?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
BSH, BSH-1 and BSH-2 are three naturally acetylated xylans obtained from bamboo shavings. The study investigated their in vitro digestibility and fermentability by intestinal bacteria. Results showed that BSH, BSH-1 and BSH-2 had low digestibility in simulated gastric and intestinal fluid, as their molecular weight decreased by 26.7%, 24.1% and 26.0%, respectively, after simulated digestion. BSH, BSH-1, BSH-2 and their corresponding digesta (BSH-D, BSH-1-D, BSH-2-D) could be fermented by Bacteroides spp. and Bifidobacterium spp. in different extent. The growth ratio of B.vulgatus and B.ovatus on the aforementioned six polysaccharides to glucose ranged from 20.8–36.3%. The growth ratio of Bi.breve, Bi.adolescentis, Bi.longum, Bi.animalis and Bi.bifidum on the previously cited six polysaccharides to glucose was 12.1%~ 28.4%. The fermentability of BSH-D, BSH-1-D and BSH-2-D was slightly enhanced as compared to their corresponding undigested sample.
PUBLIC INTEREST STATEMENT
Xylans have many useful biological effects including acting as an antioxidant, lowering cholesterol level (hypocholesterolemia) and stimulating the immune system. They can also be fermented by specific colonic bacteria to produce short-chain fatty acids that are beneficial to the body’s health. BSH, BSH-1 and BSH-2 are three naturally acetylated xylans obtained from steam-exploded bamboo shavings. The study investigated their in vitro digestibility and fermentability by intestinal bacteria. The results indicated that BSH, BSH-1 and BSH-2 had low digestibility in simulated gastric and intestinal fluid and could be fermented by Bacteroides spp. and Bifidobacterium spp. in different degrees, with a growth ratio to glucose ranged from 12.1–36.3%, and their fermentability was all slightly enhanced after simulated gastrointestinal digestion.
1. Introduction
Xylans represent the second most abundant plant cell wall polysaccharide next to cellulose in plant (Mirande et al., Citation2010). They have many useful biological effects including acting as an antioxidant, lowering cholesterol level (hypocholesterolemia) and stimulating the immune system (Deutschmann & Dekker, Citation2012). They are also reported to be served as substrates supporting microbial fermentation in the digestive tracts of ruminants and humans (Dodd, Mackie, & Cann, Citation2011). Xylans can be fermented by specific colonic bacteria to produce short-chain fatty acids that are an important energy source for the intestinal epithelium (Hu, Nie, Li, & Xie, Citation2013; Meyer, Citation2015). The fermentability of xylans by the human gut microbiota is very high in comparison with the cellulosic substrates (Slavin, Brauer, & Marlett, Citation1981). The main xylan-degrading species isolated from human faeces belong to Bacteroides (Chassard, Goumy, Leclerc, Del’Homme, & Bernalierdonadille, Citation2007; Zhang et al., Citation2014). Meanwhile, Bifidobacterium can also utilize xylans to some extent (Crittenden et al., Citation2002; Van Laere, Hartemink, Bosveld, Schols, & Voragen, Citation2000). Nevertheless, the existing research referring to the fermentability of xylans by human gut microbiota or individual intestinal bacteria so far has primarily focused on unacetylated xylans (Crittenden et al., Citation2002; Chassard et al., Citation2007; Hu et al., Citation2013; Van Laere et al., Citation2000; Wang et al., Citation2016; Zhang et al., Citation2014), information relating to the fermentation of acetylated xylans by intestinal bacteria still remains unclear.
Our research group has isolated a naturally acetylated hemicellulose from bamboo shavings, namely bamboo-shavings hemicellulose (BSH, purity of 90.2%). BSH could be separated into a neutral fraction (BSH-1, purity of 95.3%, molecular weight (Mw) of 12,800 g/mol) and an acidic fraction (BSH-2, purity of 92.5%, Mw of 11,300 g/mol) on an anion-exchange gel column (Huang et al., Citation2017a). BSH-1 was characterized as an O-acetylated arabinoxylan consisting of a linear (1→4)-β-D-xylopyranosyl backbone decorated with branches at O-2 of acetyl groups (8.4%) or at O-3 of α-L-arabinofuranosyl (2.2%) and acetyl groups (22.8%) (Huang et al., Citation2017a). BSH-2 was characterized as an O-acetylated 4-O-methyl-glucuronoarabinoxylan consisting of a linear (1→4)-β-D-xylopyranosyl backbone decorated with branches at O-2 of acetyl groups (7.0%) and 4-O-methylglucuronic acid units (6.4%) or at O-3 of α-L-arabinofuranosyl (3.2%) and acetyl groups (18.1%) or at O-2 and O-3 of acetyl groups (1.4%) (Huang et al., Citation2017a). BSH, BSH-1 and BSH-2 have shown immunostimulatory activity according to our previous studies (Huang et al., Citation2017a, Citation2017b). However, their in vitro fermentability by intestinal bacteria has not been investigated yet.
