationships between data points, a correlation matrix was constructed using the "metabolomicsR" package within the R programming environment (Han & Liang, Citation2022). Furthermore, to elucidate the interactions among variables, network analysis was conducted (Basu et al., Citation2017). For the purpose of decomposed sparse partial correlation (DSPC) analysis, data matrices were normalized via log transformation. This analysis, grounded in the decomposed graphical lasso modeling approach, aims to delineate the intricate network of relationships among a vast array of variables with a minimal sample size. Additionally, the degree of connectivity and betweenness centrality were quantified for each node within the network.
3. Results
3.1. Thermal imaging and soil enzyme activities
Thermal imaging analysis has shown that the application of vermicompost significantly decreased the leaf temperatures of the wheat plant (). The reductions in temperature have substantially encouraged the growth of the plant’s above-ground parts (). The temperature differences observed in plant leaves were statistically significant (P < 0.05) (). The T3 treatment exhibited the lowest thermal temperature values among the groups, with T4 also classified within this group. This resulted in a 19% reduction in temperature values compared to the control group. Further comparative analysis of the thermal temperature data across treatments revealed that temperatures in the T3 treatment were 15% lower than those observed in T1 and 9% lower than in T2.
Figure 1. Thermal image [A] taken from plants that clearly show the effects of treatments illustrates the pre-harvest vegetative development of plants under greenhouse conditions [B]. Error bars represent the standard error of the mean for each treatment (n = 3). Average values (n = 3) marked with different letters in the same column are significantly different according to Duncan post-hoc tests at P < 0.05.
![Figure 1. Thermal image [A] taken from plants that clearly show the effects of treatments illustrates the pre-harvest vegetative development of plants under greenhouse conditions [B]. Error bars represent the standard error of the mean for each treatment (n = 3). Average values (n = 3) marked with different letters in the same column are significantly different according to Duncan post-hoc tests at P < 0.05.](/cms/asset/2d86690e-a67d-4268-babd-19024d981c75/oafa_a_2373872_f0001_c.jpg)
Vermicompost, being a significant source of carbon, has notably enhanced the activity of the soil β-glucosidase enzyme, an essential enzyme in the carbon cycle, with this increase also found to be statistically significant (P < 0.05) (). The T4 treatment, which recorded the highest activity, exhibited a 45% higher activity compared to the control group without vermicompost application. In comparisons within the treatments, the T4 treatment showed 32% higher activity than T1, 18% higher than T2, and 12% higher than T3. Vermicompost, an important nutrient source for soil microorganisms, may also significantly increase the activity of microorganism species that produce the urease enzyme in the soil. The clearest indicator of this increase is the 84% increase produced by the T3 treatment, which recorded the highest urease activity, compared to the control group (). When comparing the vermicompost treatments applied to soils, the highest measured T3 treatment resulted in 67% higher activity from T1, 23% from T2, and 3% from T4 in the soil. The variation in urease enzyme activity caused by the treatments in the soil was also found to be statistically significant (P < 0.05).
Figure 2. The effect of vermicompost treatments on soil β-glucosidase [A], Urease [B], Catalase [C], and Dehydrogenase [D] enzyme activities investigated in the study. Error bars represent the standard error of the mean for each treatment (n = 3). Average values (n = 3) marked with different letters in the same column are significantly different according to Duncan post-hoc tests at P < 0.05.
![Figure 2. The effect of vermicompost treatments on soil β-glucosidase [A], Urease [B], Catalase [C], and Dehydrogenase [D] enzyme activities investigated in the study. Error bars represent the standard error of the mean for each treatment (n = 3). Average values (n = 3) marked with different letters in the same column are significantly different according to Duncan post-hoc tests at P < 0.05.](/cms/asset/45a15f7f-6dec-46de-ae90-1515cb75d341/oafa_a_2373872_f0002_c.jpg)
Catalase enzyme, a crucial quality parameter that maintains the balance of air and water in the soil, was significantly increased by vermicompost treatment (). This increase is clearly evidenced by a significant 54% difference in the T3 treatment, which recorded the highest activity, compared to the untreated control group. Additionally, the differences created within the treatments were found to be statistically significant (P < 0.05). The T3 treatment, which measured the highest activity, resulted in 42% more soil catalase enzyme activity than T4, 15% more than T1, and 5% more than T2. The dehydrogenase enzyme, involved in the mineralization of soil organic matter, showed a slight change with vermicompost treatment (). According to our results, the T3 treatment, which measured the highest activity in the soil, caused 13% more activity compared to the control group without vermicompost application. When comparing the activity rates caused by the treatments in the soil, with the highest activity T3 treatment, it was measured as 10% higher than T1, 6% higher than T4, and 1% higher than T2.
3.2. Macro and micro plant nutrients along with plant dry weight
Post-harvest plant dry weight measurements have shown that vermicompost treatments significantly increased plant dry weight. This increase was found to be statistically significant (). The T3 treatment, which had the highest plant dry weight, measured 39% more weight compared to the control group that did not receive any vermicompost application. When examining the weights produced by the treatments among themselves, the T3 treatment, which had the highest weight, was found to be 37% heavier than T1, 36% heavier than T4, and 13% heavier than T2. Analyses conducted on plant tissues revealed that the concentrations of macro and micro plant nutrient elements required by the wheat plant in leaf tissues have significantly increased with the vermicompost treatment (). The N concentration in plant tissues significantly increased with vermicompost treatment. This increase was seen to be 32% higher in the T3 treatment, which had the highest N values, compared to the control group that did not undergo any treatment (). The T3 treatment, which recorded the highest N values, was 21% higher than T4, 15% higher than T1, and 13% higher than T2 (P < 0.05).
