Abstract
The prevalence of hyperuricemia in patients with chronic kidney disease (CKD) is high, but the management is suboptimal under traditional treatment. This study was conducted to clarify whether febuxostat achieves better renal survival and patient outcome compared with traditional urate-lowering agents (ULAs). In total, 2,460 adults who had continuously received ULAs for at least three months before enrollment were investigated. Three groups were compared prospectively including non-conversion (n = 2,214), conversion (n = 206), and febuxostat first (n = 40). We evaluated laboratory changes, estimated glomerular filtration rate (eGFR) change, eGFR decline, renal survival, and all-cause mortality. The Cox proportional hazard risk analysis were also used for risk prediction. Multiple prescriptions for ULAs were found in both the non-conversion and conversion groups. However, improved median eGFR was noted in the febuxostat group (p < 0.001), median serum uric acid (SUA) level decreased from 9.45 to 6.7 mg/dL in the febuxostat group (p = 0.010), and median SUA decreased from 8.5 to 6.3 mg/dL in the conversion group (p < 0.001). Decline rate was retarded in the conversion (p = 0.050). Using the Cox proportional model, the multivariate analysis showed conversion group, young age, and relatively good baseline eGFR were associated with better renal outcome [Hazard ratio (HR) = 0.51, 95% confidence interval (CI) = 0.39–0.69]. Febuxostat had a beneficial effect on renal outcome and hyperuricemia in CKD patients. In summary, our results support the use of aggressive treatment with febuxostat in CKD patients.
Public Interest Statement
The prevalence of hyperuricemia in patients with advanced chronic kidney disease (CKD) is high, but the management is often suboptimal under allopurinol treatment. The prospective cohort study aimed to assess the effectiveness of febuxostat, the new non-purine based-xanthine oxidase inhibitor, for CKD patients. Febuxostat had a beneficial effect on renal outcome and hyperuricemia in CKD patients. In summary, our results support the use of aggressive treatment with febuxostat in CKD patients. The major attribution is the effects measured to the urate lowering agent used and focuses on the serum uric acid achieved. Furthermore, the increase of uric acid before febuxostat use in febuxostat start group was accompanied with increase of estimated glomerular filtration rate (eGFR) resulting in restoring baseline level after febuxostat.
Competing interests
The authors declare no competing interest.
1. Introduction
Serum uric acid (SUA) over 4 mg/dL has been known as a deleterious pro-oxidant effect in metabolic syndrome and type 2 diabetes patients (Hayden & Tyagi, Citation2004). Hyperuricemia is a strongly risk factor to be associated with cardiovascular disease, all-cause mortality, and renal outcome in a long-term follow-up study (Alderman, Cohen, Madhavan, & Kivlighn, Citation1999; Bos, Koudstaal, Hofman, Witteman, & Breteler, Citation2006; Brand, McGee, Kannel, Stokes, & Castelli, Citation1985; Liu et al., Citation2012). Febuxostat, a newly non-purine-based selective xanthine oxidase inhibitor has been proven to be safe and effective for decreasing SUA levels (Faruque et al., Citation2013). Phase III randomized control trials, including the APEX (Allopurinol-Placebo-controlled Efficacy study of febuxostat), FACT (Febuxostat vs. Allopurinol Controlled Trial), and CONFIRMS studies, lasting 6–12 months were used to evaluate the treatment efficacy in subjects with baseline serum UA ≥10.0 mg/dL (approximately 40%) who reached SUA levels <6.0 mg/dL with respect to size of the tophus, gouty attack, and adverse events (Becker et al., Citation2005; Schumacher et al., Citation2008). Two open-label extension studies (Febuxostat Open-label Clinical trial of Urate-lowering efficacy and Safety, FOCUS and Febuxostat Comparative Extension Long-term study, EXCEL) were also designed to follow up urate-lowering efficacy in patients with normal renal function or CKD patients treated with febuxostat for 3–5 years (Becker, Schumacher, MacDonald, Lloyd, & Lademacher, Citation2009; Schumacher, Becker, Lloyd, MacDonald, & Lademacher, Citation2009). The supposed lack of efficacy of uricosurics in CKD is attributed to hyperuricemia-related primary arteriolopathy of the preglomerular renal vasculature and partly atrophic proximal tubules (PTs). The traditional uricosuric agents, such as benzbromazone or probenicid, were not suitable for advanced CKD owing to the lack of viable URAT1 transporters or organic anion transporters in the atrophic PTs (Gibson, Citation2002; Mazzali et al., Citation2002). However, the reduced GFR, which is more often considered the reason and identical phenomena if the PTs is associated with still working glomeruli are not atrophic. Until now, it remains unclear what effects febuxostat had on changes in renal function.
