?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study was carried out to investigate the ability of microalgae Synechocystis sp. to high molecular weight Polycyclic Aromatic Hydrocarbon pyrene (PYR) and artificial microalgal–bacterial consortium at different concentrations. The consortium consisted of one axenic species Synechocystis sp. and two PYR-degrading bacteria with known complementary degradative capabilities viz. Pseudomonas sp. and Bacillus sp. The influence of PYR on growth in terms of chlorophyll-a were analysed, and it was found that in the presence of bacteria, Synechocystis sp. tremendously increased in growth as well as biodegradation capability, whereas Synechocystis sp. alone exhibited concentration-dependent decrease in growth and biodegradation ability. Degradation of PYR shows that the consortium could eliminate PYR by 94.1% at 50 mg/L; however, Synechocystis sp alone could degrade up to 36% at 1.5 mg/L after 16 days of incubation. The study revealed that microalgae grew better in the presence of the aerobic heterotrophic bacteria and provided them with necessary organics for efficient PYR degradation activities. Moreover, consortium JP-NKA7B2 grows efficiently on other xenobiotic compounds. The artificial consortia JP-NK is thus proven to be an effective and promising system for bioremediating PYR compound and could be suggested in degradation of PYR compound in hydrocarbon-polluted areas in situ and ex situ.
Public Interest Statement
Polycyclic aromatic hydrocarbons (PAHs) are widespread in nature soil, water and sediments because of several polluting sources through anthropogenic activities. They have been recognized as a potential health risk due to their intrinsic chemical stability, high recalcitrance to different types of degradation and high toxicity to living organisms. The fate of these compounds in the environment and the remediation of PAHs contaminated sites are, therefore, of high public interest. Microalgae play an important role in the environmental cleaning of pollutants; it provides O2 to aerobic bacteria to mineralize pollutants and then takeup the released CO2. Thus, increase in the growth of microalgae parallely increases biodegradation and detoxication capacity. Hence, in the present investigation, we have developed an artificial microalgal–bacterial consortium, which was proved to be potentially increasing the degradation capacity of model PAHs compound pyrene.
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) are widespread in nature (i.e. soil, water and sediments) because of several polluting anthropogenic activities (Samanta, Singh, & Jain, Citation2002). They have been recognized as a potential health risk due to their intrinsic chemical stability, high recalcitrance to different types of degradation and high toxicity to living organisms. The fate of these compounds in the environment and the remediation of PAH-contaminated sites are, therefore, of high public interest. It is essential to remove PAH compounds from the environment quickly and effectively, thus minimizing their adverse effects. The principal processes for their successful removal are currently believed to be microbial transformation and degradation (Gibson, Mahadevan, Jerina, Yogi, & Yeh, Citation1975). The extensive use of petroleum and petroleum products leads to serious PAHs contamination of the environment, thus considerable investigation have been carried out for biotransformation and detoxification of these pollutants by microbes and their consortium.
Microalgae play an important role in the environmental cleaning of pollutants. Microalgae provide O2 to aerobic bacteria to mineralize pollutants and then take up the released CO2 (de Godos et al., Citation2014). Furthermore, microalgae can help in co-metabolic degradation or produce biosurfactants and extracellular matters to enhance bacterial activity for increasing pollutant bioavailability (Muñoz, Guieysse, & Mattiasson, Citation2003). Additionally, microalgae can accumulate hydrocarbons, and subsequently make those compounds available to the associated hydrocarbon-utilizing bacteria (Radwan, Al-Hasan, Ali, Salamah, & Khanafer, Citation2005). The cooperation of phototrophic microalgae and heterotrophic bacteria could potentially improve the degradation of environmental contaminants, including oil pollutants (Chaillan, Gugger, Saliot, Couté, & Oudot, Citation2006; Safonova, Dmitrieva, & Kvitko, Citation1999).
PYR is a four-ring containing PAH, also one of most common PAHs in various environments. The toxicities of PYR toward a wide range of organisms, including mussels (Okay, Tolun, Tüfekçi, Telli-Karakoç, & Donkin, Citation2006), fish (Zapata-Pérez, Gold-Bouchot, Ortega, López, & Albores, Citation2002), copepods (Jensen, Nielsen, & Dahllof, Citation2008), macrophytes (Yin, Wang, Sun, Guo, & Yin, Citation2008), eukaryotic algae (Djomo et al., Citation2004; Lei, Hu, Wong, & Tam, Citation2006) as well as from microalgae species (Nirmal Kumar, Patel, Kumar, & Khan, Citation2014) have been studied. However, there is little information available about the effects of PYR on artificially constructed microalgae and bacterial consortium, which are one of most important prokaryotic organisms in aquatic ecosystem. Therefore, the present study was focused on the degradation process by the artificial microalgal–bacterial consortium.