Figure 3. The effect of vermicompost treatments on macro and micro plant nutrient uptake and plant dry weight. Error bars represent the standard error of the mean for each treatment (n = 3). Average values (n = 3) marked with different letters in the same column are significantly different according to Duncan post-hoc tests at P < 0.05.
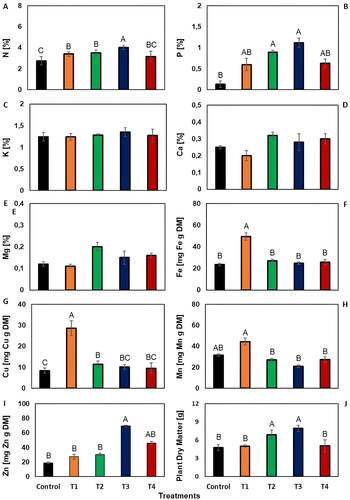
One of the most valuable results of our study was the total P content of the plant (). The T3 treatment, which had the highest plant P content, was found to have 88% more plant P content compared to the control (P < 0.05). In comparisons within the treatments, the T3 treatment, which had the highest plant P content, had 46% more P content than T1, 44% more than T4, and 21% more than T2.
The application of vermicompost has also significantly affected the uptake of microelements by plants (). The treatment with the highest iron content in plants, T1, measured 52% higher compared to the control (). In comparisons among treatments, T1, which had the highest iron content, had 49% more iron than T3, 48% more than T4, and 45% more than T2. These differences among treatments were found to be statistically significant (P < 0.05). For copper content in plants, T1, the treatment with the highest measured content, was 70% higher compared to the control (). In comparisons among treatments, T1 had 67% more copper than T4, 65% more than T3, and 60% more than T2. These differences were also statistically significant (P < 0.05). Regarding manganese content in plants, T1, the treatment with the highest content, was 29% higher compared to the control (). Among the treatments, T1 had 53% more manganese than T3, 40% more than T2, and 39% more than T4. These differences were statistically significant (P < 0.05). For zinc content, T3, the treatment with the highest content, was 73% higher compared to the control (). In comparisons among treatments, T3 had 61% more zinc than T1, 56% more than T2, and 34% more than T4. These differences among treatments were statistically significant (P < 0.05).
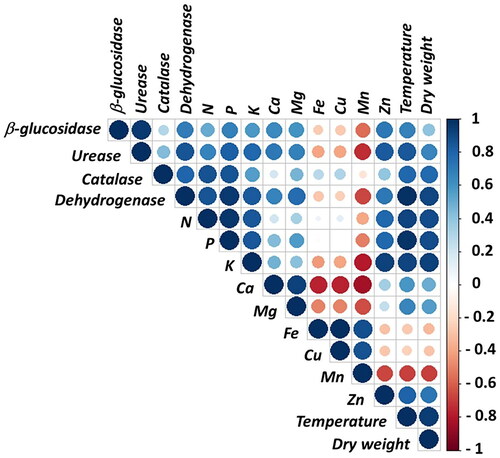
3.3. Data grouping technique: Correlation plots
In this study, a correlation graph was employed for a detailed analysis of the relationships among the examined parameters. In the correlation graph, strong positive relationships are indicated in dark blue, whereas negative relationships are shown in dark red. The size of the circles within the graph represents the magnitude of the correlation (). It was found that soil enzyme activities are highly correlated both with each other and with other examined parameters. Notably, a strong positive relationship was identified between β-glucosidase and urease enzyme activities (r = 0.95; P < 0.01), catalase enzyme activity and plant dry matter weight (r = 0.95; P < 0.01), and dehydrogenase enzyme activity and plant dry matter weight (r = 0.92; P < 0.01). Furthermore, strong positive relationships were also determined between plant leaf temperature and dehydrogenase enzyme activity (r = 0.94; P < 0.01), and urease and Zn content (r = 0.83; P < 0.01). Moderate positive relationships were observed between β-glucosidase and dehydrogenase (r = 0.66; P < 0.01), urease and catalase enzyme activities (r = 0.73; P < 0.01), and β-glucosidase and plant P content (r = 0.58; P < 0.05).
Figure 4. Correlation graph expressing the relationship among the parameters studied. Blue indicates positive correlation, while red and orange represent negative correlation. The intensity of the colors signifies the strength of the correlation, and the size of the circles denotes statistical significance.
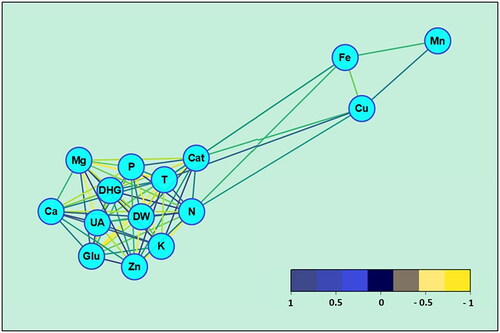
3.4. Data grouping technique: Network analysis of data
The utilization of the Debiased Sparse Partial Correlation (DSPC) Algorithm in our analysis highlighted that the DHG and UA categories exhibit prominent levels of connectivity and occupy key positions in terms of betweenness centrality within the network framework. This underscores their pivotal role in the network, where they establish robust links with various other elements (). While the Glu and T categories show some centrality, their betweenness centrality ranks lower when juxtaposed with other nodes. The centrality weights for DHG, positioned at the heart of the network, are allocated as follows: Ca (0.67), Zn (0.72), Mg (0.77), K (0.84), N (0.86), T (0.89), DW (0.91), and P (0.95). Conversely, for UA, another critical node centrally located within the network, the weights are assigned as: CAT (0.43), DW (0.66), N (0.66), Mg (0.66), Ca (0.72), K (0.78), P (0.81), T (0.83), Zn (0.84), and DHG (0.85).
