Abstract
We sought to establish the effects on cognitive performance and autonomic arousal of a multi-electrode transcutaneous electrical sensory stimulator compared with single-electrode stimulation. Progressing from a feasibility study (n = 10) to a repeated-measures, within-subject study (n = 67), healthy, right-handed participants (34 male, mean age 28 ± 11.5 yrs.) received four separate predictable (“lines” or “ring”) or unpredictable (“fly” or “random”) complex electrical stimulation patterns to the non-dominant forearm via 24 individually programmable electrodes, or single-electrode stimulation. During stimulation, participants in the main study performed a series of cognitively demanding tests or a two-point discrimination task and had their autonomic arousal (skin conductance response) monitored. Single-electrode “first felt” current intensity decreased from distal to proximal forearm, was positively correlated with forearm circumference and was lower in females. Single-electrode stimulation was associated with a significant decline in two-point discrimination performance. There were no significant autonomic arousal or cognitive performance differences between complex patterns, but all patterns were associated with greater autonomic arousal than single-electrode stimulation. In conclusion, gender and forearm location and circumference significantly influence perception-levels for electrical current. Future research should examine whether the induction of increased autonomic arousal, via multi-electrode complex patterns, has improved therapeutic application, compared with single-electrode stimulation.
Public Interest Statement
Concentrating and focusing on a task can be difficult when something else competes for your attention. “Arousal” is the bodily and mental state of being awake and alert, with the degree of arousal being related to attention and information processing ability—too little or too much arousal can adversely affect task performance. Increased attention (“selectively concentrating on a discrete aspect of information”) plays an important role in learning. In this study we investigated how mild electrical stimulation of the forearm affected people’s arousal levels and their ability to perform a mental task such as doing a “spot-the-difference” puzzle. Better understanding of attention and arousal may be beneficial in various neurological and psychiatric disorders.
Competing interests
The authors declare no competing interest.
1. Introduction
Single-electrode transcutaneous electrical stimulation has therapeutic use in neuro-rehabilitation, including foot drop (Taylor et al., Citation1999), overactive bladder (de Sèze et al., Citation2011) and the return of forearm sensorimotor function following stroke (Dimitrijevic, Citation1996; Laufer & Elboim-Gabyzon, Citation2011). At higher intensities, electrical stimulation of the skin produces motor responses in underlying muscles (functional electrical stimulation; FES), or at lower levels, sensation via stimulation of cutaneous nerves (transcutaneous electrical sensory stimulation (TESS)). In a study of stroke rehabilitation therapy (Sullivan & Hedman, Citation2004), where the goal was return of forearm function, single-electrode FES (i.e. motor stimulation) was reported to be more effective than single-electrode TESS (i.e. sensory stimulation). However, single-electrode FES has been reported to be very uncomfortable, when delivered over long periods, due to muscle fatigue (Mäenpää, Jaakkola, Sandström, Airi, & von Wendt, Citation2004). A solution to the “patient-acceptability” vs. “therapeutic-utility” trade-off may be multi-electrode TESS if this could be made as clinically efficacious as single-electrode FES.
Explanations for the efficacy of electrical stimulation as an aid to post-stroke return of function are based around learning and neuroplasticity—neuronal networks undergo a “rewiring” process, involving redistribution of postsynaptic receptors and dendritic growth (Nudo, Citation2006). Increased attention (“selectively concentrating on a discrete aspect of information”) mediated by external, physiologically (autonomically) arousing stimuli, plays an important role in learning and plasticity (O’Connell et al., Citation2008; Stefan, Wycislo, & Classen, Citation2004). Hence we reasoned that TESS capable of producing “interesting”, “attention grabbing”, “distracting” or “salient” sensations, via multiple electrodes may be as effective as, and more patient-acceptable than, single-electrode FES.
Multi-electrode TESS, was delivered by the “ShefStim” (Heller et al., Citation2013; Prenton et al., Citation2014) a device able to activate individual or subsets of electrodes, at specified current amplitudes, for very short periods of time. This allows a virtually infinite number of complex sensory “patterns” to be created, (e.g. an unpredictable spatially random sensation vs. a sensation of predictable movement). We began our study by investigating the concept of a TESS pattern being “interesting” or “salient”, by designing four identifiably different patterns.
The “first felt” current intensity was established at 24 separate forearm locations (, top) to equalise the perceived strength of stimulation at each site. Subsequently, multiple-electrode complex patterns were compared against single-electrode stimulation to explore: (1) differences in evoked skin conductance response (SCR) reflecting autonomic arousal, (2) effects on cognitive performance and a two-point discrimination task and (3) subjective experience of the cutaneous sensation.
