Abstract
The essential oils extracted from the leaves of five Eucalyptus species: E. astringens, E. camaldulensis, E. lehmannii, E. leucoxylon, and E. sideroxylon, were investigated for their antimicrobial properties. These species were growing in the same plantation area, exposed to identical conditions, and subjected to uniform agronomic practices. Processed and analyzed under consistent parameters, the essential oil yields ranged from 0.14 to 0.96% (w/w). Chromatographic analysis were resolved into 48 compounds, with 11 common to all oils. Terpenoids (oxygenated mono- and sesquiterpenes) dominated the oil profiles, constituting 55.66–76.67% of the composition. Major components identified included 1,8-cineole (21.97–50.93%), α-pinene (2.18–15.95%), p-cymene (0.83–15.94%), spathulenol (0–20.49%), globulol (4.09–14.26%), and aromadendrene (2.37–15.03%). Genetically driven interspecific variation in composition was observed through Principal Component Analysis (PCA), Hierarchical Cluster Analysis (HCA), and heatmap clustering. Moreover, distinctive components were identified for each essential oil, offering a valuable tool for discriminating between Eucalyptus species and ensuring authentication and quality control in commercial samples. Results from antimicrobial disc-diffusion assays indicated robust antimicrobial activity in all essential oils, with those derived from E. camaldulensis, E. lehmannii, and E. leucoxylon exhibiting the highest effectiveness.
1. Introduction
The genus Eucalyptus encompasses approximately 900 species and subspecies distributed worldwide (Barbosa et al., Citation2016). Members of this genus are multipurpose trees cultivated for their ornamental characteristics, timber production, and cut foliage (Caputo et al., Citation2020). Eucalyptus leaves, a by-product of tree cutting, are particularly rich in essential oil to which the antioxidant, antimicrobial, repellent, insecticidal, herbicidal, and nematicidal activities, among others are attributed (Barbosa et al., Citation2016; Batish et al., Citation2008; Mossi et al., Citation2011). Due to their numerous biological activities, Eucalyptus essential oils are widely used in various industrial sectors, including cosmeceuticals, fragrances, foods, pharmaceuticals, agrochemicals, and household products (Goldbeck et al., Citation2014). They are also employed in different traditional medicine systems to treat conditions such as colds, coughs, influenza, sore throat, and sinus congestion (Dogan et al., Citation2017). Recent applications of Eucalyptus essential oils include the treatment of gastrointestinal disorders (diarrhea, colic, and dysentery) and respiratory diseases (asthma, laryngitis, trachealgia, and pharyngitis), in addition to their anti-inflammatory, wound-healing, analgesic, anti-nociceptive, cytotoxic, and anti-diabetic properties (Aleksic Sabo & Knezevic, Citation2019).
Due to these intriguing activities and relevant applications, the main representative species of the genus Eucalyptus are extensively studied for the essential oil composition of their foliage. Previous phytochemical investigations have pointed to the presence of common compounds, including oxygenated monoterpenes (1,8-cineole, citronellol, piperitone, isopulegol, citronellal, α-terpineol, linalool, terpinyl acetate, citronellyl acetate, etc.), monoterpene hydrocarbons (α-, β-pinene, p-cymene, limonene, camphene, γ-terpinene, etc.), oxygenated sesquiterpenes (spathulenol, caryophyllene oxide, etc.), and sesquiterpene hydrocarbons (β-caryophyllene, aromadendrene, α-copaene, bicyclogermacrene, etc.) (Aleksic Sabo & Knezevic, Citation2019; Ameur et al., Citation2021; Barbosa et al., Citation2016; Limam et al., Citation2020).
However, the quality of Eucalyptus spp. essential oils and their subsequent biological activities can be somewhat variable, depending on factors such as plant species/subspecies, origin, season, organ, extraction, and analytical conditions. Consequently, different chemotypes within various populations of the same species have been described (Barbosa et al., Citation2016). Given their wide range of medicinal, agronomic, and industrial applications, analyzing Eucalyptus spp. essential oils and understanding their chemodiversity is crucial for defining potential applications and devising the best strategy for their conservation and naturalization.
In Tunisia, the genus Eucalyptus is represented by 117 species naturalized into 30 arboretums (Ben Jemâa et al., Citation2012). Most of them are cultivated for ornamental and honey trees, as well as for timber and firewood production. In folk medicine, Eucalyptus leaves are used to treat colds, coughs, and respiratory disorders, including pharyngitis, bronchitis, and sinusitis (Ameur et al., Citation2021). This fast-growing species has adapted well to the Tunisian climate and has been used to stabilize the coastal dunes of northwest Tunisia, reduce erosion, and protect roadsides (Elaieb et al., Citation2019).
Recent studies reported by Horst et al. (Citation2022b) have raised questions about the use of Eucalyptus foliage as feed for ruminants due to its low crude protein and energy content. However, results reported by Horst et al. (Citation2022a) suggest that some Eucalyptus species could be included in ruminant diets to modulate fermentation processes in the rumen. On the other hand, it is well assumed that natural extracts or essential oils rich in phytochemicals are perceived as safer and more environmentally friendly products (Beauchemin et al., Citation2022). Therefore, they have higher levels of acceptance, raising fewer animal and food safety concerns (Dey et al., Citation2021). Earlier compositional studies have reported interspecific and intraspecific variations in essential oil composition and its biological activities (Ameur et al., Citation2021; Ben Jemâa et al., Citation2012; Elaissi et al., Citation2012; Hamdi et al., Citation2015; Limam et al., Citation2020; Slimane et al., Citation2014; Yangui et al., Citation2017). However, most of these studies are focused on particular species, such as E. camaldulensis and E. globulus (Ben Jemâa et al., Citation2012; Hamdi et al., Citation2015; Slimane et al., Citation2014; Yangui et al., Citation2017), and little is known about the remaining species. The main objective of this present study was to determine the essential oil composition of five Eucalyptus species: E. astringens, E. camaldulensis, E. lehmannii, E. leucoxylon, and E. sideroxylon, and assess their chemodiversity. The antimicrobial activity against Staphylococcus aureus, Enterococcus faecium, Escherichia coli, Salmonella typhimurium and Candida albicans was also evaluated.
