Abstract
A rapid and selective liquid chromatography–mass spectrometry (LC–MS) method was developed for the evaluation of amlodipine and atorvastatin in human plasma. Detection of analytes was assigned by tandem mass spectrometry with electrospray ionization interface in positive ion mode was applied under the multiple-reaction monitoring mode (MRM). Analytes were extracted from plasma by simple liquid–liquid extraction technique using ethyl acetate. The reconstituted samples were chromatographed on C18 column by pumping acetonitrile–water (10 mM CH3COONH4, pH 3.0) = 70:30 (v/v) at a flow rate of 0.15 mL/min. The standard curves were assigned to be linear in the range of 0.2–20 ng/mL for atorvastatin and 0.1–10 ng/mL for amlodipine with mean correlation coefficient of ≥0.999 for each analyte. The intra-day and inter-day precision and accuracy results were well within the acceptable limits. The urbanized evaluate method was successfully applied to validation of amlodipine and atorvastatin in human plasma.
Keywords:
Public Interest Statement
A susceptible, discriminating, perfect and specific liquid chromatography–mass spectrometry (LC–MS) method with SIM by single quadruple mass spectrometer with ESI interface in positive ion mode with NRM mode was urbanized and assigned for evaluation of amlodipine and atorvastatin in human plasma. The intra-day and inter-day precision and accuracy results were well within the acceptable limits. The valuated method offers several compensations such as a quick and easy extraction scheme, and a short chromatographic run time, which makes the method possible for the analysis of large sample batches resulting from assay of amlodipine and atorvastatin in human plasma.
Competing interests
The authors declare no competing interest.
1. Introduction
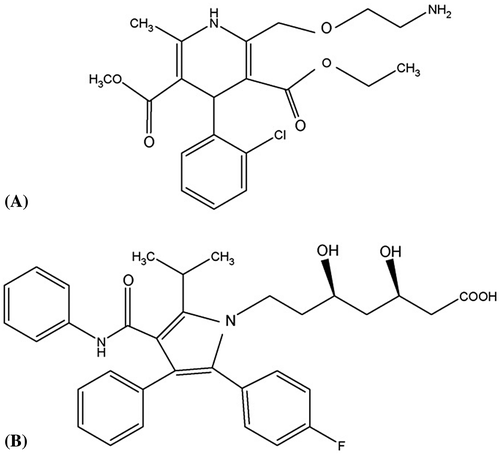
Study for the simultaneous determination of amlodipine and atorvastatin in human plasma was limited. Amlodipine (AM), 2[(2-aminoethoxy) methyl]-4-(2-chlorophenyl)-1, 4-dihydro-6-methyl-3, 5-pyridine carboxylic acid, 3-ethyl, 5-methylester () (Neil, Smith, Heckelman, & Budavari, Citation2001) is a dihydropyridine derivative with calcium antagonist activity. It is applied in the administration of hypertension, chronic stable angina pectoris and prinzmetal variant angina (Mcevoy, Citation2001). Amlodipine limits the transmembrane influx of calcium ions into vascular smooth muscle and cardiac muscle (Dollery, Citation1999; Goodman, Gilman, Rall, Nies, & Taylor, Citation1990; Sweetman & Martindale, Citation2005). Atorvastatin is chemically evaluated as [R-(R*, R*)]-2-(4-fluorophenyl)-β,δ-dihydroxy-5-(1-methylethyl)-3-phenyl-4-[(phenylamino) carbonyl]-1H-pyrrole-1-heptanoic acid ((B)) (Neil et al., Citation2001). Atorvastatin calcium is a strong inhibitor of HMG-CoA reductase, the rate-limiting enzyme in cholesterol biosynthesis and has been established to be efficient in lowering both cholesterol and triglyceride (McKeage & Siddiqui, Citation2008). It undergoes widespread first-pass metabolism and is mainly metabolized by CYP3A4. Liver metabolism produces two active hydroxy metabolites, ortho-hydroxy atorvastatin and para-hydroxy atorvastatin, and three corresponding inactive lactone metabolites (Jacobsen et al., Citation2000). Atorvastatin is widely applied in the dealing and prevention of atherosclerotic disease. However, it may cause rhabdomyolysis, the risk of which is increased by CYP3A4 inhibitors (Molden, Skovlund, & Braathen, Citation2008). There were many studies accessible for the evaluation of amlodipine or atorvastatin in human plasma (Bhatt, Singh, Subbaiah, & Shah, Citation2007; Nirogi, Mudigonda, & Kandikere, Citation2007; Nováková et al., Citation2009; Ramani, Sengupta, & Mullangi,Citation2009; Zou, Zhan, Ge, Wei, & Ouyang, Citation2009). Currently, only two papers on this topic were published. Additionally, the methods of sample preparation employed in those studies were rather complicated, which hampers the routine monitor of amlodipine or atorvastatin in plasma (Chung, GlueA, & Clin, Citation2006; Mohammadi, Rezanour, & Ansari, Citation2007; Pilli et al., Citation2011; Zhe, Fan-Yuan, & Jin, Citation2011). Our previous work was evaluation of ezetimibe by liquid chromatography–mass spectrometry (LC–MS) method in human plasma (Danafar & Hamidi, Citation2013). Consequently, an easy method that can simultaneously assay amlodipine and atorvastatin in human plasma was needed. Our goal is to expand and validate a simple and speedy LC–MS method for the validation of atorvastatin and amlodipine in human plasma.