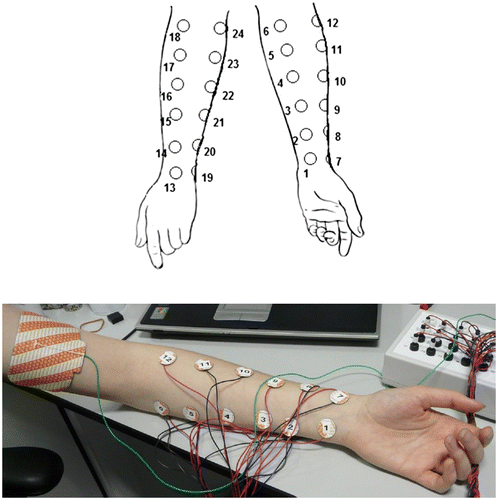
Figure 1. Anatomical placement of cathode and anode electrodes.
Notes: Schematic showing placement of 24 cathode electrodes in four columns on the volar and dorsal surfaces of the left forearm. (Bottom) Photograph of placement of the 20-mm diameter (~3.14 cm2) cathodal electrodes 1–12 (Columns 1 and 2) on the volar surface of the left forearm. The large 51 × 102 mm (~52 cm2) rectangular anodal electrode can be seen between the body of the left biceps and triceps, as can the ShefStim breakout box (top right of photograph). During actual stimulation a cohesive bandage was wrapped around the forearm to secure the cathode electrodes in position.
We investigated the influence of gender and forearm circumference on when the stimulation current was “first-felt” and described as “strong, but comfortable”. Forearm circumference was measured to investigate the effect of body size on perception thresholds, either due to sensory receptors being closer to the skin surface or because our fixed size electrodes would stimulate a different absolute proportion of skin surface, depending on the size of the individual. We hypothesised that multi-electrode TESS patterns compared with single-electrode stimulation would be associated with increased autonomic arousal and greater interference with attention-dependent cognitive performance. Ultimately, we sought to establish the “best” pattern for multi-electrode TESS, as measured by its effects on cognitive performance, autonomic arousal and self-report cutaneous sensation, reasoning that this would balance maximum rehabilitative potential with optimum patient acceptability.
2. Methods
All studies were approved by the University of Sheffield Medical School Research Ethics Committee. Participants gave written informed consent, were right-handed (Oldfield, Citation1971), had no reading difficulties (due to the nature of the neuropsychological tasks), no significant psychological or neurological condition, unbroken skin on their left forearm and no contraindications to electrical stimulation (e.g. fitted pacemaker or other electronic device).
2.1. Spatially variant perception threshold study
The stimulation kit (which meets the EU Medical Devices Directive [93/42/EEC] and harmonised standard EN 60601-1:2006) consists of the battery-operated ShefStim apparatus, a breakout accessory, an electrically isolated PC interface connection and a laptop running in-house developed software. Precisely timed and current amplitude-controlled TESS was delivered to the forearm via 24 circular hydrogel cathodal electrodes (20 mm diameter; ~3.14 cm2 [CareFusion, San Diego, CA. Ref: 019-415000]) and a single anode (51 × 102 mm; ~52 cm2 [CareFusion. Ref: 019-4222]; , bottom). The anode was much larger than the cathodes to prevent sensation due to relatively high return current from the multiple smaller electrodes (Robertson, Ward, Low, & Reed, Citation2006) and was placed between the body of the left biceps and triceps (, bottom). For single-electrode stimulation, electrode 1 was used as the cathode and electrode 2 as the anode (, top).
Twenty-four cathode electrodes were arranged into four vertical columns, distributed equidistantly in groups of 4 around the circumference of the forearm, at 6 equidistant points between the wrist and elbow. Specifically, in anatomical terms, on the forearm volar surface, Column 1 (electrodes 1–6) was placed ~2 cm medial to the palmaris longus tendon and Column 2 (electrodes 7–12) was placed ~2 cm lateral to the flexor carpi radialis longus tendon (, top). On the dorsal surface, Column 3 (electrodes 13–18) was placed between the area in which the extensor pollicis longus tendon enters the wrist and the antecubital fossa and Column 4 (electrodes 19–24) connected a point ~2 cm below the ulna styloid process with the antecubital fossa, with the electrodes lying medial to the ridge of the ulnar bone (, top).
Each electrode activation during patterned TESS consisted of five bursts of stimulation delivered 500 ms apart, with each burst consisting of three 150 μs, 100 Hz monophasic charge-balanced rectangular pulses.