2. Materials and methods
2.1. Plant materials
Leaf samples were collected from five specimens of 52-year-old trees of E. astringens, E. camaldulensis, E. lehmannii, E. leucoxylon, and E. sideroxylon growing in the arboretum of Korbous (Northeastern Tunisia, latitude: 36°50′N, longitude: 10°23′E, Altitude: 180 m above sea level; climate: sub-humid). For each species, 10–15 trees were selected within each plot (based on health status and size), and a branch (approximately 3–4 m high and 1 m long) was cut from each tree, handpicking 100 g of fresh matter sample of mature foliage. Leaves were dried at room temperature (20 ± 2 °C), ground into fine powders, and examined for their essential oil composition.
2.2. Isolation of essential oils
The dried leaf samples underwent hydrodistillation (100 g/800 mL distilled water) for three hours using a Clevenger-type apparatus. The resulting essential oil samples were dehydrated using anhydrous sodium sulfate (Na2SO4) and stored in sealed amber vials at −20 °C until analysis.
2.3. Analysis of essential oils
Samples of essential oils were diluted 20-fold in hexane and analyzed using an HP 6890 (II) gas chromatograph (Agilent Technologies, Palo Alto, CA, USA) equipped with an HP-5 (30 m × 0.32 mm ID, 0.25 µm film thickness; Supelco, Bellefonte, PA, USA) capillary column. Operating conditions were as follows: The oven temperature was programmed at 5 °C/min from an initial temperature of 40 °C (maintained isothermally for 10 minutes) to 280 °C, which was held for an additional 10 minutes. Injector and FID detector temperature were maintained at 230 °C; the injection volume was 0.5 µL; split ratio of 1:20, and the flow rate of the carrier nitrogen gas was 1.2 mL/min.
For gas chromatography-mass spectrometry (GC-MS) analysis, an HP 6890 gas chromatograph coupled to an HP 5973 mass spectrometer (Agilent Technologies, Palo Alto, CA, USA) was used. The analytical conditions and the column used for individual component separation were the same as those used for GC-FID analysis, except helium was used as the carrier gas. The mass spectrometer operated in electron-impact (EI) mode; ionization energy was set at 72 eV; ion source temperature was maintained at 270 °C; scan time was 1 second, and the mass range scanned was 50–550 amu.
The identification of constituents was based on the comparison of their retention indices (RI) relative to (C7–C20) n-alkanes (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) with those from the literature (Dey et al., Citation2021) and/or with those of authentic standards when available. Identification was also performed by matching against the NIST05a MS library. The relative content of the identified components was determined through electronic integration of the FID-peak areas without correcting for response factors.
2.4. Antimicrobial activity
The antimicrobial activity of Eucalyptus spp. essential oils was evaluated qualitatively using the disc-diffusion assay described by the National Committee for Clinical Laboratory Standards (NCCLS, 1997). The test microorganisms included the Gram-positive bacteria S. aureus (ATCC 6538) and E. faecium (ATCC 19434), the Gram-negative bacteria E. coli (ATCC 8739) and S. Typhimurium (ATCC 14028), and the yeast C. albicans (ATCC 10231). All microorganisms were obtained from the culture collection center of the Institut National de Recherche et d’Analyse Physico-Chimique (INRAP, Sidi Thabet, Tunisia). Bacterial strains were cultured in sterile Mueller Hinton agar (MHA) medium and incubated at 37 °C for 24 h, while fungal strains were cultured in Sabouraud dextrose agar (SDA) at 30 °C for 48 h.
Briefly, 100 µL of microbial suspension comprising 1–2 × 108 CFU/mL of bacterial cells or 1–5 × 106 CFU/mL for yeast were spread onto petri plates containing MHA or SDA culture mediums, respectively. Sterile filter paper discs (6 mm in diameter) were impregnated with 10 µL of essential oil (10 mg/mL in DMSO) and placed on the inoculated plates, left to stand for 2 h at 4 °C before being incubated at 37 °C for 24 h for bacteria and 30 °C for 48 h for yeast. The diameter of the inhibition zone was accurately measured. DMSO (10 µL per filter paper disc) was used as negative control while, Gentamycin and nystatin (10 µg/mL) were used as positive controls for bacteria and yeast, respectively.
2.5. Statistical analysis
The Principal Component Analysis (PCA), hierarchical cluster analysis using Euclidean distance and unweighted group method, and the heatmap clustering based on the entire composition of essential oils were conducted to elucidate the inter-relationships among all species. Antimicrobial activity data were presented as mean ± SD of triplicates. All analyses were performed using the statistical R 2.14.1 packages (Wirtschaftsuniversität Wien, Vienna, Austria).
3 Results and discussion
3.1. Yields and chemical composition of essential oils
From the leaves, pale yellowish essential oils with average yields of 0.96%, 0.35%, 0.55%, 0.32%, and 0.14% (w/w) were obtained for E. astringens, E. camaldulensis, E. lehmannii, E. leucoxylon, and E. sideroxylon, respectively (). These values align with those reported for E. oleosa (Marzoug et al., Citation2011), E. camaldulensis, E. saligna (Barbosa et al., Citation2016), E. gomphocornuta, E. paniculata (Limam et al., Citation2020), E. bosistoana, E. mellidiora, E. odorata, and E. paniculata (Kouki et al., Citation2022). However, they are considerably lower than those observed in E. globulus, E. cinerea, E. citriodora (Barbosa et al., Citation2016), E. accedens, E. cladoalyx, E. lesouefi, E. melliodora, E. punctate, E. robusta, E. wandoo (Ameur et al., Citation2021), E. melliodora, and E. maidenii, among others (Limam et al., Citation2020). These discrepancies could be attributed to genetic factors, pedoclimatic conditions, season, plant age, processing, and extraction methodology. In our case, differences in essential oil yields were unequivocally attributed to genetic differences (species) as they were cultivated and processed under the same conditions.
Table 1. Chemical composition (% total peak area) of the leaf essential oil of Eucalyptus spp.