2. Materials and methods
2.1. Media and chemicals
Bushnell Haas medium (BHM; Hi-Media, Mumbai, India) supplemented with PYR was used for the enrichment of PYR-degrading bacteria. PYR and other PAHs were procured from Sigma-Aldrich (Steinheim, Germany) (98% purity). Other chemicals used in the study were of analytical grade, such as Hi-media (Mumbai, India) and Merck (Darmstadt Hesse, Germany).
2.2. Soil sample collection for bacterial isolation
The soil samples were collected from the sampling point of common industrial effluent canal of Ankleshwar GIDC (site-A) and from ONGC pipeline leakage site near Bhadbhut (site B), Gujarat (India), where soil, sediment and water in the area have been contaminated with PAHs for many years. Subsurface soil (from 0 to 15 cm in depth) were collected and kept in ice or at 4°C until further analysis.
2.3. Enrichment and isolation and selection of potential strains of bacteria
Soil (10 g) was inoculated in 100 mL BHM amended with 50 mg/L PYR as sole carbon source and incubated at 37°C under shaking conditions (150 rpm). After one week, 10% of this culture was used as inoculum in fresh BHM amended with PYR. Repeated transfers were carried out in fresh BHM amended with PYR till a stable PYR-degrading bacteria was obtained showing consistent growth and PYR tolerance. The appropriate dilutions of the enriched consortium were spread on BHM agar plate and incubated at 37°C for bacterial growth. Based on grams staining, biochemical and morphological characteristics, 16 colonies were isolated. In order to select potential and efficient degrading strains, from amongst the 16 different isolated bacteria, a single colony was transferred to 50 mL of BHM media supplemented with 25–200 mg/L of PYR. The treated cultures were incubated at 37°C and 150 rpm shaking condition for 48 h. Bacterial growth was monitored in a UV–visible spectrophotometer by measuring absorbance at A600 nm.
2.4. PYR degradation by selected bacteria
Further, extraction of the most PYR tolerable strains and measurement of their degradation capacity towards PYR using GC-MS was carried out. The bacterial strains were treated with 100 mg/L of PYR and incubated for 48hrs at 37°C temperature and 150 rpm shaking condition. The entire content (50 mL) of the flask was taken for the determination of residual PYR in all the experiments. PYR was extracted using soxhlet apparatus in methanol: dichloromethane (1:2, v/v) of sample aliquots of about 1 g at 40°C. These extracts were concentrated to small volumes and sodium sulphate was added to remove water fraction.
2.5. GC-MS instrumental analysis for degradation studies
The constituents were detected using an autosystem XL GC apparatus (Perkin Elmer, USA). The column temperature was initially 80ºC, held for 5 min, then ramped from 80 to 290°C at 10°C/min. Helium (1.0 mL/min) was used as the carrier gas. Both Line and inject or temperatures were set at 250°C. 1 μL of each methanolic extract prepared were injected in the split mode (1:40). MS conditions were run in EI + through a Perkin Elmer Turbo Mass spectrometer as follows: ionization energy −70 eV; scan rate 1.6 scans/sec; interscan delay 0.01 s; source temperature 250°C; mass range 30–650 m/z; solvent delay 3 min. Spectra were obtained in the electron impact mode (70 eV) scanning from m/z 30 to 650 s−1. The gas chromatogram, as reproduced by the mass spectrometer, identified the mass spectra scanned at each GC peak maximum. Thus, final data was obtained by comparing the mass spectra to those in the Wiley NIST/EPA/NIH Mass Spectral Library/2005. The difference in the areas of respective peaks in the uninoculuted control (Abiotic control) and each inoculated experimental cultures was used for calculating the percentage degradation of PAHs.
The percentage of PAHs loss (D) was given by the formula
where MT was the concentration of PAHs in each treatment and MI was the initial PAHs concentration present in media (Xiaojun et al., Citation2007).
2.6. Microalgal treatment
Pure cultures of Synechocystis sp. was previously isolated from freshwater pond near Bhavnagar, Gujarat, India and grown photoautotrophically in BG11 medium (Rippka, Deruelles, Waterbury, Herdman, & Stanier, Citation1979) controlled illumination of 800 lux light for 14:10 h photo and dark period per day at 25 ± 2°C. Synechocystis sp. was subjected to different concentrations of antibiotics to accomplish axenic culture. PYR tolerances for Synechocystis sp. were recorded earlier by Nirmal Kumar et al. (Citation2014).