Although xylans are generally known to be capable of surviving digestion in the animal upper gastric tract (Deutschmann & Dekker, Citation2012; Huang et al., Citation2017a), a recent study has revealed that the Mw of a glucuronoarabinoxylan from the seeds of Plantago asiatica L was dramatically decreased after simulated gastric and intestinal digestion (Huang et al., Citation2017b). Therefore, it is necessary to study the digestibility of xylans by gastrointestinal fluid before evaluating their fermentability by intestinal bacteria. The current study first investigated the in vitro digestibility of BSH, BSH-1 and BSH-2 by a simulated gastrointestinal digestion model. Subsequently, the fermentability of BSH, BSH-1, BSH-2 and their corresponding digesta by several intestinal bacteria was evaluated.
2. Results and discussion
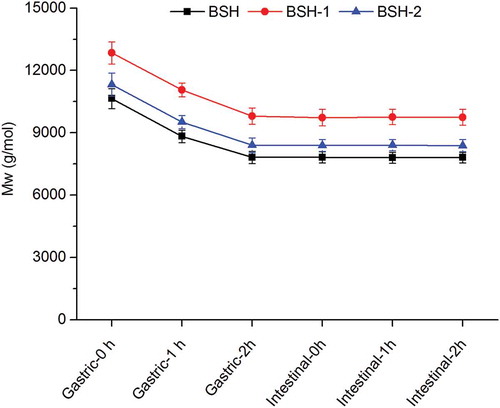
2.1. Change in Mw of BSH, BSH-1 and BSH-2 during simulated gastric and intestinal digestion
The Mw of the three polysaccharides was decreased as the gastric digestion time increased but almost remained stable during intestinal digestion (Figure ). The final Mw of BSH, BSH-1 and BSH-2 after 4 h of simulated gastrointestinal digestion was 7807 ± 254 g/mol, 9745 ± 378 g/mol and 8380 ± 299 g/mol, respectively (Figure ), which was decreased by 26.7%, 24.1% and 26.0%, respectively, as compared to their initial Mw. Meanwhile, the Mw of the three polysaccharides was almost unchanged when the digestive enzymes and bile in the gastric and intestinal medium were not added (data not shown). Considering these substances in the stomach and small intestine would not flow into the large intestine under physiological conditions, BSH, BSH-1 and BSH-2 were digested without using them and lyophilized to afford BSH-D, BSH-1-D and BSH-2-D, respectively.
2.2. Growth of intestinal bacteria on BSH, BSH-D, BSH-1, BSH-1-D, BSH-2 and BSH-2-D
Xylans were reported to be utilized well by B. vulgatus and B. ovatus (Martens et al., Citation2011; Van Laere et al., Citation2000). Especially, B. ovatus harbours several polysaccharide utilization loci that enable degradation of hemicellulosic polysaccharides (Martens et al., Citation2011). Table shows that B.vulgatus ATCC 8482 and B.ovatus ATCC 8483 were able to grow using BSH, BSH-D, BSH-1, BSH-1-D, BSH-2 or BSH-2-D as a carbon substrate, with a growth ratio of 20.8%~ 36.3% to glucose. BSH-D, BSH-1-D or BSH-2-D scored a slightly higher growth yield as compared to their corresponding undigested sample (BSH, BSH-1 and BSH-2). Of the three digested polysaccharides, BSH-2-D showed the best stimulation on the growth of B.vulgatus ATCC 8482 and B.ovatus ATCC 8483, followed by BSH-D and BSH-1-D, indicating that the two strains might prefer to ferment hemicellulose containing uronic acid and with less acetyl groups. However, growth yields of B.vulgatus ATCC 8482 and B.ovatus ATCC 8483 on the six polysaccharides were obviously lower than growth on glucose, suggesting that the acetylated xylans could not be utilized well by the two tested bacteroides. The low utilization of acetylated xylans by B.vulgatus ATCC 8482 might be due to its lack of acetylxylan esterase. Although B.ovatus ATCC 8483 was reported to contain carbohydrate acetyl esterase/feruloyl esterase precursor (Wu et al., Citation2013), the enzyme precursor might not be induced as acetylxylan esterase well by our acetylated xylans in this study. Van et al. (Citation2000) reported that two xylans (arabinoxylan from wheat flour (AXWPS) and glucuronoarabinoxylan from sorghum (AXSPS)) could be completely degraded by B.vulgatus ATCC 8482 and B.ovatus ATCC 8483, while AXSPS could be partially degraded by B.vulgatus ATCC 8482. Therefore, it might suggest that B.vulgatus ATCC 8482 and B.ovatus ATCC 8483 are better utilizer of the unacetylated xylans and have limit ability to degrade the acetylated xylans.