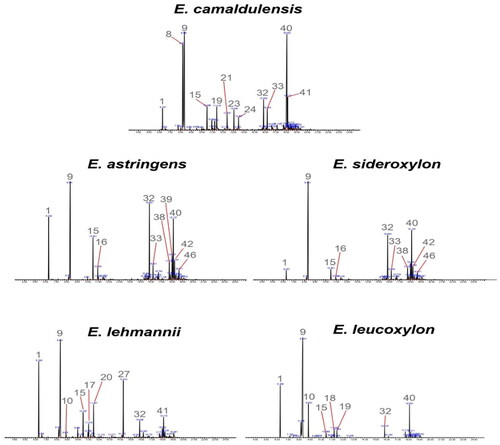
The chromatographic analysis identified 48 components covering more than 90% of the total peak area. Typical chromatographic profiles are shown in . Irrespective of the Eucalyptus species, all oil samples are terpenoid-rich essential oils (). Terpenoids (oxygenated mono- and sesquiterpenes) are particularly abundant in E. lehmannii (71.67%), E. leucoxylon (70.57%), and E. sideroxylon (76.74%).
The oxygenated monoterpene 1,8-cineole (eucalyptol) was by far the major component (22–51%) in all investigated essential oils. Therefore, the studied Eucalyptus species could be categorized as the 1,8-cineole chemotype. Other significant compounds, including aromadendrene, globulol, pinocarvone, and α-pinene, were identified in E. astringens. Aromadendrene and globulol were also detected in appreciable amounts in E. sideroxylon. In the essential oil of E. camaldulensis, spathulenol and p-cymene were abundant. E. lehmannii had the highest percentages of α-pinene, α-terpineol, and terpinyl acetate. The monoterpene hydrocarbons α-pinene, terpinolene, p-cymene, and the oxygenated sesquiterpene globulol were the most plentiful components in the essential oil of E. leucoxylon.
Compared with earlier compositional studies, the pattern of abundance of the main compounds has been reported in Eucalyptus species. For example, the profile 1,8-cineole > α-pinene has been previously described in Tunisian specimens of E. leucoxylon (Elaissi et al., Citation2012), E. lehmannii, and E. astringens (Limam et al., Citation2020). In contrast, the latter authors reported that spathulenol and o-cymene were dominants in the essential oil of E. camaldulensis. At this point, it can be inferred that this species represents different chemotypes. For instance, the p-cymene/1,8-cineole chemotype has been described in Turkish specimens of E. camaldulensis (Dogan et al., Citation2017).
Other chemotypes, including 1,8-cineole/p-cymene (Lucia et al., Citation2009); 1,8-cineole/limonene (Batista-Pereira et al., Citation2006); 1,8-cineole/α-pinene (Mediouni Ben Jemâa et al., Citation2013; Salem et al., Citation2016); α-phellandrene/β-pinene (Debbarma et al., Citation2013); linalool/1,8-cineole (Ghaffar et al., Citation2015); spathulenol/p-cymene (Verdeguer et al., Citation2009), and α-pinene/p-cymene (Chouhan et al., Citation2017), have been reported in E. camaldulensis specimens from Argentina, Brazil, Egypt, Tunisia, India, Pakistan, Spain, and Taiwan. The α-pinene/1,8-cineole chemotype has been recorded for Tunisian E. astringens (Hamdi et al., Citation2015) and E. leucoxylon (Ben Jemâa et al., Citation2012; Mediouni Ben Jemâa et al., Citation2013) specimens. Regarding E. sideroxylon from the same origin, the presence of at least two chemotypes, 1,8-cineole/globulol (in this study) and 1,8-cineole/α-pinene (Elaissi et al., Citation2012), may be confirmed. In contrast, it seems that the 1,8-cineole/α-pinene chemotype dominated the leaf essential oil of E. lehmannii species (Elaissi et al., Citation2012; Hamdi et al., Citation2015; Limam et al., Citation2020; Slimane et al., Citation2014). In general, it appears that the chemical composition of the essential oil of Eucalyptus spp. is particularly prone to qualitative and quantitative changes depending on genetic factors (species, subspecies, and cultivars), season, climate, soil type, and agronomic factors. Given their industrial importance as a source of essential oil, a better categorization of Eucalyptus species based on the definition of some distinctive specific markers will be of great importance for authentication purposes.
3.2. Specific markers and heatmap clustering
From the chemical composition of all essential oils, a list of chemically defined volatile markers has been established (). As shown, the presence of γ-maaliene and γ-gurjunene is characteristic of the essential oil of E. astringens. Fenchene, 4-carene, p-cymenene, 2-methyl-3-phenyl-propanal, phellandral, thymol, p-cymen-7-ol, and copaborneol distinguish the essential oil of E. camaldulensis.
Figure 2. Heatmap clustering using Euclidean distance and unweighted group method, and distinctive chemical markers of leaf essential oils of Eucalyptus spp. (+): presence; (−) absence.
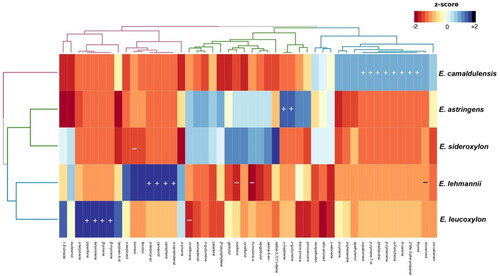
The presence of camphene, α-campholenal, carvacrol, α-terpinyl acetate, versus the absence of isocarveol, rosifoliol, and humulene epoxide II, distinguished the essential oil of E. Lehmannii from the remaining species. The essential oil of E. leucoxylon was characterized by the presence of β-pinene, β-myrcene, terpinolene, and piperitone, while being exempt from viridiflorene. The absence of borneol seems to be a characteristic of the essential oil of E. sideroxylon. From a practical standpoint, the mentioned chemical markers could provide baseline information for the quality assessment of the commercialized leaf essential oils of Eucalyptus species growing in the region of Korbous.
Additional analyses, including the heatmap clustering (with the highest percentage represented by intense blue color, while the lowest was indicated by light color) and hierarchical cluster analysis (HCA) based on Euclidean distance and the unweighted group method, allowed the separation of Eucalyptus spp. into three distinct groups: group 1—E. lehmannii and E. leucoxylon; group 2—E. astringens and E. sideroxylon; group 3—E. camaldulensis ().