2.7. Construction of an artificial consortium using axenic culture of Synechosystis sp. with isolated bacterial sp
The artificial microalgae–bacterial consortium was formed by one axenic Synechocystis sp. and two powerful PYR-degrading bacterial strains AX7 and BX2 with three different combinations. Amongst them, two were created using each of the individual bacterial strain and Synechocystis sp. designated as JP-NKA7, JP-NKB2. The third one was with mixture of both the bacterial strain and Synechocystis sp. designated as JP-NKA7B2 (Table ). Two millilitre of (chlorophyll content was 3.45 μg/mL) algae culture and 1 mL individual/mixed (v:v = 1:1, 1.0 × 107 cells/mL) bacterial culture were inoculated initially into 30 mL BG11 medium for the consortium construction, and the consortium culture were transferred into fresh BG11 medium once every 7 days (Tang et al., Citation2010). The artificial consortia were kept in controlled illumination of 800 lux light for 14:10 h photo and dark period per day at 30 ± 2°C. Synechocystis sp.
Table 1. Selected doses of PYR to Synechocystis sp. and consortium JP-NK
2.8. LC50 determination
Stock solution LC50 determination experiment was carried out with minor modification in the method of Nirmal kumar et al. (Citation2014). PYR was prepared in minimal amount of acetone, sterilized by filtration and added aseptically to the culture medium to the final concentrations indicated for each treatment. All the three exponentially growing 3 mL homogeneous consortium cultures were inoculated and treated with a range of concentrations of PYR made up to 20 mL with BG11. Without PYR treatment consortium cultures served as controls. Samples were taken after every four days up to 16 days for the measurement of microalgae growth in terms of Chlorophyll-a and calculated according to the formula of Jeffrey and Humphrey (Citation1975). Each experiment was conducted in replicates of three and their ±SE values were calculated. Growth retardation in all the three consortia was recorded for LC50 determination.
2.9. Biodegradation potential of Synechocystis sp. and consortium JP-NK using GC-MS analysis
In the biodegradation study, 2 mL of Synechocystis sp. and 1 mL microalgal–bacterial cultures were inoculated to make up the final volume of 20 mL in BG11 medium. Both the cultures were treated at different doses of PYR in addition to LC50 concentration while Abiotic control was maintained for each of the cultures tested. Samples were taken on the 0 (at the same day), 4th and 16th day for biodegradation experiment, further injected in GC with MS detector and total percentage degradation of PYR was calculated as mentioned above.
2.10. Growth on other xenobiotic compounds
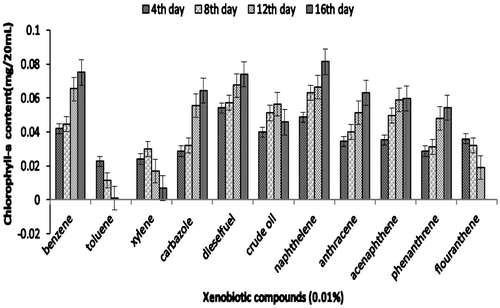
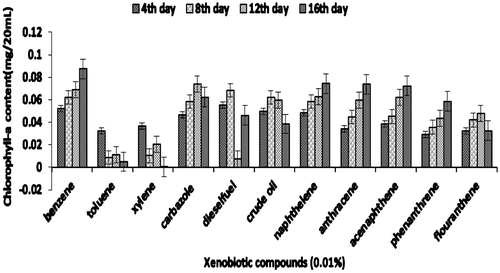
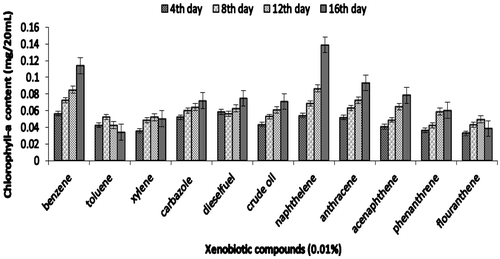
Consortium JP-NK was also tested for its ability to grow on various other xenobiotic compounds, namely benzene, toluene, xylene, phenol, carbazole, diesel fuel, crude oil, catechol, naphthalene, acenaphthalene, phenanthrene and flouranthrene at a concentration of 0.01% [(w/v)/(v/v)]. Consortium was inoculated in BG11 containing different xenobiotic compounds (0.01%) and incubated under optimized conditions (at 37°C and under shaking conditions of 150 rpm). The samples were withdrawn at every four days interval up to 16 days to monitor the growth of all the three consortia JP-NK and Synechocystis sp. in terms of chlorophyll-a content.