2. Materials and methods
2.1. Materials
Amlodipine and atorvastatin standard (99.9% purity) were supplied and identified by Pfizer (Ireland). Other chemicals and solvents were from chemical lab or HPLC purity grades, whenever needed, and were purchased locally. Drug-free human plasma was provided by Iranian Blood Transfusion Organization after routine safety evaluations.
2.2. Instrumentation and operating conditions
2.2.1. Liquid chromatography
Liquid chromatography was performed using an Agilent LC-1200 HPLC system consisting of an auto sampler (Agilent). The column was a Zorbax XDB-ODS C18 column (2.1 mm × 30 mm, 3.5 μ) and was operated at 25°C. The mobile phase consisted of acetonitrile:water (10 mM CH3COONH4, pH 3.0) = 70:30 (v/v) was set at a flow rate of 0.15 mL/min.
2.2.2. Mass spectrometry
Mass spectrometric detection was performed using an Agilent LCMS-6410 quadrupole mass spectrometer with an electrospray ionization (ESI) interface. The ESI source was put at positive ionization mode. The mass selective detector was used in the multiple reaction monitoring (MRM) modes for the highest possible selectivity and compassion. The MS operating conditions were optimized as follows: Ion spray voltage was set to 4,000 V, temperature of the ion transfer capillary was 250°C, nebulizer gas (NEB) was 30 psi, dwell time per transition (ms) 200, gas flow 8 L/min, collision gas for amlodipine 8 and for atorvastatin 20. Quantitative determinations were performed in MRM scan mode using the following transitions: m/z 409.1 → 237.9 for amlodipine, m/z 559.3 → 440.2 for atorvastatin. The quantification was applied via peak area. Data acquisition and processing were proficient using Agilent LC–MS solution Software for LCMS-6410 system.
2.3. Standard preparation
A stock solution of 0.2 mg/mL amlodipine and atorvastatin in methanol were arranged, from which the concentrations of 0.1, 0.5, 0.1, 2.5, 5, and 10 ng/mL for amlodipine and concentrations of 0.2, 2.5, 5, 5, 10, 15, and 20 ng/mL for atorvastatin were prepared by serially diluting this solution with the proper amount of mobile phase and plasma.
2.4. Sample preparation and extraction procedure
A 200 μL aliquot of the collected plasma sample from a human volunteer was pipetted into a 10 mL centrifuge tube. 2.5 mL ethyl acetate was added and then was vortexes for 3 min. After centrifugation of the sample at 15,400×g for 10 min, the organic layer was transferred to another 10 mL centrifuge tube and evaporated to dryness under a gentle stream of nitrogen gas in a water bath at 40°C. The residue was re dissolved in 200 μL mobile phase. An aliquot of 10 μL was injected into the LC–MS system.
2.5. Analysis validation tests
2.5.1. Standard curve (linear range)
The plasma samples with a series of known concentrations, ready as described, were analyzed in three separate runs and, in each case, the linear regression analysis was performed on known concentrations of amlodipine and atorvastatin against the corresponding peak heights and, then, the regression coefficient (r), slope, and y-intercept of the resulting calibration curves were determined.
2.5.2. Within-run variations
In one run, three samples with concentrations of 0.1, 5, and 10 ng/mL (from high, middle, and low regions of the standard curve) for amlodipine, three samples with concentrations of 0.2, 10, and 20 ng/mL (from high, middle, and low regions of the standard curve) for atorvastatin were prepared in triplicate and analyzed by developed LC–MS method. Then, the coefficient of variations (CV %) of the corresponding determined concentrations was determined in each case.