The four TESS-patterns (“fly”, “random”, “lines” and “ring”) were subjectively perceived very differently, despite using the same stimulation parameters—only one electrode activated at a time; and consistent (1) number of pulses per electrode activation, (2) frequency of pulses and, (3) pulse duration. The unpredictable “fly” pattern (sensation of an insect moving across the forearm) involved the stimulus moving to a pseudo-random adjacent electrode. The unpredictable “random” pattern pseudo-randomised serial activation of each electrode. The predictable “lines” pattern was felt as separate vertical columns of electrodes (i.e. 1–6, 7–12, 13–18 and 19–24; , top) being sequentially activated. The predictable “ring” pattern was felt as a moving ring or cuff travelling up and down the forearm.
2.2. Main study—Participants and questionnaires
Prior to stimulation the 67 participants (34 male, 28 ± 11.5 years; range 18–60) completed the Positive and Negative Affect Schedule (PANAS; a self-report measure of positive and negative affect; Watson & Clark, Citation1988), the Sensory Profile (Dunn, Citation2001), the Body Sensations Questionnaire (Chambless, Caputo, Bright, & Gallagher, Citation1984), the Aberrant Salience Inventory (Cicero, Kerns, & McCarthy, Citation2010) and the Auditory Hypnagogic and Hypnopompic Experiences Questionnaire (Lewis-Hanna, Hunter, Farrow, Wilkinson, & Woodruff, Citation2011). These questionnaires were chosen to investigate whether participants’ trait psychological, physical or sensory characteristics were reflected in their current perception levels or the amount of attentional interference experienced. The PANAS was readministered at the end of each subject’s testing session to monitor the effects of TESS on mood.
2.3. Multi-electrode TESS delivery
A cohesive bandage was wrapped around the forearm maintained electrode location () and ensured that the self-adhesive hydrogel electrodes stayed in good contact with the skin. Individually weighted electrode current levels were as established during piloting (), using a multiplier based on each participant’s personal perception—a current intensity described as “strong but comfortable”. Single-electrode stimulation thresholds were established separately as these were higher than for the complex TESS patterns. In a pseudo-randomized order, participants received five 8-min periods of TESS—the four complex patterns (“fly”, “random”, “lines” and “ring”) and the single-electrode stimulation condition while performing a cognitively demanding test or a two-point discrimination task (see below). At the end of each TESS session participants rated the stimulation strength and their distractibility on 10-point Likert scales (“very comfortable” to “very uncomfortable”; and “very easy to concentrate” to “very difficult to concentrate”).
Table 1. Mean “first felt” absolute and relative current intensities
2.4. SCR recording
During TESS and task performance, autonomic nervous system activity was measured using in-house built skin-conductance response (SCR) recording equipment (Farrow et al., Citation2012), based on a battery-powered, electrically isolated, same electrode configuration as a previously published method (Shastri et al., Citation2001). SCRs were sampled at 20 Hz from the medial phalange of the left index and middle fingers, using 8-mm diameter Ag/AgCl electrodes.
SCR data were analysed in Ledalab v.3.2.9 (www.ledalab.de/; Benedek & Kaernbach, Citation2010a) using the Continuous Decomposition Analysis method to distinguish the phasic (driver) information from the underlying tonic sudomotor nerve activity. Data were smoothed via convolution with a Hann window to reduce error noise, fitted to a biexponential Bateman function and optimised by a conjugated gradient descent algorithm to reduce the error between them and the inbuilt SCR model. These processing steps produced an “integrated SCR” measure, an unbiased and time-sensitive metric of continuous, phasic sympathetic activity during each 8-min TESS period (Benedek & Kaernbach, Citation2010b). Mean integrated-SCR levels and “number of SCRs” (a “typical” SCR comprising trough, peak and half-return components; trough-to-peak amplitude ≥0.15 μS) were obtained for each minute.
2.5. Cognitive/sensory tasks
The five tasks performed during the 8-min TESS periods were the Stroop test (Stroop, Citation1935), a Tangram Puzzles task (Smart Tangoes, Citation2012), two visual search tasks and a two-point discrimination task. Task order was not pseudo-randomised as we were interested in relative between TESS-pattern performance within task, rather than absolute task performance per se. The Stroop test is attentionally demanding and requires repeated inhibition of an automatic or “pre-potent” response, with participants saying the ink colour in which a colour word is written (rather than the word itself; e.g. the word BLUE printed in red ink). The Tangram Puzzles task, an ancient Chinese visuospatial dissection puzzle, requires participants to reproduce the outline of a complex shape from seven smaller geometric shapes. The first visual search task used short text paragraphs (Bartlett, Citation2004) with participants scoring through a specified letter of the alphabet. The second visual search task used pairs of pictures in a “spot-the-difference” puzzle (Smart-kit, Citation2012)—participants were presented with new picture-pairs every 2-min. For the two-point sensory discrimination task, blindfolded participants reported which fingertip of their right hand was touched by either one or two pieces of pencil lead (0.9 mm diameter; 4 mm apart, in line with previous two point discrimination literature; e.g. Sarkar, Eapen, & Adhikari, Citation2011). For all tasks, the number of correct responses during each minute, across the 8-min TESS period, was recorded.