2.11. Data analysis
The rate of PAHs degradation was measured by the application of first-order kinetic model equation (Equation 1) to the biodegradation data, which has generally been used for biodegradation of petroleum hydrocarbons (Abbassi & Shquirat, Citation2008). Applying the biodegradation data obtained for each PAHs treatment to first-order kinetic model using the linear regression made possible further evaluation and comparison of the applicability of the various biodegradation treatment strategies.(1)
(1)
Taking the natural logarithm of Equation:(2)
(2)
where S, So, k and t are the initial PAHs concentration, final PAHs concentration, specific degradation rate constant and time, respectively.
3. Results and discussion
3.1. Enrichment isolation and characterization of PYR degrading bacteria
Hydrocarbon pollution is known to cause a shift in microbial community with the emergence of new bacterial species having elevated PAH degrading capacity because of assimilation and adaptation of micro-organisms toward organic pollutants (Vilas, Siddharth, & Datta, Citation2012).
Ankleshwar industrial estate (Gujarat, India) consists of nearly 3,000 industrialized units. Majority of the industries manufacture chemicals, dyes, paints, fertilizers, pharmaceuticals, pesticides and other xenobiotic compounds. The common industrial effluent canal (Amlakhadi) is increasingly being exposed to anthropogenic pollutants, such as PAHs and heavy metals. The treated and untreated contaminated effluents from various industries are finally released in Amlakhadi which further ends up in Narmada estuary that enters the Arabian Sea, Gujarat. Kathuria (Citation2007) found many of these pollutants (PAHs) adsorb onto particulate matter due to their hydrophobic nature and settle down as sediment. Therefore, sediments (recalcitrant compounds) in the canal sink causing a major threat to the ecosystem. Hence, this site was chosen in the present study with an aim to screen for bacteria that can degrade PAHs completely and rapidly with good adaptability.
A mixed microbial population was obtained from both the contaminated sites’ soil when the soil cultures were enriched using PYR as a sole source of carbon and energy in BHM. Each successive transfer of the enrichment culture was found to contain both bacteria and fungi. By inhibiting fungal growth with cycloheximide and ketoconazole, a consortium of bacteria which grew on PYR was isolated. Each individual bacterium was further screened for their ability to tolerate PYR at various concentrations of 20–250 mg/L. Based on colony morphology and biochemical characteristics as well as gram’s staining method, 16 different bacterial strains were isolated.
3.2. Effect of different PYR concentrations on isolates
The strain AX1 grew poorly and having (A600 = 0.002) on PYR compared to other strains. Each isolate was tested for the ability to utilize PYR up to 200 mg/L. Table shows the growth pattern of chief degraders under different PYR concentrations (20–200 mg/L). Amongst them, six most powerful strains were selected having good capacity to tolerate PYR compared to other strains. Growth on PYR treatment showed that the strains BX2 and AX7 grew more efficiently than any other isolated strains and most pronounced growth of these strains was detected at 200 and 175 mg/L, respectively; besides, these other strains AX2, BX4, BX6 and BX9 also showed remarkable growth on PYR. Similarly, the lowest optimum growth in isolate BX22 was 0.05 at 20 mg/L, while the highest was noted for BX2 i.e. 0.45 when PYR level was 75 mg/L (A600). The result that strains isolated from site B were able to grow better on the PYR than site A might be due to the prolonged exposure of complex hydrocarbon mixture of crude oil. The growth of the isolates on PYR was concentration dependent.
Table 2. Tolerance level of isolated bacterial strains to PYR at 37°C temperature 150 rpm shaking condition after 48 h
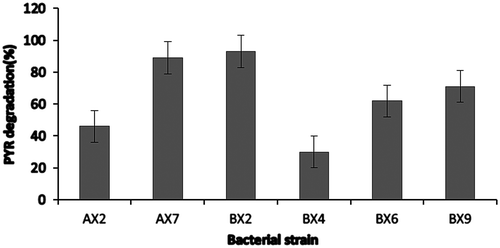
3.3. PYR degradation by bacterial isolates
Amongst 16 isolated bacterial strains, six most powerful PYR tolerable strains were selected and further inoculated in BHM for their individual PYR degradation capacity. The degradation study revealed that strain BX4 can degrade PYR by 30% followed by 46% and 62% of strain AX2 and BX6, respectively (Figure ). However, strain BX2and AX7 can degrade PYR up to 93 and 87% within 48 h of incubation period, which were further identified by morphological and 16S rRNA-based identification technique as Pseudomonas indoxyladons (Gene bank accession number: BX2-236735)and Bacillus benzoevorans (Gene bank accession number: AX7-1346516), respectively. The genus Pseudomonas is one of the most studied genera for the degradation of PAHs and other xenobiotic compounds, out of which P. putida and P. aeruginosa are the most studied species for PAH degradation. Jacques et al. (Citation2005) studied anthracene degradation by strains P. aeruginosa and P. citronellolis isolated from petrochemical sludge as well as biosurfactants production from these two strains which increased solubilization of anthracene in medium. Said et al. (Citation2007) isolated PAH-degrading A. xylosoxidans and many other strains of Pseudomonas genera from Bizerte lagoon sediment. Moreover, Luo et al. (Citation2009) reported various communities that belong to Bacillus sp. in biodegradation of benzo (a) pyrene.