2.5.3. Between-run variations
On three different runs, samples from upper, intermediate, and lower concentration regions used for construction of standard curve (the same as within-run variations test) were organized and analyzed by LC–MS method. Then, the corresponding CV% values were calculated.
2.5.4. Absolute recovery (accuracy)
For each sample tested for within- and between-run variations, the absolute recovery of the method was determined as the percent ratio of the measured concentration (determined using standard curve) to the corresponding nominal added concentration.
2.5.5. Relative recovery (matrix effect)
Three samples with concentrations 0.1, 5, and 10 ng/mL (from high, middle, and low regions of the standard curve) for amlodipine and three samples with concentrations of 0.2, 10, and 20 ng/mL (from high, middle, and low regions of the standard curve) for atorvastatin were prepared in triplicate and analyzed by developed LC–Mass method. Then, the ratio of the recorded peak heights to the peak heights resulting from the direct injection of the aqueous solutions of amlodipine and atorvastatin with the same concentrations were determined as percentage in each case.
2.5.6. Limits of detection and quantitation
Limit of detection (LOD) of the method was calculated as the lowest amlodipine and atorvastatin concentration producing a signal-to-noise (S/N) ratio of about 3:4, respectively. Limit of quantitation (LOQ) was determined as the lowest amlodipine and atorvastatin concentration capable of being quantitated with enough accuracy and precision.
2.5.7. Stability
2.5.7.1. Freeze and thaw stability
Three concentration levels of QC plasma samples were stored at the storage temperature (−20°C) for 24 h and thawed unassisted at room temperature. When completely thawed, the samples were refrozen for 24 h under the same conditions. The Freeze–Thaw Cycle was repeated twice, then the samples were tested after three freeze (−20°C)-thaw (room temperature) cycles.
2.5.7.2. Short-term temperature stability
Three concentration levels of QC plasma samples were kept at room temperature for a period that exceeded the routine preparation time of samples (around 6 h).
2.5.7.3. Long-term stability
Three concentration levels of QC plasma samples kept at low temperature (−20°C) were studied for a period of four weeks.
2.5.7.4. Post-preparative stability
The auto sampler stability was showed reanalyzing extracted QC samples kept under the auto sampler conditions (4°C) for 12 h.
4. Results and discussion
4.1. Sample preparation
Liquid–liquid extraction was necessary and important because this technique can not only purify but also concentrate the sample. n-Hexane-isopropanol (95:5, v/v), ethyl acetate and methylene chloride-ethyl acetate (20:80, v/v) were all attempted and ethyl acetate was finally assumed because of its high extraction efficiency and less interference.
4.2. Separation
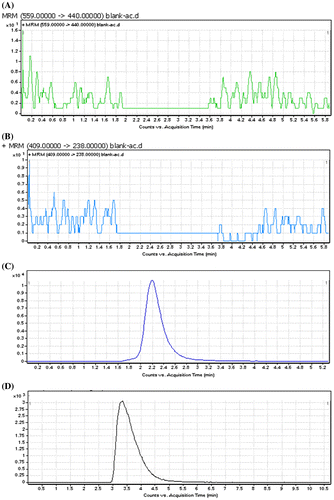
Detection of analytes was achieved by tandem mass spectrometry with ESI interface in positive ion mode was operated under the MRM. The MRM (+) chromatograms extracted from supplemented plasma are depicted in . As shown, the retention times of amlodipine and atorvastatin were 2.5 and 3.8 min, respectively. The total HPLC–MS analysis time was 5 min per sample.
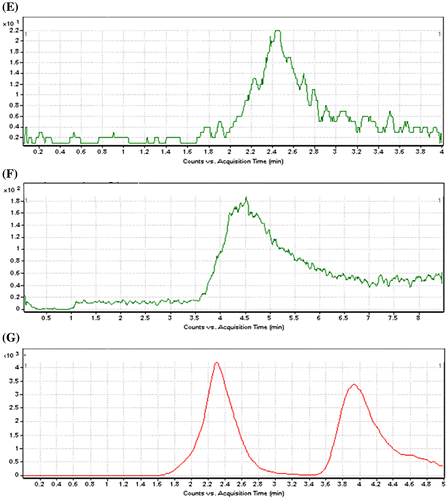
Figure 2. The MRM (+) chromatograms of amlodipine and atorvastatin (A) blank plasma of atorvastatin, (B) blank plasma, of amlodipine, (C) supplemented plasma (concentration of amlodipine = 5 ng/mL), (D) supplemented plasma (concentration of atorvastatin = 5 ng/mL), (E) LOQ (concentration of amlodipine = 0.1 ng/mL), (F) LOQ (concentration of atorvastatin = 0.2 ng/mL), (G) the MRM (+) chromatograms for plasma sample. The concentration of amlodipine and atorvastatin were 5 ng/mL.