3. Results
3.1. Spatially variant, single-electrode perception threshold study
Mean “first felt” current across all participants (n = 10; 5 male, age range 23–62 years) and all electrodes, when stimulated individually was 2.7 ± 0.81 mA (range 1.0–7.4 mA) with a significant gender difference (males = 3.1 mA, females = 2.3 mA; p < 0.001; independent-samples t-test). Wrist circumference was positively correlated with “first felt” perception threshold (p = 0.04, r = 0.42; Pearson product-moment correlation coefficient).
Anatomically, perception thresholds showed a consistent spatial variance across participants, with generally decreasing current intensities from distal to proximal forearm ().
3.2. Determining factors for pattern thresholds
There were significant gender-related differences for TESS pattern “first felt” levels (males = 2.5 mA, females = 1.8 mA; p < 0.001; independent-samples t-test), single electrode “first felt” levels (males = 2.9 mA, females = 2.2 mA; p < 0.001), pattern “strong but comfortable” levels (males = 3.8 mA, females = 3.3 mA; p = 0.012) and single electrode “strong but comfortable” levels (males = 4.8 mA, females = 3.7 mA; p < 0.001). Wrist circumference was positively correlated with pattern “first felt” threshold (p = 0.001, r = 0.406; Pearson product-moment correlation coefficient), pattern “strong but comfortable” level (p = 0.05, r = 0.244) and single electrode “first felt” level (p = 0.003, r = 0.362), but not with single electrode “strong but comfortable” level (p > 0.05). There were significant gender-related differences in Likert-scale self-report ratings (1 = “very comfortable”; 10 = “very uncomfortable”) of TESS strength for both the patterns (males = 5.2, females = 5.8; p = 0.05) and the single-electrode stimulation (males = 4.9, females = 5.6; p = 0.032). Hence, although females received a significantly lower absolute current than males, they reported a significantly higher subjective (though personally selected) intensity of stimulation.
3.3. Quantitative sensitivity scores and task performance
Sensitivity questionnaire scores (see Section 2.2) were not significantly correlated with task performance, “first felt” threshold or “strong but comfortable” levels for either the TESS patterns or the single electrode stimulation conditions (p > 0.1). “First felt”, and “strong but comfortable” current levels, and sensitivity questionnaire scores did not significantly differ as to whether a participant had experienced a hypnagogic or hypnopompic hallucination (p > 0.05; independent-samples t-test). PANAS “positive” and “fear” scores, were significantly lower post-experiment compared with pre-experiment (p < 0.001; paired-samples t-test).
3.4. Effect of TESS patterns on task performance
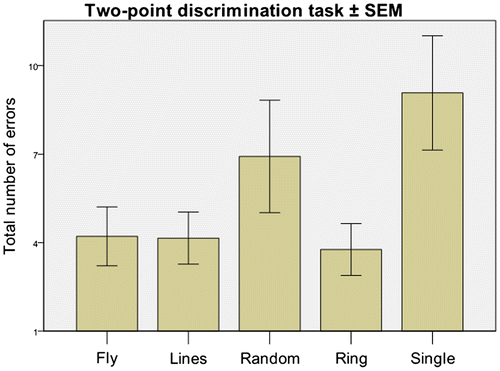
For the four cognitive tasks (Stroop test, Tangram Puzzles, letter-search and “spot-the-difference”) there was no main effect of TESS pattern on performance (p > 0.1; multi-variate ANOVA). There was however a significant difference between TESS patterns as a whole and the single-electrode stimulation condition for the sensory two-point discrimination task (p = 0.023; post hoc Fisher LSD test; ). Specifically, two-point discrimination task performance in the single-electrode stimulation condition (9.0 ± 1.8 errors) was significantly worse than for the unpredictable “fly” pattern (4.2 ± 1.0; p = 0.005; Student’s t-test), the predictable “lines” pattern (4.2 ± 1.9; p = 0.009) and the predictable “ring” pattern (3.8 ± 0.8; p = 0.01), but not significantly different from the unpredictable “random” pattern (6.9 ± 1.9; p = 0.06). There was no significant correlation between participants’ self-report level of attentional distraction (“difficulty concentrating” on the tasks) elicited by each pattern and their actual task performance (p > 0.1; Pearson product-moment correlation coefficient).