3.4. Effect of PYR on microalgae growth
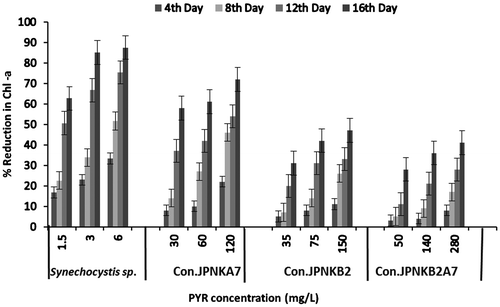
The finalized LC50 doses of PYR after 16 days of exposure to selected consortium strains were 3.0 mg/L for Synechocystis sp., 40 mg/L for Consortium JP-NKA7, 70 mg/L for JP-NKB2 and 100 mg/L for JP-NKA7B2. Thus, based upon the LC50 doses, three concentrations, i.e. One LC50 concentration, another concentration is lower and the third concentration is higher to LC50, for each species were selected (Table ).
The effect of PYR over the strain tested was measured by estimating the chlorophyll-a concentration at 4 days interval up to 16 days with that of the control (Figure ). The minimum chlorophyll-a concentration was 0.33 μg/20 mL recorded at 150.0 mg/L of PYR for consortiumJP-NKA7 and 0.11 μg/20 mL concentrations at 6.0 mg/L for Synechocystis sp. alone after 16 days. However, consortium JP-NKB2A7 showed maximum growth of 3.45 mg/20 mL on the 16th day in the control cells, which substantiated the findings of Abed (Citation2010), who stated that microalgae grew well in presence of heterotrophic bacteria. The mechanism of toxicity is generally believed to be the disruption of biological membrane. The lypophillic character of aromatic hydrocarbon can alter the membrane fluidity, permeability of the membrane, cause lipid bilayer disruption and diminish the energy transduction and affect the activity of membrane-associated proteins (Sikkema, De Bont, & Poolman, Citation1995). It was observed that chlorophyll-a content fluctuated from 0.43 to 0.13 μg/20 mL when treated to Synechocystis sp., whereas in JP-NKB2, up to 2.1 to 0.24 μg/20 mL at the end of the 4th day was noted Moreover, total decrease by 48, 62 and 74% was observed at 1.5, 3.0 and 6.0 mg/L doses, respectively. However, after 16 days of PYR treatments, growth in consortium JP-NKA7 were depleted by 36, 58, 66% at 20, 40 and 80 mg/L doses, respectively. Besides that in consortium JP-NKB2, it fluctuates from 0.19 to 0.016 mg/20 mL at 140.0 mg/L of PYR, where total fall in chlorophyll-a of Synechocystis sp was observed by 48, 62 and 94% at 1.5, 3.0 and 6.0 mg/L of PYR treatments after the 16th day, respectively. Moreover, consortium JP-NKB2A7 has shown depletion by 28, 38 and 56% at 50, 100.0 and 200.0 mg/L doses, respectively, after the 16th day. Growth of Synechocystis sp. was adversely affected by PYR at higher concentrations compared to all the three consortia, which was corroborated with the finding of Tang et al. (Citation2010) that stated that growth of unialgal GH2 declined more rapidly compared to bacteria–unialgal consortium in oil degradation.