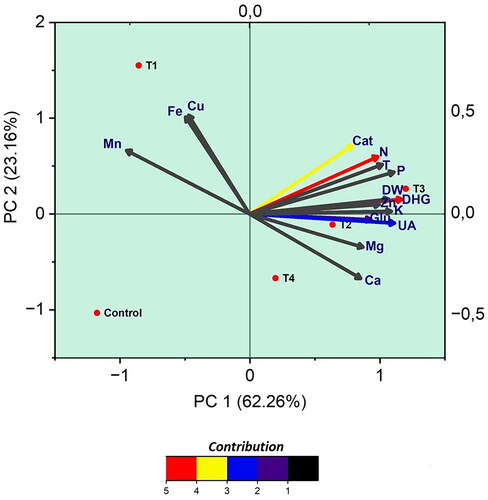
3.5. Data grouping technique: Principal component analysis (PCA)
The analysis of a dataset encompassing 15 distinct parameters through Principal Component Analysis (PCA) was aimed at pinpointing the primary sources of variance within the dataset. Illustrated in are the outcomes from the PCA. The initial principal component (PC1) is responsible for encapsulating 63.26% of the data’s variance. The variables that contribute most significantly to PC1, listed in descending order of their loadings, include β-glucosidase, urease, dehydrogenase, phosphorus (P), potassium (K), zinc (Zn), temperature, and the dry weight of plant matter. These factors are deemed pivotal in delineating the overall variability observed in the soil’s biological characteristics. On the other hand, the secondary principal component (PC2) elucidates 23.16% of the data’s variance, with copper (Cu), manganese (Mn), iron (Fe), nitrogen (N), and catalase (Cat) showing the highest loadings. These elements are identified as key in illustrating the overarching variance related to plant growth. The tertiary principal component (PC3), accounting for 9.67% of the variance, is predominantly influenced by calcium (Ca) and magnesium (Mg). These components are highlighted as essential in shedding light on the variation in macro elements crucial for plant development. The PCA results succinctly convey that a few principal components can effectively encapsulate the soil properties, with the foremost two components representing the bulk of the dataset’s variance. This underscores their significance in comprehending the impact on soil biological attributes.
4. Discussion
4.1. Thermal imaging and soil enzyme activities
Observing the temperature of plants allows for the assessment of the crop’s water potential, facilitating the adjustment of irrigation schedules. This leads to a reduction in water consumption and an increase in crop yield, thereby enabling sustainable agricultural practices (Gautam & Pagay, Citation2020). Our results indicate that vermicompost treatment caused a decrease in plant temperature. Similarly, on egg plant it has been shown that application of biochar and vermicompost has benefited plant water status under deficit irrigation (Ebrahimi et al., Citation2021). Lower leaf temperatures are considered an indicator of high transpiration rates (Yang et al., Citation2020). During this transpiration process, the evaporation occurring at the leaf surface absorbs a significant amount of heat from the leaf, resulting in a marked decrease in leaf temperature (Xu et al., Citation2020). Thermal cameras can clearly observe these temperature changes resulting from heat absorption (Wan et al., Citation2021). Mulero (Mulero et al., Citation2023) identified lower leaf temperatures associated with increased leaf water content in wheat plants grown under watered conditions and exposed to different CO2 levels, using thermal imaging, whereas higher leaf temperatures were observed under dry conditions. Akin and Kaya (Citation2024) reported a significant decrease in wheat yield under drought conditions due to the adverse effects on the photosynthesis process caused by the closing of stomata, leading to increased canopy temperatures of the wheat plant. The application of organic residues to soil notably enhances the activity of microorganisms involved in the enzymatic production processes, thereby directly augmenting microbial activity. Numerous studies have documented that the applicationof organic fertilizers, such as vermicompost, significantly elevates the activity of the β-glucosidase enzyme within soils (Albiach et al., Citation2000; Deng & Tabatabai, Citation1997; Srivastava et al., Citation2012). This incremental rise in β-glucosidase enzyme activity can be linked to the enzyme’s role in generating simple substrates for soil microorganisms, which these organisms readily exploit as an energy source (Adetunji et al., Citation2017; Mulidzi & Wooldridge, Citation2016). The diminished activity observed in the control group, characterized by the lowest urease enzyme activity, can be attributed to the experimental soil’s inherent low enzymatic activity, a consequence of its scant organic matter content (1.1%), alkaline pH level (7.9), high lime content (30%), and the repetitive nature of the cultivation processes applied (Leinweber et al., Citation2008; Skujin̦š, Citation1973). It is established that urease enzyme exhibits heightened activity in soils with a pH range of 6.5–7.0 and a lower lime content (Wang et al., Citation2008). The determination of the highest urease enzyme activity in the T3 treatment can be explained by the application of vermicompost to calcareous soils with low alkalinity and organic matter levels, which enhances the soil’s organic matter content, thereby providing an energy source for microorganisms. Furthermore, the introduction of carbon-rich vermicompost to soils acts as a stimulant for soil microorganisms, leading to an increase in the soil’s urease enzyme activity (Bandick & Dick, Citation1999; Pramanik et al., Citation2007; Rowell et al., Citation1973). There exists a pronounced interplay between the C:N ratio of vermicompost and the soil urease enzyme activity. Following this interaction, it has been proposed that soil urease enzyme activities can serve as a crucial biological quality indicator, reflecting the beneficial impact of vermicompost applications on soil health (Cayuela et al., Citation2008; Karmegam et al., Citation2019). Catalase enzyme activity is intricately linked to the aeration of soil. The utilization of soil with a high clay content (58%) and a clayey texture, as indicated in , resulted in reduced catalase