Figure 2. Effect of TESS pattern on two-point discrimination scores.
Notes: Mean two point discrimination total errors stratified by TESS pattern. Error bars show +/−2 standard errors. A significant between-pattern difference in task performance was seen with the single electrode stimulation condition (electrode 1, ) resulting in an increased number of errors (p = 0.023 post hoc Fisher LSD test; multivariate ANOVA).
3.5. Effects of TESS patterns on SCR
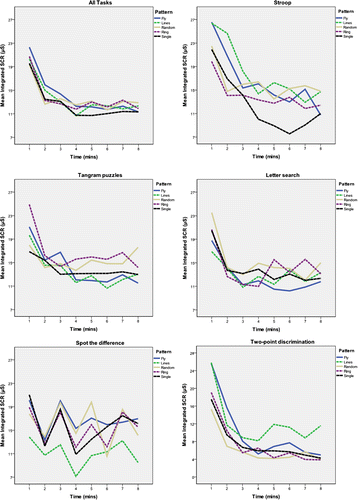
There was a significant, habituation-related, main effect of time, for four of the five tasks, within each TESS-pattern for mean integrated-SCR () and number of SCRs. The exception was for the “spot-the-difference” task (, bottom, middle)—which reflects the autonomically arousing effect of presenting new picture-pairs every 2-min. There were no significant between-TESS pattern differences in integrated-SCR for any of the five tasks (p > 0.1; ). However, qualitatively, single-electrode stimulation was associated with reduced integrated-SCR during the Stroop task compared with the TESS patterns overall (; top, middle). Finally, mirroring task order, there was a significant, between-task, main effect of time for mean integrated-SCR and number of SCRs (p < 0.001, repeated-measures ANOVA).
Figure 3. Mean integrated-SCR (μS) across eight, 1-min epochs, for all tasks, stratified by TESS pattern.
Notes: All tasks collapsed. (Top middle) Stroop. (Top right) Tangram puzzles. (Bottom left) letter search. (Bottom middle) spot the difference. (Bottom right) two-point discrimination. A significant main effect of time is seen during all TESS patterns and during all tasks (except during spot the difference, where a new set of picture-pairs was presented every two minutes). There was no significant between TESS pattern effect on integrated-SCR on any of the tasks.
4. Discussion
Contrary to our main hypothesis, we were unable to demonstrate a single “best” complex TESS pattern which had the greatest impact on cognitively demanding task performance or autonomic arousal. However, our piloting and experimental results from the ShefStim have provided some valuable insights into the design and testing of multi-electrode TESS protocols, to optimise their therapeutic utility and patient acceptability. Specifically, we report (1) a clear distal-proximal gradient on the forearm in “first felt” current intensity; (2) that such complex TESS patterns are tolerable to receive over extended periods of time; (3) self-report and SCR differences between patterns as a whole when compared with a standard single-electrode stimulation condition; (4) that females objectively had significantly lower absolute cutaneous perception and tolerability current intensity thresholds, but subjectively reported significantly higher stimulation intensity (to self-selected levels); (5) that the variability in cutaneous perception and tolerability current intensities is sufficiently large as to require subject-specific current-level setting; and (6) that despite all complex TESS patterns objectively delivering the same total current at the same electrode activation frequency, that different patterns (“fly”, “random”, “lines” and “ring”) produced very different subjective qualities and experiences.
One possible reason that female participants had significantly lower “first felt” and “strong but comfortable” current levels than males (in agreement with previous findings; de Jesus Guirro, de Oliveira Guirro, & de Sousa, Citation2014) is the increased amount of subcutaneous fat in women (Maffiuletti, Citation2008), which creates a layer of higher electrical resistance, thereby decreasing the current flow to deeper layers. Although males required higher absolute stimulation current levels, it is interesting to note that women gave significantly higher subjective ratings of the strength of stimulation. Although we specifically designed our study to avoid participants experiencing levels of electrical stimulation that would be described as “painful”, we note that there is consistent evidence from meta-analyses that females show heightened sensitivity to experimentally induced pain compared with males (e.g. Paller, Campbell, Edwards, & Dobs, Citation2009). Such reporting differences may be due to social or cultural pressures regarding gender differences in expression of pain or discomfort, with males being less likely to report discomfort (Weisse, Foster, & Fisher, Citation2005). Whether the biological and/or psychological mechanism(s) for these different thresholds for “first felt”, “strong but comfortable” and painful stimuli are the same or overlapping, is to our knowledge unknown. Our confirmed hypothesis of a positive correlation between forearm circumference and perception thresholds was based on the assumption (for which we have, to date, been unable to find any scientific support or refutation) that each area of skin, as represented in the somatosensory homunculus, contains a fixed number of cutaneous receptors. Hence, our standard 20-mm diameter (~3.14 cm2) electrodes would stimulate more receptors on a smaller forearm, due to increased receptor density (albeit at a lower per-receptor current). Furthermore, a smaller arm would have sensory endings closer to the surface.