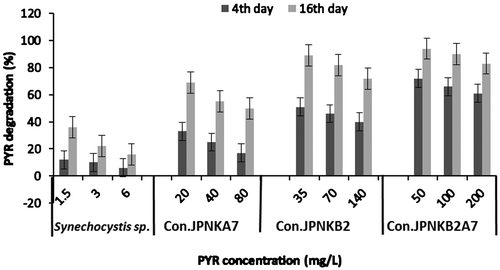
3.5. PYR degradation
The percentage degradation of PAHs by Synechocystis sp. and JP-NK throughout the study period is shown in Figure . There was a drastically reduction in PYR concentration during the study period in all treatments as compared to those of control. In the current study, PYR concentration was reduced by 12.3, 10.1, 6.2% on the 4th day and 36, 22.2, 16% on the 16th day at 1.5, 3.0 and 6.0 mg/L treatments, respectively. However, consortium JP-NKB2A7 performed higher biodegradation of PYR showing a sharp reduction by 94% in 50 mg/L, followed by 90% at 100 mg/L and 83% at 200 mg/L concentration. Kafilzadeh and HoshyariPour (Citation2012) have isolated different bacterial species Mycobacterium sp., Corynebacterium sp., Nocardia sp., Pseudomonas sp., Rhodococcus sp. and Micrococcus sp. capable of degrading PYR by 89.1, 79.4, 75.3, 68.2, 62.3 and 56.8%, respectively, after 10 days of incubation. Sarma, Duraja, Deshpande, and Lal (Citation2010) also observed 61.5% degradation of PYR by an enteric bacterium, Leclercia adecarboxylata PS4040 within 20 days when used as a sole source of carbon and energy. Cheung and Kinkle (Citation2005) observed the higher initial rates of PYR degradation with shortest acclimation periods when soils were pre-exposed with a mixture of phenanthrene and PYR for 14 weeks. In the present investigation, it has been revealed that compared to all three consortia, JP-NKB2A7 is more efficient and has high potential for degrading and removing PYR compared to Synechocystis sp. alone.
3.6. Kinetic study
The first-order kinetic model using the linear regression showedthe highest “k” value of Consortium JP-NKA7B2 (0.174 day−1) for the biodegradation of PYR, while for the Synechocystis sp. alone, it was (0.028 day−1), which shows that in the presence of bacteria, Synechocystis sp. show efficient degradation (Table ). Therefore, value of the kinetic parameter suggested that the degree of effectiveness of these bioremediation strategies in the clean-up of PYR using different consortia are in the following order: Consortium JP-NKA7B2 > Consortium JP-NKB2 > Consortium JP-NKA7.
Table 3. Specific degradation rate constants (k) in the PYR-treated organisms after 16 days
3.7. Growth on other xenobiotic compound
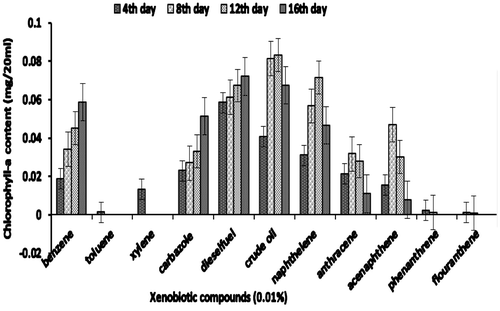
The efficiency of consortium to utilize other xenobiotic compounds is presented in Figures 4aFigure 4bFigure 4c–4d. Besides being able to utilize PYR, consortium JP-NKA7B2 was also able to utilize other PAHs, such as naphthalene, anthracene, acenaphthalene and phenanthrene, after 16 days. However, consortium JP-NKA7B2 and JP-NKA7 had shown slower growth in the presence of flouranthene after 8 days. On the contrary, Synechocystis sp. was not able to utilize other PAHs except naphthalene, acenaphthalene and anthracene after 8 days of incubation (Figure ). However, it shows moderate growth on crude oil and diesel oil, the reason being the presence of lower molecular weight hydrocarbon compounds. Previously, many Pseudomonas sp. have been reported to degrade alkane and PAHs, such as strains belonging to P. aeruginosa and P. citronellolis was able to utilize anthracene (Jacques et al., Citation2005). Besides PAHs, consortia JP-NKA7B2, JP-NKB2 and JP-NKA7 were also capable of utilizing other petroleum hydrocarbons, such as diesel oil, crude oil, carbazole, benzene and phenol, although Synechocystis sp. as well as consortia JP-NKA7B2, JP-NKB2 and JP-NKA7 failed to grow in the presence of xylene and toluene. Comparing the results of the present study with those available in the literature suggested that broad catabolic activities may be common in PAHs-degrading strains. Due to structural similarity in many aromatic hydrocarbons, it could be expected that functionally similar oxygenase enzyme present in micro-organisms is capable of degrading more than one aromatic hydrocarbon (Vilas et al., Citation2012). Alternatively, coexistence and cooperation of different bacterial strains might promote utilization of a broad range of xenobiotic compounds. Ascon-Cabrera and Lebeault (Citation1993) stated that no single organism has all the characteristics required for degradation of the different pollutants, whereas these characteristics might be found in microbial consortia.
4. Conclusions
The present study reveals that Synechocystis sp. alone cannot grow better in the presence of PYR, but in the presence of constructed artificial bacterial consortium, JP-NKA7B2 produced different organics during efficient degradation activities. Moreover, increase in degradation capacity increases metabolite content of JP-NKA7B2. Additionally, growth on other xenobiotic compounds shows efficacy of the consortium towards polluted sites. Thus, artificial microalgae–bacterial consortium may further be recommended for degrading PYR-contaminated sites in situ and ex situ.