3.3. Principal component analysis (PCA)
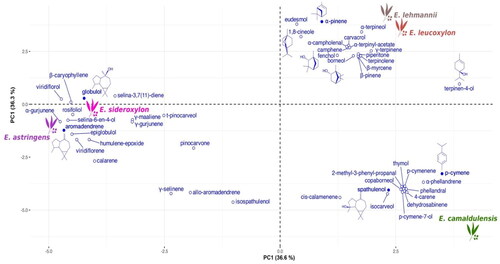
To validate the aforementioned classification, a PCA analysis () based on the entire volatile profile was conducted. The PCA biplot, explaining 73.3% of the total variance (with 36.6% and 36.3% for PC1 and PC2, respectively), distinctly reveals three groups. The first group unites E. lehmannii and E. leucoxylon, characterized by high levels of α-pinene, γ-terpinene, and α-terpineol. These components, along with other monoterpenes, showed positive loading on PC1.
The second group, represented by the essential oils of E. astringens and E. sideroxylon, exhibits similar profiles primarily consisting of sesquiterpenes, both oxygenated and hydrocarbons. These include aromadendrene, globulol, viridiflorene, viridiflorol, rosifoliol, selina-6-en-4-ol, humulene epoxide II, α-gurjunene, and β-caryophyllene, all of which were negatively loaded on PC1. E. camaldulensis is distinctly separated from other species due to its elevated content of spathulenol, cis-calamenene, isocarveol, and p-cymene, in addition to the previously mentioned marker components.
Considering that all Eucalyptus species are of the same age and cultivated and processed under identical conditions (i.e. collection of leaves, drying, extraction of essential oils, and their analysis), the genetic dissimilarity between Eucalyptus spp. is reaffirmed based on their essential oil composition.
Given that the bioactivity of an essential oil is primarily determined by its chemical composition, it will be highly significant to evaluate the antimicrobial activity of Eucalyptus spp.
3.4. Antimicrobial activity of Eucalyptus spp. Essential oils
The results of the antimicrobial activity of the five Eucalyptus spp. essential oils are summarized in . All essential oils strongly inhibited the growth of the tested strains, with the Gram-positive strains S. aureus (inhibition zone diameter: 25–35 mm) and E. faecium (inhibition zone diameter: 29.5–34.5 mm) being the most sensitive. They were notably inhibited by the essential oils of E. camaldulensis, E. lehmannii, and E. sideroxylon. Additionally, the essential oils from E. camaldulensis and E. leucoxylon were particularly effective against the Gram-negative bacteria E. coli and S. typhimurium. The former essential oil (E. camaldulensis) also demonstrated high efficiency against the yeast C. albicans, with an inhibition halo similar to that of the standard antibiotic nystatin.
Table 2. Antimicrobial activity (expressed as zone of inhibition in mm) of the leaf essential oils of Eucalyptus spp.
These results align with previous reports showcasing the potent antimicrobial activity of Eucalyptus essential oils against gram-positive bacterial strains, especially S. aureus, and the yeast C. albicans (Barbosa et al., Citation2016). The sensitivity of Gram-positive bacteria is attributed to the presence of a thick peptidoglycan wall associated with the lipophilic ends of lipoteichoic acid, facilitating the entry of hydrophobic components into the cell membrane (Chouhan et al., Citation2017). Numerous studies have linked the antimicrobial activity of Eucalyptus essential oils to their main components. For instance, it has been reported that the essential oil of E. camaldulensis strongly inhibits the growth of the Gram-positive S. aureus and Bacillus cereus (Dogan et al., Citation2017). E. camaldulensis essential oil, described as the most active among Eucalyptus species, has also been found effective against the yeast C. albicans (Aleksic Sabo & Knezevic, Citation2019), supporting our findings. Similar results have been reported for essential oils derived from E. sideroxylon (Ashour, Citation2008), E. astringens, E. lehmannii (Limam et al., Citation2020), and E. leucoxylon (Elaissi et al., Citation2012), among others.
Direct evidence of the antimicrobial activity of the main components of Eucalyptus essential oils has also been provided. Particularly active compounds include 1,8-cineole (Wang et al., Citation2022), α-pinene (Dhar et al., Citation2014), terpinyl acetate (Badr et al., Citation2021; Fidan et al., Citation2019), α-terpineol (Li et al., Citation2014), globulol (Tan et al., Citation2008), aromadendrene (Mulyaningsih et al., Citation2010), p-cymene (Marchese et al., Citation2017), spathulenol (Dzul-Beh et al., Citation2019), trans-pinocarveol (Viljoen et al., Citation2002), and terpinen-4-ol (Cordeiro et al., Citation2020), among others. The synergistic and additive effects of these compounds have been described for the essential oil of E. globulus (Mulyaningsih et al., Citation2010). In their checkerboard assay, the study authors successfully demonstrated that the combination of 1,8-cineole and aromadendrene greatly enhanced the antimicrobial effect against methicillin-resistant S. aureus (MRSA) and vancomycin-resistant E. faecalis through additive or synergistic interactions. Four years later, Johansen et al. (Citation2022) showed that combinations involving p-cymene, terpinen-4-ol, α-terpineol, and linalool exhibited an additive antibacterial effect against some food-borne pathogens, including E. coli O157:H7, S. aureus, S. mutans, S. sanguinis, S. enterica, Listeria monocytogenes, and Vibrio parahaemolyticus. More recently, it has been demonstrated that the combination of terpinen-4-ol and α-terpineol synergistically inhibits the growth of S. aureus, MRSA, E. coli, and Pseudomonas aeruginosa (Johansen et al., Citation2022).
From a mechanistic standpoint, the identified components (either individually or in combination) could exert their antimicrobial activity by interfering with the lipophilic core of the membrane, leading to increased fluidity, and ultimately causing the leakage of vital macromolecules (e.g. nucleic acids and proteins), potassium ions, and protons (Zomorodian et al., Citation2017). Other mechanisms of action include the alteration of fatty acid composition, impairment of metabolic pathways, inhibition of the cellular respiratory chain with a concomitant interruption of oxidative phosphorylation, a decrease in ATP pool, interference with glucose and oxygen uptake, denaturation of cell proteins, disruption of nucleic acid synthesis, installation of oxidative stress, and inhibition of enzyme activity (Angane et al., Citation2022; Dhar et al., Citation2014; Li et al., Citation2014; Melkina et al., Citation2021; Xiang et al., Citation2018). The disruption of biofilm formation and basic bacterial metabolism have been proposed as the main mechanisms underlying the antibacterial activities of 1,8-cineole-rich essential oils of E. bicostata, E. gigantea, E. intertexta, E. obliqua, E. pauciflora, and E. tereticornis against S. aureus, L. monocytogens, acinetobacter baumannii, Pseudomonas aeruginosa and E. coli (Polito et al., Citation2022).