Apart from a main effect of time on integrated-SCR mean amplitude and number of SCRs, we reported no significant difference between complex TESS patterns on autonomic arousal either collapsed across tasks or within each task (). The effect of TESS on task performance was limited to an increased error rate in the two-point discrimination task during the single-electrode stimulation condition compared with three of the four complex patterns (). It may be relevant that the two-point discrimination task was the only passive task undertaken; all the cognitive tasks were active and associated with low numbers of errors and low distraction scores, suggesting high participant motivation and engagement (supported by comments during participant debriefing which revealed a high level of competitive drive). Such prioritising of cognitive task performance may have allowed participants to override the distracting effects of TESS. It is possible therefore that the TESS current levels administered (subjectively “strong but comfortable”) were attentionally distracting (i.e. salient) at rest, but insufficient to impact on willed or focused task performance. The lack of a significant relationship between scores on the Auditory Hypnagogic and Hypnopompic Experiences Questionnaire and “first felt” current levels, or other sensory questionnaire scores, suggests that over-attention/over-sensitivity to sensorial stimuli may be dissociable between auditory and somatosensory modalities.
We could not include a “no-stimulation” control group as this would have been impossible to deliver “blind” to participants. Our decision to not randomise task order minimised within-task variance, and was based on the fact that our focus was on the modulatory effect of complex TESS patterns on task performance (rather than absolute task performance). Though we chose four identifiably different TESS patterns, the range of possible patterns and parameter settings (e.g. number of electrodes simultaneously activated, number of pulses per electrode activation, frequency of pulses, pulse duration, current amplitude, etc.) is virtually infinite.
Future research could investigate the use of passive, less willed (focused) cognitive tasks such as unexpected recall of previously read text. A longer pulse train may produce more intense sensations, increasing cognitive interference and thereby shifting attentional priority from the cognitive task to the complex TESS pattern. Identification of a complex multi-channel TESS pattern, which affects task performance, would allow improvements to the conventional sensory stimulation currently used in stroke rehabilitation. Once an appropriate current intensity level (either individually set or gender-specific) had been established, the electrodes could be fitted into a sleeve (e.g. Keller, Kuhn, & Morari, Citation2006) delivering more patient-acceptable TESS with minimal interference of normal activities of daily living.
In conclusion, we were unable to establish a single “best” multi-channel TESS pattern, though our neurophysiological results suggest that multiple-electrode patterns as a whole are superior to conventional single-electrode stimulation. The current intensity required to produce “first felt” sensation decreased from distal to proximal forearm, with the lateral-dorsal forearm (electrodes 13–18; ) requiring the greatest intensity. SCR is a feasible methodology to use as a measure of autonomic arousal in response to TESS. More “passive” tasks than those used in the present study may be more amenable to interference by TESS (and represent greater ecological validity as a comparator for “salience”). Different patterns of TESS, although delivering the same total current (i.e. only one electrode activated at a time, same number of pulses per electrode activation, frequency of pulses and pulse duration) are subjectively experienced very differently. We believe that our findings have further illuminated the complex concept of “salience” and contributed towards the development of more patient-friendly multi-channel TESS devices for therapeutic use.
| Abbreviations | ||
| FES | = | functional electrical stimulation |
| TESS | = | transcutaneous electrical sensory stimulation |
| SCR | = | skin conductance response |
| µs | = | microseconds |
| mA | = | milliamperes |
Acknowledgements
The authors would like to thank all the study participants.
Additional information
Funding
Notes on contributors
Tabitha Mufti
Tabitha Mufti was a 3rd year medical student at the time this research was conducted and undertook the project as an intercalated BMedSci degree.
Martin Slovak
Martin Slovak is a PhD student in Medical Physics and provided technical and hardware support to the project as well as programming the ShefStim “complex pattern” sequences.