Acknowledgement
Authors are thankful to Sophisticated Instrumentation Centre for Applied Research & Testing (SICART) V.V. Nagar, Gujarat, for analysis of samples on GC/MS.
Additional information
Funding
Notes on contributors
J.I. Nirmal Kumar
Authors have documented some intermediate biotransformants formed during flouranthrene and phenanthrene degradation by Aulosira fertilissima Rao as well as biodegradation capability and enzymatic variation of potentially hazardous Polycyclic Aromatic Hydrocarbons—Anthracene and Pyrene by Anabaena fertilissima. The response of nitrogen-fixing enzymes of Anabaena fertilissima, Aulosira fertilissima and Westeillopsis fertilissima towards 2, 4, D and pencycuron was also carried out. Besides, authors have explored enzymatic evaluation during biodegradation of kerosene and diesel, by locally isolating fungi from petroleum-contaminated soils of Western India.
References
- Abbassi, B. E., & Shquirat, W. D. (2008). Kinetics of indigenous isolated bacteria used for ex-situ bioremediation of petroleum contaminated soil. Water, Air, and Soil Pollution, 192, 221–226.10.1007/s11270-008-9649-4
- Ascon-Cabrera, M., & Lebeault, J. M. (1993). Selection of xenobiotic degrading microorganisms in a biphasic aqueous-organic system. Applied and Environmental Microbiology, 59, 1717–1724.
- Chaillan, F., Gugger, M., Saliot, A., Couté, A., & Oudot, J. (2006). Role of cyanobacteria in the biodegradation of crude oil by a tropical cyanobacterial mat. Chemosphere, 62, 1574–1582.10.1016/j.chemosphere.2005.06.050
- Cheung, P. Y., & Kinkle, B. K. (2005). Changes in Mycobacterium spp. population structure and pyrene mineralization in polycyclic aromatic hydrocarbon-amended soils. Soil Biology and Biochemistry, 37, 1929–1937.10.1016/j.soilbio.2005.02.029
- de Godos, I., Vargas, V. A., Guzmán, H. O., Soto, R., García, B., García, P. A., & Muñoz, R. (2014). Assessing carbon and nitrogen removal in a novel anoxic–aerobic cyanobacterial–bacterial photobioreactor configuration with enhanced biomass sedimentation. Water Research, 61, 77–85.10.1016/j.watres.2014.04.050
- Djomo, J. E., Dauta, A., Ferrier, V., Narbonne, J. F., Monkiedje, A., & Njine, T. (2004). Toxic effects of some major polyaromatic hydrocarbons found in crude oil and aquatic sediments on Scenedesmus subspicatus. Water Research, 38, 1817–1821.10.1016/j.watres.2003.10.023
- Gibson, D. T., Mahadevan, V., Jerina, D., Yogi, H., & Yeh, H. J. C. (1975). Oxidation of the carcinogens benzo [a] pyrene and benzo [a] anthracene to dihydrodiols by a bacterium. Science, 189, 295–297.10.1126/science.1145203
- Jacques, R. J. S., Santos, E. C., Bento, F. M., Peralba, M. C. R., Selbach, P. A., Sá, E. L. S., & Camargo, F. A. O. (2005). Anthracene biodegradation by Pseudomonas sp. isolated from a petrochemical sludge landfarming site. International Biodeterioration & Biodegradation, 56, 143–150.10.1016/j.ibiod.2005.06.005
- Jeffrey, S. W., & Humphrey, G. F. (1975). New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural populations. Biochemie und Physiologie der Pflanzen, 167, 191–194.
- Jensen, M. H., Nielsen, T. G., & Dahllof, I. (2008). Effects of pyrene on grazing and reproduction of Calanus finmarchicus and Calanus glacialis from Disko Bay, West Greenland. Aquatic Toxicology, 87, 99–107.10.1016/j.aquatox.2008.01.005
- Kafilzadeh, F., & HoshyariPour, F. (2012). Degradation of naphthalene, phenanthrene and pyrene by Pseudomonas sp. and Corynebacterim sp. in the landfills. International Journal of Biosciences, 2, 77–84.
- Kathuria, V. (2007). Informal regulation of pollution in a developing country: Evidence from India. Ecological Economics, 63, 403–417.10.1016/j.ecolecon.2006.11.013
- Lei, A., Hu, Z., Wong, Y., & Tam, N. F. (2006). Antioxidant responses of microalgal species to pyrene. Journal of Applied Physiology, 18, 67–78.