Serum uric acid is commonly elevated in subjects with CKD (Weng, Tarng, et al., Citation2014), and its secondary effect contributes to progression of renal and non-renal disease (Johnson et al., Citation2013; Park et al., Citation2011; Weng, Shu, et al., Citation2014). To date, few clinical interventional trials for hyperuricemia in CKD patients have been conducted, partly because no new drugs for treating patients with hyperuricemia have been developed. We therefore conducted an observational study with three groups of patients, including non-conversion, drug conversion, and febuxostat first. We assessed the association of different effects of urate-lowering drugs (ULAs) on SUA levels, eGFR decline rate, renal survival, and patient survival to clarify whether febuxostat was superior to traditional ULAs in reducing CKD progression.
2. Materials and methods
2.1. Study design and populations
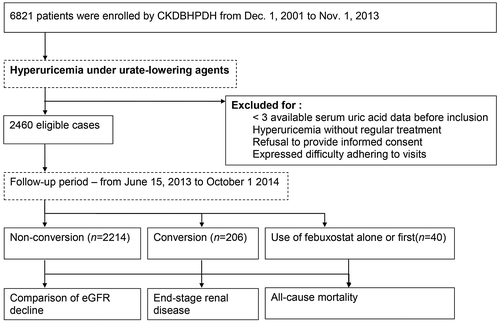
Using administrative data from the Chronic Kidney Disease division of the Bureau of Health Promotion, Department of Health, R.O.C. (CKDBHPDH), we retrieved the medical records of all CKD patients (n = 6,821) who had serial SUA and collected related laboratory findings. Febuxostat was approved for use in treating hyperuricemia and gout history in our hospital on June 15, 2013. In total, 2,460 cases were eligible to receive ULAs and at least three tests of SUA were conducted before inclusion in the study, irrespective of grouping, in June, 2013 (). The exclusion criteria were as follows: fewer than three tests of SUA before inclusion, hyperuricemia without regular treatment, refusal to provide informed consent, and difficulty adhering to visits. The follow-up period was from June 15, 2013 to October 1, 2014. We divided these patients into three groups, including the non-conversion group (always traditional ULAs, n = 2,214), drug conversion group (shifting traditional ULAs to febuxostat, n = 206), and febuxostat first (n = 40). This study was approved by the Institutional Review Board of Taichung Veterans General Hospital (No. CE12252, CE12252–1, CE12252–2, and CE12218). Written informed consent was given by participants (or next of kin/ caregiver in the case of children) allowing their clinical records to be used in this study, and consent in each case was obtained before the patient’s information was anonymized and de-identified prior to analysis. Besides, all participants were consenting for simply follow-up.
SUA level was obtained by averaging all measurements, once per month for three months, before the study began. The same calculation was applied for median values of SUA data in the conversion point and after the conversion. For each patient, the eGFR was calculated by two equations: (1) a 4-variable composite index (serum creatinine, age, race, and gender)–Modification of Diet in Renal Disease (MDRD) equation (Park et al., Citation2011; Smith, Díaz-Torné, Perez-Ruiz, & March, Citation2010) accompanied with linear regression analysis, (2) Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) 2009 equation (Levey et al., Citation2009; Matsushita et al., Citation2012). If a patient had at least three outpatient eGFR measures in a specified interval, the eGFR decline for the corresponding group would be used. Analyzed data included chronic diseases, serial laboratory results, and effects of different ULAs. We investigated the primary outcomes, with respect to retardation of renal function progression, eGFR decline, renal survival, and patient death, were better following febuxostat treatment or a conversion prescriptive pattern compared with traditional ULAs.