Table 1. Growth ratio of several intestinal bacteria on bamboo shavings-derived xylans to glucose
Within the group of Bifidobacterium spp., Bi.breve ATCC 15700, Bi.adolescentis ATCC 15703, Bi.longum ATCC 15707, Bi.animalis ATCC 27536 and Bi.bifidum ATCC 29521 could ferment BSH, BSH-D, BSH-1, BSH-1-D, BSH-2 or BSH-2-D to some extent, with a growth ratio to glucose ranged from 12.1–28.4% (Table ). Meanwhile, the growth of the tested bifidobacteria strains on BSH-D, BSH-1-D and BSH-2-D was slightly higher as compared to their corresponding undigested sample (BSH, BSH-1 and BSH-2). However, the growth yields of the tested bifidobacteria strains on the six polysaccharides were considerably lower than growth on glucose, which indicated that our acetylated xylans could not be utilized well by bifidobacteria. This finding also supported the opinion that bifidobacteria was mainly oligosaccharide utilizers and could not ferment polysaccharides well (Van Laere et al., Citation2000).
On the whole, the result of Table suggested that BSH, BSH-1, BSH-2 and their corresponding digesta (BSH-D, BSH-1-D, BSH-2-D) could be fermented by Bacteroides spp. and Bifidobacterium spp. to some extent, and the fermentability of BSH-D, BSH-1-D and BSH-2-D on the aforementioned strains was slightly enhanced as compared to their corresponding undigested sample (BSH, BSH-1 and BSH-2). This might be due to the decrease in Mw of the polysaccharides after simulated gastrointestinal digestion.
Table shows a decrease in pH of the medium for almost all strains tested when grown on the six polysaccharides. The acidification of the medium after fermentation of the six substrates was almost in line with their enhancement for the growth of the tested strain (Table ).
Table 2. pH value of the medium after fermentation of bamboo shavings-derived xylans by several intestinal bacteria
3. Experimental
3.1. Materials
BSH, BSH-1 and BSH-2 were prepared using our previous published method (Huang et al., Citation2017a). Pepsin from porcine gastric mucosa, trypsin, chymotrypsin, porcine pancreatic lipase and pancreatic amylase were from Sigma-Aldrich Corp. (St. Louis, USA). Bacterial strains were obtained from ATCC (American Type Culture Collection). Most strains were isolated from the human (animal) gastrointestinal tracts or faeces. All other reagents used were of analytical grade and purchased from China National Pharmaceutical Group Corp.(Beijing, China).
3.2. Simulated gastric and intestinal digestion in vitro
Digestion experiments were carried out using a flow-through dissolution system, ZRS-8GD (Tianda Tianfa Technology Co., Ltd, Tianjin, China) equipped with a temperature circulator-controller and eight stirring paddles.