enzyme activity within the control group. A secondary factor contributing to this reduced activity is the increment in soil bulk density, or in other terms, soil compaction. This increase in soil bulk density diminishes oxygen availability by displacing air with water in the soil’s air-filled pores, adversely affecting catalase enzyme activity which is notably lower in clayey soils with diminished soil aeration and in sandy soils with excessive soil aeration (Alef & Nannipieri, Citation1995; Leirós et al., Citation1999; Li et al., Citation2016). The observed gradual decrease in catalase enzyme activity in the T3 and T4 treatments can be attributed to an excess of air beyond what is required by aerobic microorganisms in the soil, a condition facilitated by the enhanced porosity attributable to vermicompost addition (Guangming et al., Citation2017). These findings concerning catalase enzyme activity align with the research conducted by Ye (Ye et al., Citation2014). In soils treated with vermicompost, an initial increase in dehydrogenase enzyme activity is observed, attributable to the readily accessible carbon compounds for soil microorganisms. Subsequently, dehydrogenase enzyme activity reaches a state of equilibrium after a certain period (Parastesh et al., Citation2019). In line with this dynamic, Uz and Tavali (Citation2014) documented in a calcareous soil incubation trial that dehydrogenase enzyme activity peaked during the first four weeks following vermicompost application, attributed to heightened microbial activity, before trending downwards towards equilibrium. This observation is in harmony with the outcomes of the present study, which utilized soil with a high lime content () and spanned a duration of 120 days. It’s important to note that dehydrogenase enzyme activity in soils is influenced by a multitude of factors, including soil organic matter, soil colloids, clay, and environmental conditions (W. A. Dick & Tabatabai, Citation1992).
4.2. Macro and micro plant nutrients along with plant dry weight
The incorporation of vermicompost significantly influenced the levels of nitrogen and phosphorus, key macro plant nutrients derived from the soil, as well as all examined micronutrients (copper, manganese, iron, and zinc) with statistical significance (P < 0.05). However, the levels of calcium and potassium, which are also considered macro plant nutrients, remained unaffected by vermicompost treatment (P > 0.05). Notably, the T3 vermicompost treatment yielded the highest concentrations of N, P, and K among macro nutrients, while the highest levels of Fe, Cu, and Mn were observed in the T1 vermicompost treatment, and zinc peaked in the T3 treatment (). The nutrient-rich nature of vermicompost, when applied to soil, significantly promotes plant growth. A pivotal impact of vermicompost on plant growth is its enhancement of vital soil properties, such as mineralization and nitrification, thus providing plants with a greater quantity of mineral nutrients compared to other organic fertilizers (Karlsons et al., Citation2016). Vermicompost application is documented to elevate soil organic matter content and nitrogen concentration, a critical nutrient for plant growth (Akhzari & Pessarakli, Citation2017; Erdal & Ekinci, Citation2020). The augmentation of phosphorus availability in plants is attributed to the solubilization of phosphorus by organic acids released during microbial decomposition, particularly in calcareous soils, facilitated significantly by the enhanced activity of soil microorganisms through vermicompost application (Brucker et al., Citation2020). Thus, vermicompost positively affects the concentrations of nitrogen and phosphorus in plants, essential mineral nutrients during the growth phase (Pourranjbari Saghaiesh et al., Citation2019). The constrained uptake of potassium by plants is linked to the dominance of 2:1 type clays in the experimental soil and its high clay content. In soils treated with vermicompost, potassium fixation by clay-rich soils occurs due to the interaction between clay colloids and potassium, with the plant’s access to potassium varying according to the soil’s fixation capacity (Mahanta et al., Citation2012). The bioavailability of micronutrients generally diminishes in calcareous soils; however, the application of vermicompost can enhance micronutrient availability in such soils. Khosravi et al. (Citation2018) highlighted that the ability of vermicompost to chelate micronutrient elements in calcareous soils, preventing their conversion into forms unavailable to plants, is a significant factor in increasing micronutrient availability. Numerous studies have consistently demonstrated that the application of vermicompost significantly enhances the dry matter content of plants, a finding supported by various researchers (Edwards, Citation1995; Erdal & Ekinci, Citation2017; Nagavallemma et al., Citation2004). The principal mechanism behind this substantial increase in plant dry matter content is attributed to the slow decomposition rate of vermicompost in soil, enabling plants to assimilate the essential nutrients required throughout their growth period (Catanzaro et al., Citation1998). Additionally, the experiment’s soil having a relatively high pH value of 7.9 (), in contrast to the lower pH value of 7.2 for the applied vermicompost (), is considered a secondary factor contributing to the augmented dry matter accumulation. In this context, Sharma (S. Sharma et al., Citation2005) reported in their comparative study of vermicompost and chemical fertilizers that vermicompost application led to a reduction in soil pH and resulted in a higher dry matter content in plants relative to those fertilized chemically.