Although, the exact mechanism of the antimicrobial effect of Eucalyptus essential oils is not fully understood, the implication of one or more of the mechanisms mentioned above could explain the strong antimicrobial activity of the studied Eucalyptus species. In any case, these data provide evidence for the current use of their essential oils as a natural antiseptic and food preservative.
4. Conclusions
The compositional analysis of the leaf essential oils of Eucalyptus spp. revealed significant chemical polymorphism, primarily determined by genetic factors (species effect). Essential oils rich in 1,8-cineole were categorized using distinctive chemical markers, enabling differentiation between various Eucalyptus spp. and their corresponding essential oils extracted under the same conditions. This chemical identification method can serve as a means to determine the specific Eucalyptus species of origin for the oils.
The studied oils exhibited robust antimicrobial activity, likely attributed to their high 1,8-cineole contents and/or other potential compounds acting synergistically or additively. Based on these findings, it is suggested that the essential oils from the studied Eucalyptus spp. could serve as candidates for natural flavors and preservatives in food/feed, cosmetic, pharmaceutical, agrochemical, and household applications, particularly for highly perishable items and products susceptible to microbial contamination. Further studies exploring additional activities of Eucalyptus essential oils and providing details on the mechanisms of their actions should be conducted and reported.
Author contributions
Conceptualization, H.A. and K.H.; methodology, H.A., Y.M. and K.H.; software, K.H.; validation, H.A., S.L. and K.H.; formal analysis, H.A., Y.M. and K.H.; investigation, H.A., Y.M., S.H., W.S., S.A., and K.H.; resources, H.A., S.H. and K.H.; data curation, H.A. and K.H.; writing—original draft preparation, H.A. and K.H.; writing—review and editing, H.A., S.H., M.C., M.d.H.-M., W.S., S.A., S.L. and K.H.; visualization, H.A., S.H., M.C., M.d.H.-M., S.A., S.L. and K.H.; supervision, H.A., M.C., M.d.H.-M. and K.H., project administration, H.A., W.S. and K.H.; funding acquisition, W.S. All authors have read and agreed to the published version of the manuscript.
Informed consent statement
Not applicable.
Institutional review board statement
Not applicable.
Acknowledgements
Authors are thankful to the International Center for Agricultural Research in the Dry Areas (ICARDA) and the Livestock and Climate CGIAR Initiatives of the OneCGIAR. The opinions expressed in this work do not necessarily reflect the views of ICAR, ICARDA, or the One CGIAR. The authors extend their appreciation to Researchers Supporting Project number (RSP2024R390), King Saud University, Riyadh, Saudi Arabia.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data presented in this study are available on request from the corresponding author.
Additional information
Funding
Notes on contributors
Hajer Ammar
Hajer Ammar PhD in Animal Production Science, Nutrition, from the University of Leon (Spain). Her researches are focused on ruminant nutrition, feed staff evaluation and innovative staregies to reduce methane emissions. She is a membership (expert) in Technical Advisor Group (TAG) Circular bioeconomy & Ecosystem services (FAO Livestock Environmental Assessment and Performance).
Yassine M’Rabet
Yassine M’Rabet PhD at the Institut National de Recherche et d’Analyse Physico-Chimiques, Sidi Thabet (Tunisia). His researches are focused on oil analysis, phytochemical analysis, secondary metabolites and their bioactive molecules and biological activities, analytical HPLC, R Programming, python scripting, GNU/Linux and multivariate analysis methods.
Sawsan Hassan
Sawsan Hassan She is Ph.D. in Cactus Pear Agronomy from the University of Palermo, Italy. She is an accomplished forage agronomist at The International Center for Agricultural Research in the Dry Areas with 15 years of experience in forage systems and their impact on livestock production in arid and semi-arid regions of West Asia, South Asia, and North Africa. Her expertise lies in cactus pear and forage crop agronomy and management, with a strong background in field-based research, quantitative analysis, and statistics. Additionally, she possesses advanced proficiency in data management and analysis, backed by an impressive publication record of over 100 scientific works, including journal articles, conference papers, scientific reports, blogs, databases, and manuals.
Mireille Chahine
Mireille Chahine is a Professor and Extension Dairy Specialist in the Animal, Veterinary and Food Sciences Department at the University of Idaho. She is stationed and works out of the Twin Falls Research & Extension Center in Twin Falls, Idaho. Originally from Lebanon, Dr. Chahine received a B.S.degree in agricultural engineering from the Lebanese University, a specialized post-graduate degree in Animal Sciences from the International Center for Agronomic Studies in Zaragoza Spain, a M.S. in Animal Sciences from the Autonomous University of Barcelona, Spain and a Ph.D. degree in Animal Sciences with an emphasis on Dairy Science from the University of Minnesota. Dr. Chahine coordinates dairy Extension programming throughout Idaho and serves as a resource for stakeholders including dairy producers, dairy employees, dairy managers, allied industries representatives, veterinarians, Extension educators and the general public. In addition to Extension, Dr. Chahine, conducts on-farm/applied research addressing applied dairy management, nutrition and environmental issues. She is a member of NC-2042 a multi-state research group titled “Management Systems to Improve the Economic and Environmental Sustainability of Dairy Enterprises”. Dr. Chahine has published more than 65 peer-reviewed articles and has given more than 300 presentations nationally and internationally.
Mario de Haro-Marti
Mario de Haro-Marti PhD is a professor and Extension educator in Gooding County. He provides multi-county environmental management education and applied research in his areas of specialization, including livestock waste and nutrient management, air and water quality, livestock production sustainability, agricultural pollution prevention, dairy production and bilingual training of the dairy workforce. Mario’s applied research and education programs include dairy composting improvement, vermicomposting, reduction of air emissions from livestock operations, nutrient and manure management, and helping livestock producers to increase production efficiencies while reducing their environmental impact. He has worked with the University of Idaho Extension since summer 2007.
Walid Soufan
Walid Soufan Ph.D. degree in Crop Science from Göttingen University-Germany (2008). Currently he is faculty member (Associate Professor) in Plant Production Department, Collage of Food and Agricultural Sciences, King Saud University. He is the author of more than 113 article in ISI journals. His research interests revolve around the production and physiology of crops and modern trends in the production of fodder crops, crop production in the hydroponic system, biotic and abiotic stresses on crops, organic agriculture and environmental changes and their impact on agriculture and plants.