Anthony T. Barker
Anthony Barker is an associate professor of Medical Physics. He led the groups which invented and first demonstrated the technique of transcranial magnetic stimulation (TMS) and which developed the ShefStim self-optimising multichannel stimulator. He has a long-standing interest in the effects on the body of low level electromagnetic fields such as those from mobile phones and powerlines.
Tom F.D. Farrow
Tom Farrow is a senior lecturer in Neuroimaging with an interest in the neurophysiology underlying empathy, deception, theory of mind and social cognition, all of which rely on salience detection—“a stimulus’s state or quality of standing out relative to neighbouring stimuli”; hence his interest in the ShefStim device.
References
- Bartlett, D. (2004). Typeonline. Tadley, Hampshire. Retrieved April 18, 2012, from https://typingchamp.com/
- Benedek, M., & Kaernbach, C. (2010a). A continuous measure of phasic electrodermal activity. Journal of Neuroscience Methods, 190, 80–91. doi:10.1016/j.jneumeth.2010.04.028.
- Benedek, M., & Kaernbach, C. (2010b). Decomposition of skin conductance data by means of nonnegative deconvolution. Psychophysiology, 47, 647–658. doi:10.1111/j.1469-8986.2009.00972.x.
- Chambless, D. L., Caputo, G. C., Bright, P., & Gallagher, R. (1984). Assessment of fear of fear in agoraphobics: The body sensations questionnaire and the agoraphobic cognitions questionnaire. Journal of Consulting and Clinical Psychology, 52, 1090–1097. doi:10.1037/0022-006X.52.6.1090.
- Cicero, D. C., Kerns, J. G., & McCarthy, D. M. (2010). The aberrant salience inventory: A new measure of psychosis proneness. Psychological Assessment, 22, 688–701. doi:10.1037/a0019913.
- de Jesus Guirro, R. R., de Oliveira Guirro, E. C., & de Sousa, N. T. A. (2014). The sensory and motor thresholds of transcutaneous electrical stimulation are influenced by gender and age. Archives of Physical Medicine and Rehabilitation, 7, 42–47. doi:10.1016/j.pmrj.2014.07.004.
- de Sèze, M., Raibaut, P., Gallien, P., Even-Schneider, A., Denys, P., Bonniaud, V., … Amarenco, G. (2011). Transcutaneous posterior tibial nerve stimulation for treatment of the overactive bladder syndrome in multiple sclerosis: Results of a multicenter prospective study. Neurourology and Urodynamics, 30, 306–311. doi:10.1002/nau.20958.
- Dimitrijevic, M. M. (1996). Modification of motor control of wrist extension by mesh glove electrical afferent stimulation in stroke patients. Archives of Physical Medicine and Rehabilitation, 77, 252–258. doi:10.1016/S0003-9993(96)90107-0.
- Dunn, W. (2001). The sensations of everyday life: Empirical, theoretical, and pragmatic considerations. American Journal of Occupational Therapy, 55, 608–620. doi:10.5014/ajot.55.6.608.
- Farrow, T. F. D., Johnson, N. K., Hunter, M. D., Barker, A. T., Wilkinson, I. D., Woodruff, P. W. R. (2012). Neural correlates of the behavioural-autonomic interaction response to potentially threatening stimuli. Frontiers in Human Neuroscience, 6, 1–17, Article 349. doi:10.3389/fnhum.2012.00349
- Heller, B. W., Clarke, A. J., Good, T. R., Healey, T. J., Nair, S., Pratt, E. J. … Barker, A. T. (2013). Automated setup of functional electrical stimulation for drop foot using a novel 64 channel prototype stimulator and electrode array: Results from a gait-lab based study. Medical Engineering and Physics, 35, 74–81. doi:10.1016/j.medengphy.2012.03.012.
- Keller, T. L., Kuhn, A., & Morari, M. (2006). New multi-channel transcutaneous electrical stimulation technology for rehabilitation. Conference Proceedings: IEEE Engineering in Medicine and Biology Magazine, 1, 194–197. doi:10.1109/IEMBS.2006.259399.
- Laufer, Y., & Elboim-Gabyzon, M. (2011). Does sensory transcutaneous electrical stimulation enhance motor recovery following a stroke? A systematic review. Neurorehabilitation and Neural Repair, 25, 799–809. doi:10.1177/1545968310397205.
- Lewis-Hanna, L. L., Hunter, M. D., Farrow, T. F. D., Wilkinson, I. D., & Woodruff, P. W. R. (2011). Enhanced cortical effects of auditory stimulation and auditory attention in healthy individuals prone to auditory hallucinations during partial wakefulness. NeuroImage, 57, 1154–1161. doi:10.1016/j.neuroimage.2011.04.058.