5. Conclusions
This research has meticulously examined the positive effects of vermicompost on the growth of wheat plants, soil enzyme activities, and nutrient uptake, demonstrating its critical role in sustainable agriculture. Vermicompost has facilitated the reduction of leaf temperatures, thus promoting the development of the plant’s above-ground parts and significantly increasing the activity of key soil enzymes such as β-glucosidase, urease, and catalase. This enhancement has improved soil health and nutrient accessibility. Correlation and network analyses have revealed that vermicompost establishes strong relationships between biological properties in soil and plant systems, thereby contributing to holistic improvement across the system. Consequently, the use of vermicompost emerges as a key element in sustainable agriculture, aiming to increase plant productivity, improve soil health, and reduce environmental impacts. The findings of this study indicate that the application of vermicompost can significantly enhance both plant health and soil quality, contributing to agricultural sustainability.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available on request from the corresponding author.
References
- Adetunji, A. T., Lewu, F. B., Mulidzi, R., & Ncube, B. (2017). The biological activities of β-glucosidase, phosphatase and urease as soil quality ındicators: a review. Journal of Soil Science and Plant Nutrition, 17(3), 794–807. https://doi.org/10.4067/S0718-95162017000300018
- Akhzari, D., & Pessarakli, M. (2017). Effects of vermicompost and urea fertilizers on qualitative and quantitative characteristics of vetiveria zizanioides stapf. grown under drought stress conditions. Journal of Plant Nutrition, 40(14), 2063–2075. https://doi.org/10.1080/01904167.2017.1346126
- Akin, S., & Kaya, C. (2024). Impact of salicylic acid and sodium hydrosulfide applied singly or in combination on drought tolerance and grain yield in wheat plants. Food and Energy Security, 13(1), e532. https://doi.org/10.1002/fes3.532
- Albiach, R., Canet, R., Pomares, F., & Ingelmo, F. (2000). Microbial biomass content and enzymatic activities after the application of organic amendments to a horticultural soil. Bioresource Technology, 75(1), 43–48. https://doi.org/10.1016/S0960-8524(00)00030-4
- Alef, K., & Nannipieri, P. (1995). ‘Methods in applied soil microbiology and biochemistry (p. c1995). Academic Press.
- Ali, S., Xu, Y., Ma, X., Ahmad, I., Jia, Q., Akmal, M., Hussain, Z., Arif, M., Cai, T., Zhang, J., Jia, Z., Manzoor. 2019. Deficit ırrigation strategies to ımprove winter wheat productivity and regulating root growth under different planting patterns. Agricultural Water Management, 219(June), 1–11. https://doi.org/10.1016/j.agwat.2019.03.038
- Bandick, A. K., & Dick, R. P. (1999). Field management effects on soil enzyme activities. Soil Biology and Biochemistry, 31(11), 1471–1479. https://doi.org/10.1016/S0038-0717(99)00051-6
- Basu, S., Duren, W., Evans, C. R., Burant, C. F., Michailidis, G., & Karnovsky, A. (2017). Sparse network modeling and metscape-based visualization methods for the analysis of large-scale metabolomics data. Bioinformatics (Oxford, England), 33(10), 1545–1553. https://doi.org/10.1093/bioinformatics/btx012
- Beck, T. H. (1971). The determination of catalase activity in soils. Journal of Plant Nutrition and Soil Science, 130, 68–81.
- Bremner. (1982). Total nitrogen. In Methods of soil analysis (pp. 595–624). https://cir.nii.ac.jp/crid/1570291226053723136.
- Brucker, E., Kernchen, S., & Spohn, M. (2020). Release of phosphorus and silicon from minerals by soil microorganisms depends on the availability of organic carbon. Soil Biology and Biochemistry, 143(April), 107737. https://doi.org/10.1016/j.soilbio.2020.107737
- Cañizares, R., Benitez, E., & Ogunseitan, O. A. (2011). Molecular analyses of β-glucosidase diversity and function in soil. European Journal of Soil Biology, 47(1), 1–8. https://doi.org/10.1016/j.ejsobi.2010.11.002
- Catanzaro, C. J., Williams, K. A., & Sauve, R. J. (1998). Slow release versus water soluble fertilization affects nutrient leaching and growth of potted chrysanthemum. Journal of Plant Nutrition, 21(5), 1025–1036. https://doi.org/10.1080/01904169809365461
- Cayuela, M. L., Mondini, C., Sánchez-Monedero, M. A., & Roig, A. (2008). Chemical properties and hydrolytic enzyme activities for the characterisation of two-phase olive mill wastes composting. Bioresource Technology, 99(10), 4255–4262. https://doi.org/10.1016/j.biortech.2007.08.057
- Chen, J., Zhou, S., Rong, Y., Zhu, X., Zhao, X., & Cai, Z. (2017). Pyrosequencing reveals bacterial communities and enzyme activities differences after application of novel chiral ınsecticide paichongding in aerobic soils. Applied Soil Ecology, 112(April), 18–27. https://doi.org/10.1016/j.apsoil.2016.12.007
- Cordero, I., Snell, H., & Bardgett, R. D. (2019). High throughput method for measuring urease activity in soil. Soil Biology & Biochemistry, 134(July), 72–77. https://doi.org/10.1016/j.soilbio.2019.03.014
- Deng, S. P., & Tabatabai, M. A. (1997). Effect of tillage and residue management on enzyme activities in soils: III. phosphatases and arylsulfatase. Biology and Fertility of Soils, 24(2), 141–146. https://doi.org/10.1007/s003740050222
- Dick, R. G., & Burns, R. P. eds. (2002). Enzymes in the environment: activity, ecology, and applications. CRC Press. https://doi.org/10.1201/9780203904039
- Dick, W. A., & Tabatabai, M. A. (1992). Significance and potential uses of soil enzymes. In Soil microbial ecology: applications in agricultural and environmental management (pp. 95–127). https://www.cabdirect.org/cabdirect/abstract/19931976431.