Sonia Andres
Sonia Andres PhD degree in Animal Production Science from the University of Leon (Spain). Actually she is a researcher in the University of Leon “Consejo Superior de Investigaciones Científicas (CSIC), Instituto de Ganadería de Montaña”. Her researches are focused on ruminant nutrition, Food science and chemistry, meat and milk quality. She is a member in different European projects EU-PRIMA MilkQua; EU-PRIMA MedGoat; EU-Erasmus+ INNOMEATEDU project).
Secundino López
Secundino López PhD degree in Animal Production Science from the University of Leon (Spain). The main research topics are ruminant nutrition and feeding, feed chemistry, development of mathematical models for feed evaluation. Actually he is professor of animal nutrition in the department of Animal Production (University of León, Spain). His teaching courses are related to animal nutrition and feed science at the Faculty of Veterinary and the School of Agronomy.
Karim Hosni
Karim Hosni is PhD in Biological from the University of Tunis El Manar. Actually he is professor at the National Institute for Research and Physico-Chemical Analysis. His researches are focused on the development of functional foods, valorization of food by-products and the application of green and sustainable extraction methods for the recovery of highly valuable chemicals from residuals matrices. He authored and co-authored more than 110 articles and 10 chapter books. Actually, He serves as associated editor in Frontiers in Pharmacology.
References
- Aleksic Sabo, V., & Knezevic, P. (2019). Antimicrobial activity of Eucalyptus camaldulensis Dehn. plant extracts and essential oils: A review. Industrial Crops and Products, 132, 413–429. https://doi.org/10.1016/j.indcrop.2019.02.051
- Ameur, E., Sarra, M., Yosra, D., Mariem, K., Nabil, A., Lynen, F., & Larbi, K. M. (2021). Chemical composition of essential oils of eight Tunisian Eucalyptus species and their antibacterial activity against strains responsible for otitis. BMC Complementary Medicine and Therapies, 21(1), 209. https://doi.org/10.1186/s12906-021-03379-y
- Angane, M., Swift, S., Huang, K., Butts, C. A., & Quek, S. Y. (2022). Essential oils and their major components: An updated review on antimicrobial activities, mechanism of action and their potential application in the food industry. Foods (Basel, Switzerland), 11(3), 464. https://doi.org/10.3390/foods11030464
- Ashour, H. M. (2008). Antibacterial, antifungal, and anticancer activities of volatile oils and extracts from stems, leaves, and flowers of Eucalyptus sideroxylon and Eucalyptus torquata. Cancer Biology & Therapy, 7(3), 399–403. https://doi.org/10.4161/cbt.7.3.5367
- Badr, M. M., Badawy, M. E. I., & Taktak, N. E. M. (2021). Characterization, antimicrobial activity, and antioxidant activity of the nanoemulsions of Lavandula spica essential oil and its main monoterpenes. Journal of Drug Delivery Science and Technology, 65, 102732. https://doi.org/10.1016/j.jddst.2021.102732
- Barbosa, L. C. A., Filomeno, C. A., & Teixeira, R. R. (2016). Chemical variability and biological activities of Eucalyptus spp. essential oils. Molecules (Basel, Switzerland), 21(12), 1671. https://doi.org/10.3390/molecules21121671
- Batish, D. R., Singh, H. P., Kohli, R. K., & Kaur, S. (2008). Eucalyptus essential oil as a natural pesticide. Forest Ecology and Management, 256(12), 2166–2174. https://doi.org/10.1016/j.foreco.2008.08.008
- Batista-Pereira, L. G., Fernandes, J. B., da Silva, M. F. G. E., Vieira, P. C., Bueno, O. C., & Corrêa, A. G. (2006). Electrophysiological responses of Atta sexdens rubropilosa workers to EOs of Eucalyptus and its chemical composition. Zeitschrift Fur Naturforschung. C, Journal of Biosciences, 61(9–10), 749–755. https://doi.org/10.1515/znc-2006-9-1023
- Beauchemin, K. A., Ungerfeld, E. M., Abdalla, A. L., Alvarez, C., Arndt, C., Becquet, P., Benchaar, C., Berndt, A., Mauricio, R. M., McAllister, T. A., Oyhantçabal, W., Salami, S. A., Shalloo, L., Sun, Y., Tricarico, J., Uwizeye, A., De Camillis, C., Bernoux, M., Robinson, T., & Kebreab, E. (2022). Current enteric methane mitigation options. Journal of Dairy Science, 105, 9297–9326.