4.3. Method validation
4.3.1. Assay specificity
No interferences of the analytes were observed. (A, B) shows an HPLC chromatogram for a blank plasma sample indicating no endogenous peaks at the retention positions of amlodipine and atorvastatin.
4.3.2. Linearity and LOQ
The method produced linear responses throughout the amlodipine and atorvastatin concentration range of 0.1–10 ng/mL for amlodipine and concentration range of 0.2–20 ng/mL for atorvastatin, which is suitable for intended purposes. A typical linear regression equation of the method was: y = y = 2753x + 1090, for amlodipine and y = 655.8x + 319, for atorvastatin, with x and y representing concentration (in ng/mL) and peak height (in arbitrary units), respectively, and the regression coefficient (r) of 0.999. The lower limit of quantification for amlodipine and atorvastatin were proved to be 0.1 and 0.2 ng/mL respectively. The lower LOD for amlodipine and atorvastatin were 0.05 and 0.1 ng/mL, respectively. (E, F) shows the chromatogram of an extracted sample that contained (LOQ) of amlodipine and atorvastatin. (C, D) shows the chromatogram of an extracted sample that contained amlodipine and atorvastatin with concentrations of 5 ng/mL. (G) shows the chromatogram of an extracted sample that contained amlodipine and atorvastatin with concentrations of 3 ng/mL.
4.3.3. Within-run variations and accuracy
The within-run variations of the developed LC–Mass method as well as the corresponding absolute recoveries are shown in Tables and .
Table 1. Within-run variations and accuracy of the LC–Mass method for quantitation of amlodipine (n = 3)
Table 2. Within-run variations and accuracy of the LC–Mass method for quantitation of atorvastatin (n = 3)
4.3.4. Between-run variations and accuracy
The between-run variations of the developed LC–Mass method as well as the corresponding absolute recoveries are shown in Tables and .
Table 3. Between-run variations and accuracy of the LC–Mass method for quantitation of amlodipine (n = 3)
Table 4. Between-run variations and accuracy of the LC–Mass method for quantitation of atorvastatin (n = 3)
4.3.5. Extraction recovery
The extraction recovery determined for amlodipine and atorvastatin were shown to be consistent, precise and reproducible. Data were shown below in Tables and .
Table 5. Relative recovery of amlodipine by the LC–Mass method (n = 3)
Table 6. Relative recovery of atorvastatin by the LC–Mass method (n = 3)
4.3.6. Stability
Tables and summarize the freeze and thaw stability, short-term stability, long-term stability, and post-preparative stability data of amlodipine and atorvastatin. All the results showed the stability behavior during these tests and there were no stability related problems during the samples routine analysis for the pharmacokinetic, bioavailability or bioequivalence studies. The stability of working solutions was tested at room temperature for 6 h. Based on the results obtained, these working solutions were stable within 6 h.
Table 7. Data showing stability of amlodipine in human plasma at different QC levels (n = 5)
Table 8. Data showing stability of atorvastatin in human plasma at different QC levels (n = 5)
5. Conclusion
A sensitive, selective, accurate, and precise HPLC method with triple quadruple mass spectrometer with ESI interface in positive ion mode with MRM mode was developed and validated for determination of amlodipine and atorvastatin in human plasma. The described method offers several advantages such as a rapid and simple extraction scheme, and a short chromatographic run time, which makes the method suitable for the analysis of large sample batches resulting from study of amlodipine and atorvastatin.
Additional information
Funding
Notes on contributors
Hossein Danafar
Dr. Hossein Danafar is Assistant Professor of Medicinal Chemistry and Dean of Department of Medicinal Chemistry at the School of Pharmacy, Zanjan University of Medical Sciences, Zanjan, Iran. He received his PhD (2013) degree in Medicinal Chemistry from Tabriz University of Medical Sciences, Tabriz, Iran. His current research interests are the analytical chemistry, pharmaceutical analysis, design, synthesis and characterization of polymeric drug delivery systems, including micelles, nanogels and molecularly imprinted carriers, bioconjugates, and magnetic targeted drug delivery systems. The future study is analysis of other formulation drugs by HPLC, LC–MS, and GC-MS in human plasma.