- Ebrahimi, M., Mousavi, A., Souri, M. K., & Sahebani, N. (2021). Can vermicompost and biochar control Meloidogyne javanica on eggplant? Nematology, 23(9), 1053–1064. https://doi.org/10.1163/15685411-bja10094
- Ebrahimi, M., Souri, M. K., Mousavi, A., & Sahebani, N. (2021a). Biochar and vermicompost improve growth and physiological traits of eggplant (Solanum melongena L.) under deficit irrigation. Chemical and Biological Technologies in Agriculture, 8(1), 1–14. https://doi.org/10.1186/s40538-021-00216-9
- Edwards, C. A. (1995). Historical overview of vermicomposting. Historical Overview of Vermicomposting, 36(6), 56–58.
- Erdal, İ., & Ekinci, K. (2017). Effects of vermicomposts obtained from rose oil processing wastes, dairy manure, municipal open market wastes and straw on plant growth, mineral nutrition, and nutrient uptake of corn. Journal of Plant Nutrition, 40(15), 2200–2208. https://doi.org/10.1080/01904167.2017.1346677
- Erdal, İ., & Ekinci, K. (2020). Effects of composts and vermicomposts obtained from forced aerated and mechanically turned composting method on growth, mineral nutrition and nutrient uptake of wheat. Journal of Plant Nutrition, 43(9), 1343–1355. https://doi.org/10.1080/01904167.2020.1727506
- Gautam, D., & Pagay, V. (2020). A review of current and potential applications of remote sensing to study the water status of horticultural crops. Agronomy, 10(1), 140. https://doi.org/10.3390/agronomy10010140
- Grant, O. M., Tronina, L., Jones, H. G., & Chaves, M. M. (2007). Exploring thermal ımaging variables for the detection of stress responses in grapevine under different ırrigation regimes. Journal of Experimental Botany, 58(4), 815–825. https://doi.org/10.1093/jxb/erl153
- Guangming, L., Xuechen, Z., Xiuping, W., Hongbo, S., Jingsong, Y., & Xiangping, W. (2017). Soil enzymes as ındicators of saline soil fertility under various soil amendments. Agriculture, Ecosystems & Environment, 237(January), 274–279. https://doi.org/10.1016/j.agee.2017.01.004
- Han, X., & Liang, L. (2022). metabolomicsR: A streamlined workflow to analyze metabolomic data in R. Bioinformatics Advances, 2(1), vbac067. https://doi.org/10.1093/bioadv/vbac067
- Helmke, P. A., & Sparks, D. L. (1996). Lithium, sodium, potassium, rubidium, and cesium. In Methods of soil analysis (pp. 551–574). John Wiley & Sons, Ltd. https://doi.org/10.2136/sssabookser5.3.c19
- Jami, N., Rahimi, A., Naghizadeh, M., & Sedaghati, E. (2020). Investigating the use of different levels of mycorrhiza and vermicompost on quantitative and qualitative yield of saffron (Crocus Sativus L.). Scientia Horticulturae, 262(February), 109027. https://doi.org/10.1016/j.scienta.2019.109027
- Jing, C., Xu, Z., Zou, P., Tang, Q., Li, Y., You, X., & Zhang, C. (2019). Coastal halophytes alter properties and microbial community structure of the saline soils in the Yellow River Delta, China. Applied Soil Ecology, 134(February), 1–7. https://doi.org/10.1016/j.apsoil.2018.10.009
- Jones, J. B., Jr., & Case, V. W. (1990). Sampling, handling, and analyzing plant tissue samples. In Soil testing and plant analysis (pp. 389–427). John Wiley & Sons, Ltd. https://doi.org/10.2136/sssabookser3.3ed.c15
- Karlsons, A., Osvalde, A., Andersone-Ozola, U., & Ievinsh, G. (2016). Vermicompost from municipal sewage sludge affects growth and mineral nutrition of winter rye (secale cereale) plants. Journal of Plant Nutrition, 39(6), 765–780. https://doi.org/10.1080/01904167.2015.1087566
- Karmegam, N., Vijayan, P., Prakash, M., & John Paul, J. A. (2019). Vermicomposting of paper ındustry sludge with cowdung and green manure plants using eisenia fetida: a viable option for cleaner and enriched vermicompost production. Journal of Cleaner Production, 228(August), 718–728. https://doi.org/10.1016/j.jclepro.2019.04.313
- Khosravi, A., Zarei, M., & Ronaghi, A. (2018). Effect of PGPR, phosphate sources and vermicompost on growth and nutrients uptake by lettuce in a calcareous soil. Journal of Plant Nutrition, 41(1), 80–89. https://doi.org/10.1080/01904167.2017.1381727
- Leinweber, P., Jandl, G., Baum, C., Eckhardt, K.-U., & Kandeler, E. (2008). Stability and composition of soil organic matter control respiration and soil enzyme activities. Soil Biology and Biochemistry, Special Section: Functional Microbial Ecology: Molecular Approaches to Microbial Ecology and Microbial Habitats, 40(6), 1496–1505. https://doi.org/10.1016/j.soilbio.2008.01.003
- Leirós, M. C., Trasar-Cepeda, C., Camiña, F., & Gil-Sotres, F. (1999). An ımproved method to measure catalase activity in soils. Soil Biology and Biochemistry, 31(3), 483–485. https://doi.org/10.1016/S0038-0717(98)00153-9
- Li, Y., Niu, W., Wang, J., Liu, L., Zhang, M., & Xu, J. (2016). Effects of artificial soil aeration volume and frequency on soil enzyme activity and microbial abundance when cultivating greenhouse tomato. Soil Science Society of America Journal, 80(5), 1208–1221. https://doi.org/10.2136/sssaj2016.06.0164