A simulation of gastric and intestinal digestion was performed according to the method of Minekus et al. (Minekus et al., Citation2014), with some minor modifications. For the gastric digestion test, three sample cells were loaded with 100 ml of polysaccharide (concentration: 4 mg/ml) and 100 ml of simulated gastric fluid (SGF). An additional cell loaded with an equal amount of water and SGF was used as a reference cell. The SGF consisted of KCl 6.9 mmol/L, KH2PO4 0.9 mmol/L, NaHCO3 25 mmol/L, NaCl 47.2 mmol/L, MgCl2·(H2O)6 0.1 mmol/L, (NH4)2CO3 0.5 mmol/L and CaCl2·(H2O)2 0.15 mmol/L and pepsin 2000 U/ml. The pH of SGF was adjusted to 3.0 by addition of 0.1 N HCl. The gastric digestion test was performed at 37°C for 2 h. Samples (ca. 2 ml) were collected for further analysis at the beginning and after 1 and 2 h of gastric digestion. The digested solution was subsequently added with 194 ml of simulated intestinal fluid (SIF) for the intestinal digestion test. The SIF consisted of KCl 6.8 mmol/L, KH2PO4 0.8 mmol/L, NaHCO3 85 mmol/L, NaCl 38.4 mmol/L, MgCl2·(H2O)6 0.33 mmol/L, CaCl2·(H2O)2 0.6 mmol/L, trypsin 100 U/ml, chymotrypsin 25 U/ml, porcine pancreatic lipase 2000 U/ml, pancreatic amylase 200 U/ml and sodium taurocholate 10 mmol/L. The pH of SIF was adjusted to 7.0 by addition of 0.1 N HCl. The reference cell was loaded with an equal amount of water and pumped with the same volume of SIF used for the sample cells. The intestinal digestion was also performed at 37°C for 2 h. Samples (ca. 2 ml) were collected for further analysis at the beginning and after 1 and 2 h of intestinal digestion. Three independently replicated collections were made for each sample. It was worth to note that the enzymes and sodium taurocholate were added after adjustment of pH in SGF and SIF. Meanwhile, control reactions, without digestive enzymes and sodium taurocholate added, were performed. The digested fluid (without digestive enzymes and sodium taurocholate added) was dialyzed against distilled water (cut-off molecular weight 1000 g/mol) at 4°C for 2 days and lyophilized for 7 days to afford BSH-D, BSH-1-D and BSH-2-D, respectively.
The Mw of the digested sample was analyzed using high performance gel permeation chromatography as described previously (Huang et al., Citation2017a).
3.3. In vitro fermentation by intestinal bacteria
Bacteria strains (B.vulgatus ATCC 8482, B.ovatus ATCC 8483, Bi.breve ATCC 15700, Bi.adolescentis ATCC 15703, Bi.longum ATCC 15707, Bi.animalis ATCC 27536, Bi.bifidum ATCC 29521) were pre-cultured in ATCC#2107 broth. The sugar-free broth was sterilized at 121°C for 15 min. The sugar-free broth supplemented with 5 mg/ml of glucose or polysaccharide was filtered through a 0.22 μm filter under aseptic conditions and prepared freshly for use. All the aforementioned broth was inoculated with 1% (v/v) of an overnight full-grown strain for 48 h at 37°C. All strains grew in an anaerobic jar. Glucose was used as a control, sugar-free medium served as a negative control. After 48 h of fermentation, the pH was measured using a micro-pH meter (METTLER TOLEDO, Switzerland). The optical density (OD) of samples was measured at 600 nm using a multi-scan microplate reader (Bio Tek, USA). The growth yield for each bacteria strain on the various substrates was calculated relative to its growth yield on glucose. The calculation was performed using the following formula:
3.4. Statistical analysis
Data are expressed as means ± standard deviations of triplicate measurements. Statistical analyses were performed using SPSS for Windows (version 17.0). The differences between various groups were tested by one-way analysis of variance (ANOVA) followed with Duncan test.
4. Conclusion
In summary, the naturally acetylated xylans obtained from steam-exploded bamboo shavings (BSH, BSH-1 and BSH-2) had low digestibility in simulated gastric and intestinal fluid and could be fermented by the individual human intestinal bacteria (Bacteroides spp. and Bifidobacterium spp.) to some extent. Further research will investigate their fermentability by multiple intestinal bacteria (i.e. human gut microbiota) and the cross-feeding of different intestinal bacteria using acetylated xylan as a carbon source.
Competing interests
The authors declare no competing interests.
Additional information
Funding
Notes on contributors
Bin Lin
Juqing Huang obtained her Doctor’s degree in Food Science from Zhejiang University (Hangzhou, China) in 2015. She currently works as a research assistant in Fujian Academy of Agricultural Sciences (Fuzhou, China). Her research interests include bioactive carbohydrates and intestinal microbiota.
References
- Chassard, C., Goumy, V., Leclerc, M., Del’Homme, C., & Bernalierdonadille, A. (2007). Characterization of the xylan-degrading microbial community from human faeces. FEMS Microbiologic Ecology, 61(1), 121–131.