- Mäenpää, H., Jaakkola, R., Sandström, M., Airi, T., & von Wendt, L. (2004). Electrostimulation at sensory level improves function of the upper extremities in children with cerebral palsy: A pilot study. Developmental Medicine and Child Neurology, 46, 84–90. doi:10.1017/S0012162204000180.
- Maffiuletti, N. A. (2008). Differences in electrical stimulation thresholds between men and women. Annals of Neurology, 63, 507–512. doi:10.1002/ana.21346.
- Nudo, R. J. (2006). Plasticity. Neurotherapeutics, 3, 420–427. doi:10.1016/j.nurx.2006.07.006.
- O’Connell, R. G., Bellgrove, M. A., Dockree, P. M., Lau, A., Fitzgerald, M., & Robertson, I. H. (2008). Self-alert training: Volitional modulation of autonomic arousal improves sustained attention. Neuropsychologia, 46, 1379–1390. doi:10.1016/j.neuropsychologia.2007.12.018.
- Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9, 7–113. doi:10.1016/0028-3932(71)90067-4.
- Paller, C. J., Campbell, C. M., Edwards, R. R., & Dobs, A. S. (2009). Sex-based differences in pain perception and treatment. Pain Medicine, 10, 289–299. doi:10.1111/j.1526-4637.2008.00558.x.
- Prenton, S., Kenney, L. P., Stapleton, C., Cooper, G., Reeves, M. L., Heller, B. W. … Williamson, T. (2014). Feasibility study of a take-home array-based functional electrical stimulation system with automated setup for current functional electrical stimulation users with foot-drop. Archives of Physical Medicine and Rehabilitation, 95, 1870–1877. doi:10.1016/j.apmr.2014.04.027.
- Robertson, V. J., Ward, A. R., Low, J., Reed, A. (2006). Electrotherapy explained: Principles and practice (4th ed.). Oxford: Butterworth-Heinemann (Elsevier).
- Sarkar, S., Eapen, C., & Adhikari, P. (2011). Sensory changes in the upper limb in type 2 diabetic patients—A case control study. Journal of Clinical and Diagnostic Research, 5, 96–100. doi: JCDR/2011/1154.
- Shastri, A., Lomarev, M. P., Nelson, S. J., George, M. S., Holzwarth, M. R., & Bohning, D. E. (2001). A low-cost system for monitoring skin conductance during functional MRI. Journal of Magnetic Resonance Imaging, 14, 187–193. doi:10.1002/jmri.1171.
- Smart-kit. (2012). Smart-kit: School games and puzzles (USA: Author). Retrieved October 15, 2012, from http://www.smart-kit.com/
- Smart Tangoes. (2012). Rex Games Inc. (San Francisco, USA: Speciality Toys Network). Retrieved January 5, 2015, from http://www.smarttangoes.com/buy/tbl102/ultra-tangoes-black/
- Stefan, K., Wycislo, M., & Classen, J. (2004). Modulation of associative human motor cortical plasticity by attention. Journal of Neurophysiology, 92, 66–72. doi:10.1152/jn.00383.2003.
- Stroop, J. R. (1935). Studies of interference in serial verbal reactions. Journal of Experimental Psychology, 18, 643–662. doi:10.1037/h0054651.
- Sullivan, J. E., & Hedman, L. D. (2004). A home program of sensory and neuromuscular electrical stimulation with upper-limb task practice in a patient 5 years after a stroke. Physical Therapy, 84, 1045–1054. doi:10.1177/1545968308329922.
- Taylor, P. N., Burridge, J. H., Dunkerley, A. L., Wood, D. E., Norton, J. A., Singleton, C., & Swain, I. D. (1999). Clinical use of the Odstock dropped foot stimulator: Its effect on the speed and effort of walking. Archives of Physical Medicine and Rehabilitation, 80, 1577–1583. doi:10.1016/S0003-9993(99)90333-7.
- Watson, D., & Clark, L. A. (1988). Development and validation of brief measures of positive and negative affect: The PANAS Scales. Journal of Personality and Social Psychology, 54, 1063–1070. doi:10.1037/0022-3514.54.6.1063.
- Weisse, C. S., Foster, K. K., & Fisher, E. A. (2005). The influence of experimenter gender and race on pain reporting: Does racial or gender concordance matter? Pain Medicine, 6, 80–87. doi:10.1111/j.1526-4637.2005.05004.x.