- Lindsay, W. L., & Norvell, W. A. (1978). Development of a DTPA soil test for zinc, ıron, manganese, and copper. Soil Science Society of America Journal, 42(3), 421–428. https://doi.org/10.2136/sssaj1978.03615995004200030009x
- Liu, M., Wang, C., Wang, F., & Xie, Y. (2019). Maize (Zea Mays) growth and nutrient uptake following ıntegrated ımprovement of vermicompost and humic acid fertilizer on coastal saline soil. Applied Soil Ecology, 142(October), 147–154. https://doi.org/10.1016/j.apsoil.2019.04.024
- Ma, S., Liu, S., Gao, Z., Wang, X., Ma, S., & Wang, S. (2024). Water deficit diagnosis of winter wheat based on thermal ınfrared ımaging. Plants (Basel, Switzerland), 13(3), 361. https://doi.org/10.3390/plants13030361
- Mahanta, K., Jha, D. K., Rajkhowa, D. J., & Manoj-Kumar. (2012). ‘Microbial enrichment of vermicompost prepared from different plant biomasses and their effect on rice (Oryza Sativa L.) growth and soil fertility’. Biological Agriculture & Horticulture 28(4), 241–250. https://doi.org/10.1080/01448765.2012.738556
- Mulero, G., Jiang, D., Bonfil, D. J., & Helman, D. (2023). Use of thermal ımaging and the photochemical reflectance ındex (PRI) to detect wheat response to elevated CO2 and drought. Plant, Cell & Environment, 46(1), 76–92. https://doi.org/10.1111/pce.14472
- Mulidzi, A. R., & Wooldridge, J. (2016). Effect of ırrigation with diluted winery wastewater on enzyme activity in four western cape soils. Sustainability in Environment, 1(2), 141. https://doi.org/10.22158/se.v1n2p141
- Nagavallemma, K. P., Wani, S. P., Lacroix, S., Padmaja, V. V., Vineela, C., Rao, M. B., & Sahrawat, K. L. (2004). Vermicomposting: Recycling wastes into valuable organic fertilizer.global theme on agroecosystems report no. 8’. monograph. International Crops Research Institute for the Semi-Arid Tropics. 2004. https://oar.icrisat.org/3677/.
- Nelson, D. W., & Sommers, L. E. (1996). Total carbon, organic carbon, and organic matter. In Methods of soil analysis (pp. 961–1010). John Wiley & Sons, Ltd. https://doi.org/10.2136/sssabookser5.3.c34
- Parastesh, F., Ali Alikhani, H., & Etesami, H. (2019). Vermicompost enriched with phosphate–solubilizing bacteria provides plant with enough phosphorus in a sequential cropping under calcareous soil conditions. Journal of Cleaner Production, 221(June), 27–37. https://doi.org/10.1016/j.jclepro.2019.02.234
- Partey, S. T., Zougmore, R. B., Thevathasan, N. V., & Preziosi, R. F. (2019). Effects of plant residue decomposition on soil N availability, microbial biomass and β-glucosidase activity during soil fertility ımprovement in Ghana. Pedosphere, 29(5), 608–618. https://doi.org/10.1016/S1002-0160(17)60433-8
- Piñero, J. C., Shivers, T., Byers, P. L., & Johnson, H.-Y. (2020). Insect-based compost and vermicompost production, quality and performance. Renewable Agriculture and Food Systems, 35(1), 102–108. https://doi.org/10.1017/S1742170518000339
- Pourranjbari Saghaiesh, S., Souri, M. K., & Moghaddam, M. (2019). Characterization of nutrients uptake and enzymes activity in Khatouni Melon (Cucumis Melo Var. Inodorus) seedlings under different concentrations of nitrogen, potassium and phosphorus of nutrient solution. Journal of Plant Nutrition, 42(2), 178–185. https://doi.org/10.1080/01904167.2018.1551491
- Pramanik, P., Ghosh, G. K., Ghosal, P. K., & Banik, P. (2007). Changes in organic – C, N, P and K and enzyme activities in vermicompost of biodegradable organic wastes under liming and microbial ınoculants. Bioresource Technology, 98(13), 2485–2494. https://doi.org/10.1016/j.biortech.2006.09.017
- Rowell, M. J., Ladd, J. N., & Paul, E. A. (1973). Enzymically active complexes of proteases and humic acid analogues. Soil Biology and Biochemistry, 5(5), 699–703. https://doi.org/10.1016/0038-0717(73)90062-X
- Rupasinghe, I. S. U., & Leelamanie, D. A. L. (2020). Comparison of municipal and agriculture-based solid waste composts: short-term crop-yield response and soil properties in a tropical ultisol. Biologia, 75(6), 809–818. https://doi.org/10.2478/s11756-020-00464-4
- Serri, F., Souri, M. K., & Rezapanah, M. (2021). Growth, biochemical quality and antioxidant capacity of coriander leaves under organic and inorganic fertilization programs. Chemical and Biological Technologies in Agriculture, 8(1), 1–8. https://doi.org/10.1186/s40538-021-00232-9
- Sharma, K., & Garg, V. K. (2018). Comparative analysis of vermicompost quality produced from rice straw and paper waste employing earthworm Eisenia Fetida (Sav.). Bioresource Technology, 250(February), 708–715. https://doi.org/10.1016/j.biortech.2017.11.101
- Sharma, S., Pradhan, K., Satya, S., & Vasudevan, P. (2005). Potentiality of earthworms for waste management and in other uses – a review.