- Crittenden, R., Karppinen, S., Ojanen, S., Tenkanen, M., Fagerström, R., Mättö, J., … Poutanen, K. (2002). In vitro fermentation of cereal dietary fibre carbohydrates by probiotic and intestinal bacteria. Journal of the Science of Food and Agriculture, 82(8), 781–789. doi:10.1002/jsfa.1095
- Deutschmann, R., & Dekker, R. F. H. (2012). From plant biomass to bio-based chemicals: Latest developments in xylan research. Biotechnology Advancement, 30(6), 1627–1640. doi:10.1016/j.biotechadv.2012.07.001
- Dodd, D., Mackie, R. I., & Cann, I. K. (2011). Xylan degradation, a metabolic property shared by rumen and human colonic bacteroidetes. Molecular Microbiologic, 79(2), 292–304. doi:10.1111/mmi.2011.79.issue-2
- Hu, J. L., Nie, S. P., Li, C., & Xie, M. Y. (2013). In vitro fermentation of polysaccharide from the seeds of plantago asiatica L. by human fecal microbiota. Food Hydrocolloid, 33(2), 384–392. doi:10.1016/j.foodhyd.2013.04.006
- Huang, J. Q., Pang, M. R., Li, G. Y., Wang, N., Jin, L., & Zhang, Y. (2017a). Alleviation of cyclophosphamide-induced immunosuppression in mice by naturally acetylated hemicellulose from bamboo shavings. Food and Agricultural Immunology, 28(2), 328–342. doi:10.1080/09540105.2016.1272553
- Huang, J. Q., Qi, R. T., Pang, M. R., Liu, C., Li, G. Y., & Zhang, Y. (2017b). Isolation, chemical characterization, and immunomodulatory activity of naturally acetylated hemicelluloses from bamboo shavings. Journal of Zhejiang University-SCIENCE B (Biomedicine & Biotechnology), 18(2), 138–151. doi:10.1631/jzus.B1500274
- Martens, E. C., Lowe, E. C., Chiang, H., Pudlo, N. A., Wu, M., Mcnulty, N. P., … Eisen, J. A. (2011). Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biology, 9(12), 631-641. doi:10.1371/journal.pbio.1001221
- Meyer, D. (2015). Health benefits of prebiotic fibers. Advances in Food and Nutrition Research, 74, 47–91. doi:10.1016/bs.afnr.2014.11.002
- Minekus, M., Alminger, M., Alvito, P., Ballance, S., Bohn, T., Bourlieu, C., … Brodkorb, A. (2014). A standardised static in vitro digestion method suitable for food – An international consensus. Food & Function, 5(6), 1113–1124. doi:10.1039/C3FO60702J
- Mirande, C., Kadlecikova, E., Matulova, M., Capek, P., Bernalier-Donadille, A., Forano, E., & Béra-Maillet, C. (2010). Dietary fibre degradation and fermentation by two xylanolytic bacteria Bacteroides xylanisolvens XB1A and Roseburia intestinalis XB6B4 from the human intestine. Journal of Applied Microbiology, 109(2), 451–460. doi:10.1111/j.1365-2672.2010.04671.x
- Slavin, J. L., Brauer, P. M., & Marlett, J. A. (1981). Neutral detergent fiber, hemicellulose and cellulose digestibility in human subjects. Journal of Nutrition, 111(2), 287-297. doi:10.1093/jn/111.2.287
- Van Laere, K. M., Hartemink, R., Bosveld, M., Schols, H. A., & Voragen, A. G. (2000). Fermentation of plant cell wall derived polysaccharides and their corresponding oligosaccharides by intestinal bacteria. Journal of Agricultural and Food Chemistry, 48(5), 1644-1652. doi:10.1021/jf990519i
- Wang, K., Pereira, G. V., Cavalcante, J. J. V., Zhang, M., Mackie, R., & Cann, I. (2016). Bacteroides intestinalis DSM 17393, a member of the human colonic microbiome, upregulates multiple endoxylanases during growth on xylan. Scientific Reports, 29(6), 34360. doi: 10.1038/srep34360
- Wu, M., Mcnulty, N. P., Rodionov, D. A., Khoroshkin, M. S., Griffin, N. W., Cheng, J., … Henrissat, B. (2013). Genetic determinants of in vivo fitness and diet responsiveness in multiple human gut Bacteroides. Science, 4(2), 127-143. doi:10.1126/science.aac5992
- Zhang, M., Chekan, J. R., Dodd, D., Hong, P. Y., Radlinski, L., Revindran, V., … Cann, I. (2014). Xylan utilization in human gut commensal bacteria is orchestrated by unique modular organization of polysaccharide-degrading enzymes. Proceedings of the National Academy of Sciences of the United States of America, 111(35), E3708-E3717. doi:10.1073/pnas.1406156111