- Ben Jemâa, J. M., Haouel, S., Bouaziz, M., & Khouja, M. L. (2012). Seasonal variations in chemical composition and fumigant activity of five Eucalyptus EOs against three moth pests of stored dates in Tunisia. Journal of Stored Products Research, 48, 61–67. https://doi.org/10.1016/j.jspr.2011.10.001
- Caputo, L., Smeriglio, A., Trombetta, D., Cornara, L., Trevena, G., Valussi, M., Fratianni, F., De Feo, V., & Nazzaro, F. (2020). Chemical composition and biological activities of the essential oils of Leptospermum petersonii and Eucalyptus gunnii. Frontiers in Microbiology, 11, 409. https://doi.org/10.3389/fmicb.2020.00409
- Chouhan, S., Sharma, K., & Guleria, S. (2017). Antimicrobial activity of some essential oils—Present status and future perspectives. Medicines, 4(3), 58. https://doi.org/10.3390/medicines4030058
- Cordeiro, L., Figueiredo, P., Souza, H., Sousa, A., Andrade-Júnior, F., Medeiros, D., Nóbrega, J., Silva, D., Martins, E., Barbosa-Filho, J., & Lima, E. (2020). Terpinen-4-ol as an antibacterial and antibiofilm agent against Staphylococcus aureus. International Journal of Molecular Sciences, 21(12), 4531. https://doi.org/10.3390/ijms21124531
- Debbarma, J., Kishore, P., Nayak, B. B., Kannuchamy, N., & Gudipati, V. (2013). Antibacterial activity of ginger, Eucalyptus and sweet orange peel EOs on fish-borne bacteria. Journal of Food Processing and Preservation, 37(5), 1022–1030. https://doi.org/10.1111/j.1745-4549.2012.00753.x
- Dey, A., Attri, K., Dahiya, S. S., & Paul, S. S. (2021). Influence of dietary phytogenic feed additives on lactation performance, methane emissions and health status of Murrah buffaloes (Bubalus bubalis). Journal of the Science of Food and Agriculture, 101(10), 4390–4397. https://doi.org/10.1002/jsfa.11080
- Dhar, P., Chan, P., Cohen, D. T., Khawam, F., Gibbons, S., Snyder-Leiby, T., Dickstein, E., Rai, P. K., & Watal, G. (2014). Synthesis, antimicrobial evaluation, and structure-activity relationship of alpha-pinene derivatives. Journal of Agricultural and Food Chemistry, 62(16), 3548–3552. https://doi.org/10.1021/jf403586t
- Dogan, G., Kara, N., Bagci, E., & Gur, S. (2017). Chemical composition and biological activities of leaf and fruit essential oils from Eucalyptus camaldulensis. Zeitschrift Fur Naturforschung. C, Journal of Biosciences, 72(11–12), 483–489. https://doi.org/10.1515/znc-2016-0033
- Dzul-Beh, A., García-Sosa, K., Uc-Cachón, A. H., Bórquez, J., Loyola, L. A., Barrios-García, H. B., Peña-Rodríguez, L. M., & Molina-Salinas, G. M. (2019). In vitro growth inhibition and bactericidal activity of spathulenol against drug-resistant clinical isolates of Mycobacterium tuberculosis. Revista Brasileira de Farmacognosia, 29(6), 798–800. https://doi.org/10.1016/j.bjp.2019.06.001
- Elaieb, M. T., Ayed, S. B., Ouellani, S., Khouja, M. L., Touhami, I., & Candelier, K. (2019). Collapse and physical properties of native and pre-steamed Eucalyptus camaldulensis and Eucalyptus saligna wood from Tunisia. Journal of Tropical Forest Science, 31(2), 162–174. https://doi.org/10.26525/jtfs2019.31.2.162174
- Elaissi, A., Rouis, Z., Salem, N. A. B., Mabrouk, S., Ben Salem, Y., Salah, K. B. H., Aouni, M., Farhat, F., Chemli, R., & Harzallah-Skhiri, F. (2012). Chemical composition of 8 Eucalyptus species’ essential oils and the evaluation of their antibacterial, antifungal and antiviral activities. BMC Complementary and Alternative Medicine, 12, 1–15.
- Fidan, H., Stefanova, G., Kostova, I., Stankov, S., Damyanova, S., Stoyanova, A., & Zheljazkov, V. D. (2019). Chemical composition and antimicrobial activity of Laurus nobilis L. essential oils from Bulgaria. Molecules (Basel, Switzerland), 24(4), 804. https://doi.org/10.3390/molecules24040804
- Ghaffar, A., Yameen, M., Kiran, S., Kamal, S., Jalal, F., Munir, B., Saleem, S., Rafiq, N., Ahmad, A., Saba, I., & Jabbar, A. (2015). Chemical composition and in-vitro evaluation of the antimicrobial and antioxidant activities of essential oils extracted from seven Eucalyptus species. Molecules (Basel, Switzerland), 20(11), 20487–20498. https://doi.org/10.3390/molecules201119706
- Goldbeck, J. C., do Nascimento, J. E., Jacob, R. G., Fiorentini, Â. M., & da Silva, W. P. (2014). Bioactivity of essential oils from Eucalyptus globulus and Eucalyptus urograndis against planktonic cells and biofilms of Streptococcus mutans. Industrial Crops and Products, 60, 304–309. https://doi.org/10.1016/j.indcrop.2014.05.030
- Hamdi, S. H., Hedjal-Chebheb, M., Kellouche, A., Khouja, M. L., Boudabous, A., & Ben Jemâa, J. M. (2015). Management of three pests’ population strains from Tunisia and Algeria using Eucalyptus essential oils. Industrial Crops and Products, 74, 551–556. https://doi.org/10.1016/j.indcrop.2015.05.072
- Horst, E. H., Ammar, H., Ben Rhouma, R., Khouja, M., Khouja, M. L., Giráldez, F. J., & López, S. (2022a). Seasonal and species variations in the nutritive value of Eucalyptus foliage as a potential feed resource for ruminants in silvopastoral systems. Agroforestry Systems, 96(8), 1189–1198. https://doi.org/10.1007/s10457-022-00777-0
- Horst, E. H., Ammar, H., Khouja, M. L., Vargas, J. E., Andrés, S., & López, S. (2022b). In vitro screening of the foliage of Eucalyptus species harvested in different seasons for modulating rumen fermentation and methane production. Agriculture, 12(12), 2153. https://doi.org/10.3390/agriculture12122153
- Johansen, B., Duval, R. E., & Sergere, J. C. (2022). First evidence of a combination of terpinen-4-ol and α-terpineol as a promising tool against ESKAPE pathogens. Molecules (Basel, Switzerland), 27(21), 7472. https://doi.org/10.3390/molecules27217472
- Kouki, H., Polito, F., De Martino, L., Mabrouk, Y., Hamrouni, L., Amri, I., Fratianni, F., De Feo, V., & Nazzaro, F. (2022). Chemistry and bioactivities of six Tunisian Eucalyptus species. Pharmaceuticals, 15(10), 1265. https://doi.org/10.3390/ph15101265