- Skujin̦š, J. (1973). Dehydrogenase: An ındicator of biological activities in arid soils. Bulletins from the Ecological Research Committee, 17, 235–241. https://www.jstor.org/stable/20111567.
- Sparks, D. L. (1996). Methods of soil analysis. Part 3, chemical methods. Soil Science Society of America Book Series. Madison, Wis.: Soil Science Society of America : American Society of Agronomy.
- Srivastava, P. K., Gupta, M., Upadhyay, R. K., Sharma, S., Singh, N., Tewari, S. K., Singh, B., & Shikha. (2012). Effects of combined application of vermicompost and mineral fertilizer on the growth of Allium Cepa L. and soil fertility. Journal of Plant Nutrition and Soil Science 175(1), 101–107. https://doi.org/10.1002/jpln.201000390
- Tabatabai, M. A. (1982). Soils enzymes dans methods of soil analysis. Part 2 chemical and microbial properties’. Agronomy.
- Tabatabai, M. A., & Bremner, J. M. (1972). Assay of urease activity in soils. Soil Biology and Biochemistry, 4(4), 479–487. https://doi.org/10.1016/0038-0717(72)90064-8
- Tao, R., Li, J., Guan, Y., Liang, Y., Hu, B., Lv, J., & Chu, G. (2018). Effects of urease and nitrification ınhibitors on the soil mineral nitrogen dynamics and nitrous oxide (N2O) emissions on calcareous soil. Environmental Science and Pollution Research İnternational, 25(9), 9155–9164. https://doi.org/10.1007/s11356-018-1226-9
- Uz, I., & Tavali, I. E. (2014). Short-term effect of vermicompost application on biological properties of an alkaline soil with high lime content from mediterranean region of Turkey. TheScientificWorldJournal, 2014(August), e395282–11. https://doi.org/10.1155/2014/395282
- Wan, Q., Brede, B., Smigaj, M., & Kooistra, L. (2021). Factors ınfluencing temperature measurements from miniaturized Thermal Infrared (TIR) cameras: A laboratory-based approach. Sensors (Basel, Switzerland), 21(24), 8466. https://doi.org/10.3390/s21248466
- Wang, Q. K., Wang, S. L., & Liu, Y. X. (2008). Responses to N and P fertilization in a young eucalyptus dunnii plantation: microbial properties, enzyme activities and dissolved organic matter. Applied Soil Ecology, 40(3), 484–490. https://doi.org/10.1016/j.apsoil.2008.07.003
- Xu, K., Zheng, C., & Ye, H. (2020). The transpiration characteristics and heat dissipation analysis of natural leaves grown in different climatic environments. Heat and Mass Transfer, 56(1), 95–108. https://doi.org/10.1007/s00231-019-02701-2
- Yang, Y., Zhang, Q., Huang, G., Peng, S., & Li, Y. (2020). Temperature responses of photosynthesis and leaf hydraulic conductance in rice and wheat. Plant, Cell & Environment, 43(6), 1437–1451. https://doi.org/10.1111/pce.13743
- Ye, N., Li, H., Zhu, G., Liu, Y., Liu, R., Xu, W., Jing, Y., Peng, X., & Zhang, J. (2014). Copper suppresses abscisic acid catabolism and catalase activity, and ınhibits seed germination of rice. Plant & Cell Physiology, 55(11), 2008–2016. https://doi.org/10.1093/pcp/pcu136
- Zelazny, L. W., Marion, L., Jackson, C. H., & Lim. (1986). Oxides, hydroxides, and aluminosilicates. In Methods of soil analysis (pp. 101–150). John Wiley & Sons, Ltd. https://doi.org/10.2136/sssabookser5.1.2ed.c6
- Zhang, F., Wang, R., Yu, W., Liang, J., & Liao, X. (2020). Influences of a vermicompost application on the phosphorus transformation and microbial activity in a paddy soil. Soil and Water Research, 15(4), 199–210. https://doi.org/10.17221/91/2019-SWR