- Luo, Y. R., Tian, Y., Huang, X., Yan, C. L., Hong, H. S., Lin, G. H., & Zheng, T. L. (2009). Analysis of community structure of a microbial consortium capable of degrading benzo(a)pyrene by DGGE. Marine Pollution Bulletin, 58, 1159–1163.10.1016/j.marpolbul.2009.03.024
- Muñoz, R., Guieysse, B., & Mattiasson, B. (2003). Phenanthrene biodegradation by an algal–bacterial consortium in two-phase partitioning bioreactors. Applied Microbiology and Biotechnology, 61, 261–267.
- Nirmal Kumar, J. I., Patel, J. G., Kumar, R. N., & Khan, S. R. (2014). Chronic response of three different cyanobacterial species on growth, pigment, and metabolic variations to the high molecular weight polycyclic aromatic hydrocarbon—Pyrene. Polycyclic Aromatic Compounds, 34, 143–160.10.1080/10406638.2013.867514
- Okay, O. S., Tolun, L., Tüfekçi, V., Telli-Karakoç, F., & Donkin, P. (2006). Effects of pyrene on mussels in different experimental conditions. Environment International, 32, 538–544.10.1016/j.envint.2005.12.005
- Radwan, S. S., Al-Hasan, R. H., Ali, N., Salamah, S., & Khanafer, M. (2005). Oil-consuming microbial consortia floating in the Arabian Gulf. International Biodeterioration & Biodegradation, 56, 28–33.10.1016/j.ibiod.2005.03.007
- Raeid, M. M. A. (2010). Interaction between microalgae and aerobic heterotrophic bacteria in the degradation of hydrocarbons. International Biodeterioration & Biodegradation, 64, 58–64.
- Rippka, R., Deruelles, J., Waterbury, J. B., Herdman, M., & Stanier, R. Y. (1979). Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Journal of General Microbiology, 111, 1–61.10.1099/00221287-111-1-1
- Safonova, E. T., Dmitrieva, I. A., & Kvitko, K. V. (1999). The interaction of algae with alcanotrophic bacteria in black oil decomposition. Resources Conservation and Recycling, 27, 193–201.10.1016/S0921-3449(99)00014-2
- Said, O. B., Gon, U., Bour, M. E., Dellali, M., Aissa, P., & Duran, R. (2007). Characterization of aerobic polycyclic aromatic hydrocarbon-degrading bacteria from Bizerte lagoon sediments Tunisi. Journal of Applied Microbiology, 104, 987–997.
- Samanta, S. K., Singh, O. V., & Jain, R. K. (2002). Polycyclic aromatic hydrocarbons: Environmental pollution and bioremediation. Trends in Biotechnology, 20, 243–248.10.1016/S0167-7799(02)01943-1
- Sarma, P. M., Duraja, P., Deshpande, S., & Lal, B. (2010). Degradation of pyrene by an enteric bacterium, Leclercia adecarboxylata PS4040. Biodegradation, 21, 59–69.10.1007/s10532-009-9281-z
- Sikkema, J., De Bont, J. A., & Poolman, B. (1995). Mechanisms of membrane toxicity of hydrocarbons. Microbiology and Molecular Biology Reviews, 59, 201–222.
- Tang, X., He, L. Y., Tao, X. Q., Dang, Z., Guo, C. L., Lu, G. N., & Yi, X. Y. (2010). Construction of an artificial microalgal–bacterial consortium that efficiently degrades crude oil. Journal of Hazardous Materials, 181, 1158–1162.10.1016/j.jhazmat.2010.05.033
- Vilas, P., Siddharth, J., & Datta, M. (2012). Naphthalene degradation by bacterial consortium (DV-AL) developed from Alang-Sosiya ship breaking yard, Gujarat, India. Bioresource Technology, 107, 122–130.
- Xiaojun, L., Peijun, L., Xin, L., Chungui, Z., Li, Q., & Zongqiang, G. (2007). Biodegradation of aged polycyclic aromatic hydrocarbons (PAHs) by microbial consortia in soil and slurry phases. Journal of Hazardous Materials, 150, 21–26. doi:10.1016/j.jhazmat.2007.04.04
- Yin, Y., Wang, X. R., Sun, Y. Y., Guo, H. Y., & Yin, D. Q. (2008). Bioaccumulation and oxidative stress in submerged macrophyte Ceratophyllum demersum L. upon exposure to pyrene. Environmental Toxicology, 23, 328–336.10.1002/(ISSN)1522-7278
- Zapata-Pérez, O., Gold-Bouchot, G., Ortega, A., López, T., & Albores, A. (2002). Effect of pyrene on hepatic cytochrome P450 1A (CYP1A) expression in Nile Tilapia (Oreochromis niloticus). Archives of Environmental Contamination and Toxicology, 42, 477–485.10.1007/s00244-001-0018-1