- Li, L., Shi, C., Yin, Z., Jia, R., Peng, L., Kang, S., & Li, Z. (2014). Antibacterial activity of α-terpineol may induce morphostructural alterations in Escherichia coli. Brazilian Journal of Microbiology: [Publication of the Brazilian Society for Microbiology], 45(4), 1409–1413. https://doi.org/10.1590/s1517-83822014000400035
- Limam, H., Ben Jemaa, M., Tammar, S., Ksibi, N., Khammassi, S., Jallouli, S., Del Re, G., & Msaada, K. (2020). Variation in chemical profile of leaves essential oils from thirteen Tunisian Eucalyptus species and evaluation of their antioxidant and antibacterial properties. Industrial Crops and Products, 158, 112964. https://doi.org/10.1016/j.indcrop.2020.112964
- Lucia, A., Licastro, S., Zerba, E., Gonzalez, A. P., & Masuh, H. (2009). Sensitivity of Aedes aegypti adults (Diptera: Culicidae) to the vapors of Eucalyptus EOs. Bioresource Technology, 100(23), 6083–6087. https://doi.org/10.1016/j.biortech.2009.02.075
- Marchese, A., Arciola, C., Barbieri, R., Silva, A., Nabavi, S., Tsetegho Sokeng, A., Izadi, M., Jafari, N., Suntar, I., Daglia, M., & Nabavi, S. (2017). Update on monoterpenes as antimicrobial agents: A particular focus on p-cymene. Materials, 10(8), 947. https://doi.org/10.3390/ma10080947
- Marzoug, H. N. B., Romdhane, M., Lebrihi, A., Mathieu, F., Couderc, F., Abderraba, M., Khouja, M. L., & Bouajila, J. (2011). Eucalyptus oleosa essential oils: Chemical composition and antimicrobial and antioxidant activities of the oils from different plant parts (stems, leaves, flowers and fruits). Molecules (Basel, Switzerland), 16(2), 1695–1709. https://doi.org/10.3390/molecules16021695
- Mediouni Ben Jemâa, J., Haouel, S., & Khouja, M. L. (2013). Efficacy of Eucalyptus EOs fumigant control against Ectomyelois ceratoniae (Lepidoptera: Pyralidae) under various space occupation conditions. Journal of Stored Products Research, 53, 67–71. https://doi.org/10.1016/j.jspr.2013.02.007
- Melkina, O. E., Plyuta, V. A., Khmel, I. A., & Zavilgelsky, G. B. (2021). The mode of action of cyclic monoterpenees (−)-limoneneand (+)-α-pinene on bacterial cells. BioMolecules, 11(6), 806. https://doi.org/10.3390/biom11060806
- Mossi, A. J., Astolfi, V., Kubiak, G., Lerin, L., Zanella, C., Toniazzo, G., Oliveira, D. d., Treichel, H., Devilla, I. A., Cansian, R., & Restello, R. (2011). Insecticidal and repellency activity of essential oil of Eucalyptus sp. against Sitophilus zeamais Motschulsky (Coleoptera, Curculionidae). Journal of the Science of Food and Agriculture, 91(2), 273–277. https://doi.org/10.1002/jsfa.4181
- Mulyaningsih, S., Sporer, F., Zimmermann, S., Reichling, J., & Wink, M. (2010). Synergistic properties of the terpenoids aromadendrene and 1,8-cineole from the essential oil of Eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology, 17(13), 1061–1066. https://doi.org/10.1016/j.phymed.2010.06.018
- Polito, F., Kouki, H., Khedhri, S., Hamrouni, L., Mabrouk, Y., Amri, S., Nazzaro, F., Fratianni, F., & De Feo, V. (2022). Chemical composition and phytotoxic and antibiofilm activity of the essential oils of E. bicostata, E. gigantea, E. intertexta, E. obliqua, E. pauciflora, and E. tereticornis. Plants, 11(22), 3017. https://doi.org/10.3390/plants11223017
- Salem, M. Z. M., Zidan, Y. E., Mansour, M. M. A., El Hadidi, N. M. N., & Abo Elgat, W. A. A. (2016). Antifungal activities of two essential oils used in the treatment of three commercial woods deteriorated by five common mold fungi. International Biodeterioration & Biodegradation, 106, 88–96. https://doi.org/10.1016/j.ibiod.2015.10.010
- Slimane, B. B., Ezzine, O., Dhahri, S., & Jamaa, M. L. B. (2014). Essential oils from two Eucalyptus from Tunisia and their insecticidal action on Orgyia trigotephras (Lepidotera, Lymantriidae). Biological Research, 47(1), 29. https://doi.org/10.1186/0717-6287-47-29
- Tan, M., Zhou, L., Huang, Y., Wang, Y., Hao, X., & Wang, J. (2008). Antimicrobial activity of globulol isolated from the fruits of Eucalyptus globulus labill. Natural Product Research, 22(7), 569–575. https://doi.org/10.1080/14786410701592745
- Verdeguer, M., Blázquez, M. A., & Boira, H. (2009). Phytotoxic effects of Lantana camara, Eucalyptus camaldulensis and Eriocephalus africanus EOs in weeds of Mediterranean summer crops. Biochemical Systematics and Ecology, 37(4), 362–369. https://doi.org/10.1016/j.bse.2009.06.003
- Viljoen, A. M., Klepser, M. E., Ernst, E. J., Keele, D., Roling, E., Van Vuuren, S., Demirci, B., Baser, K. F. C., van Wyk, B.-E., & Jäger, A. K. (2002). The composition and antimicrobial activity of the essential oil of the resurrection plant Myrothamnus flabellifolius. South African Journal of Botany, 68(1), 100–105. https://doi.org/10.1016/S0254-6299(15)30450-6
- Wang, Y., Zhang, Y., Song, X., Fang, C., Xing, R., Liu, L., Zhao, X., Zou, Y., Li, L., Jia, R., Ye, G., Shi, F., Zhou, X., Zhang, Y., Wan, H., Wei, Q., & Yin, Z. (2022). 1,8-Cineole inhibits biofilm formation and bacterial pathogenicity by suppressing luxS gene expression in Escherichia coli. Frontiers in Pharmacology, 13, 988245. https://doi.org/10.3389/fphar.2022.988245
- Xiang, F., Bai, J., Tan, X., Chen, T., Yang, W., & He, F. (2018). Antimicrobial activities and mechanism of the essential oil from Artemisia argyi Levl. et Van. var. argyi cv. Qiai. Industrial Crops and Products, 125, 582–587. https://doi.org/10.1016/j.indcrop.2018.09.048
- Yangui, I., Zouaoui Boutiti, M., Boussaid, M., & Messaoud, C. (2017). Essential oils of Myrtaceae species growing wild in Tunisia: Chemical variability and antifungal activity against Biscogniauxia mediterranea, the causative agent of charcoal canker. Chemistry & Biodiversity, 14(7), e1700058. https://doi.org/10.1002/cbdv.201700058
- Zomorodian, K., Moein, M., Pakshir, K., Karami, F., & Sabahi, Z. (2017). Chemical composition and antimicrobial activities of the essential oil from Salvia mirzayanii leaves. Journal of Evidence-Based Complementary & Alternative Medicine, 22(4), 770–776. https://doi.org/10.1177/2156587217717414