References
- Bhatt, J., Singh, S. G., Subbaiah, G., & Shah, B. (2007). A rapid and sensitive liquid chromatography–tandem mass spectrometry (LC–MS/MS) method for the estimation of amlodipine in human plasma. Biomedical Chromatography, 21, 169–175.10.1002/(ISSN)1099-0801
- Chung, M., GlueA, Bramson P., & Clin, C. J. (2006). Bioavailability of amlodipine besylate/atorvastatin calcium combination tablet. Pharmacology, 46, 1030–1037.
- Danafar, H., & Hamidi, M. (2013). A rapid and sensitive LC–MS method for determination of ezetimibe concentration in human plasma: Application to a bioequivalence study. Chromatographia, 76, 1667–1675.10.1007/s10337-013-2548-x
- Dollery, C. (1999). Therapeutic drugs (2nd ed., pp. 151–160). London: Churchill Livingstone.
- Goodman, A. G., Gilman, L. S, Rall, T. W., Nies, A. S., & Taylor, P. (1990). The pharmacological basis of therapeutics (8th ed., pp. 774–780). Oxford: Pergamon Press.
- Jacobsen, W., Kuhn, B.Soldner, A., Kirchner, G., Sewing, K. F., Kollman, P. A., … Chritians, U. (2000). It is important in the metabolism of many drugs including paclitaxel, rosiglitazone, mass spectrometer SCIEX API. Drug Metabolism and Disposition, 28, 1369–1378.
- Mcevoy, G. K. (2001). Determination of amlodipine besylate by charge-transfer complex formation with p-chloranilic acid (American Hospital Formulary Service®, pp. 488–865). Bethesda: American Society of Health-System Pharmacists.
- McKeage, K., & Siddiqui, M. A. (2008). Amlodipine/atorvastatin fixed-dose combination. American Journal of Cardiovascular Drugs, 8, 51–67.10.2165/00129784-200808010-00007
- Mohammadi, A., Rezanour, N., & Ansari, M. (2007). A stability-indicating high performance liquid chromatographic (HPLC) assay for the simultaneous determination of atorvastatin and amlodipine in commercial tablets. Journal of Chromatography B, 846, 215–221.10.1016/j.jchromb.2006.09.007
- Molden, E., Skovlund, E., & Braathen, P. (2008). Risk management of simvastatin or atorvastatin interactions with CYP3A4 inhibitors. Drug Safety, 31, 587–596.
- Neil, M. J. O., Smith, A., Heckelman, P. E., & Budavari, S. (2001). The Merck index, an encyclopedia of chemicals, drugs and biologicals (13th ed., pp. 488–865). White House Station, NJ: Merck.
- Nirogi, R., Mudigonda, K., & Kandikere, V. (2007). Chromatography–mass spectrometry methods for the quantitation of statins in biological samples. Journal of Pharmaceutical and Biomedical Analysis, 44, 379–387.10.1016/j.jpba.2007.02.008
- Nováková, L., Vlčková, H., Šatínský, D., Sadílek, P., Solichová, D., Bláha, M., & Bláha, V. (2009). Ultra high performance liquid chromatography tandem mass spectrometric detection in clinical analysis of simvastatin and atorvastatin. Journal of Chromatography B, 877, 2093–2103.10.1016/j.jchromb.2009.05.052
- Pilli, N. R., Inamadugu, J. K., Mullangi, R., Karra, V. K., Vaidya, J. R., & Seshagiri Rao, J. V. L. N. (2011). Simultaneous determination of atorvastatin, amlodipine, ramipril and benazepril in human plasma by LC–MS/MS and its application to a human pharmacokinetic study. Biomedical Chromatography, 25, 439–449.10.1002/bmc.v25.4
- Ramani, A. V., Sengupta, P., & Mullangi, R. (2009). Development and validation of a highly sensitive and robust LC-ESI-MS/MS method for simultaneous quantitation of simvastatin acid, amlodipine and valsartan in human plasma: Application to a clinical pharmacokinetic study. Biomedical Chromatography, 23, 615–622.10.1002/bmc.v23:6
- Sweetman, S. C., & Martindale, D. (2005). The complete drug reference (34th ed., pp. 862–866). London: Pharmaceutical Press.
- Zhe, H. U., Fan-Yuan, Z., & Jin, Z. (2011). HPLC–MS–MS for the simultaneous determination of atorvastatin and amlodipine in plasma of hypertensive patients. Chromatographia, 73, 257–262.
- Zou, Q., Zhan, Y., Ge, Z., Wei, P., & Ouyang, P. (2009). Sensitive HPLC method with fluorescence detection and on-line wavelength switching for simultaneous determination of valsartan and amlodipine in human plasma. Arzneimittelforschung, 59, 383–391.