2.2. Statistical analyses
Proportions for categorical variables were analyzed using the Pearson χ2 test. Descriptive analysis was summarized by mean ± standard deviation and differences were tested by one-way ANOVA if the normality assumption was satisfied, or by Kruskall-Wallis test when the normal assumption was violated. A multiple imputation method was used to estimate the missing values for SUA, serum creatinine, and eGFR (Haririan et al., Citation2011; Little & Rubin, Citation2002). Normality of continuous variables was tested using the Kolmogorov-Smirnov method. The power was tested with population mean and number of cases in the relatively small group of patients. The median tested laboratory data for events of interest were analyzed by non-parametric sample t test. Regression models for eGFR decline using ordinary least-squares regression of the corresponding values were used for analyses (Al-Aly et al., Citation2010; Coresh et al., Citation2014; Levin, Djurdjev, Beaulieu, & Er, Citation2008; Matsushita et al., Citation2009). Finally, Cox proportional hazard analysis was used for risk prediction. Analyses were performed using the Statistical Package for the Social Sciences (version 15.1; SPSS, Inc., Chicago, IL, USA).
3. Results
3.1. Patient characteristics
shows the baseline characteristics of the recruited patients. Patients in the non-conversion group were older (69.9 ± 15.8) than those in the other two groups, p < 0.001). The percentage of diabetes mellitus was high (p = 0.004) and abnormal body mass index (BMI) was also high in the febuxostat first group vs. the other two groups (p = 0.042). SUA level was high 9.2 ± 1.6 mg/dL in the febuxostat group. Serum blood urea nitrogen (p = 0.034) and creatinine (p = 0.001) were high in the conversion group among these three groups. Duration of drug exposure revealed median 2.1 [interquartile range (IQR): 1.0–3.9] years in non-conversion group, 3.2 (IQR: 1.4–4.8) years in conversion group, and 0.5 (IQR: 0.0–2.5) years in febuxostat first group.
Table 1. Baseline characteristics of the study population
3.2. The characteristics of urate-lowering agents (ULAs) prescription
Most patients had more than one ULAs (e.g. uricosuric agents or allopurinol) in the non-conversion and conversion groups (). Colchicine, which is more likely used in these patients for prophylaxis either chronically or when initiating urate lowering therapy, was also frequently used in the non-conversion group (39.7% patients; dose: 0.5 ± 0.1 mg/day) and conversion group (74.8% patients; dose: 0.5 ± 0.1 mg/day). However, frequency of colchicine use was less marked in the febuxostat group (5.0% patients; dose: 0.5 ± 0.0 mg/day). Patients were maintained on a daily dose of around 40 mg febuxostat during the follow-up period, while 3.3% patients had their dosage of febuxostat increased from 40 to 80 mg/day in the long-term follow-up (data not shown).
Table 2. Characteristics of patients with chronic kidney diseases and hyperuricemia with medications
3.3. Effect of different groups of patients on serum uric acid, eGFR change, and eGFR decline
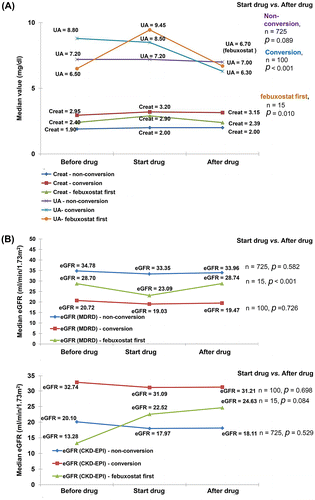
In the conversion group, median SUA level improved from 8.5 to 6.3 mg/dL (p < 0.001) after drug conversion. The first use of febuxostat group was also found to have significantly reduced median serum UA level from 9.45 to 6.7 mg/dL (p = 0.010) (A). With respect to eGFR change (B), the analysis using the MDRD equation showed no change in the conversion group. However, there was significant improvement in eGFR in the first use of febuxostat group, with an increase from 23.1 to 28.7 ml/min/1.73 m2 (p < 0.001). But in the analysis using the CKD-EPI equation, there was still no improvement in eGFR in the conversion group. There was near borderline significant improvement in eGFR in the febuxostat first group, with an increase from 22.5 ml/min/1.73 m2 to 24.6 ml/min/1.73 m2 (p = 0.084).
Figure 2. Serial change of laboratory data, including (A) change of median serum uric acid concentration and serum creatinine, and (B) estimated glomerular filtration rate by the Modification of Diet in Renal Disease (MDRD) equation and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.
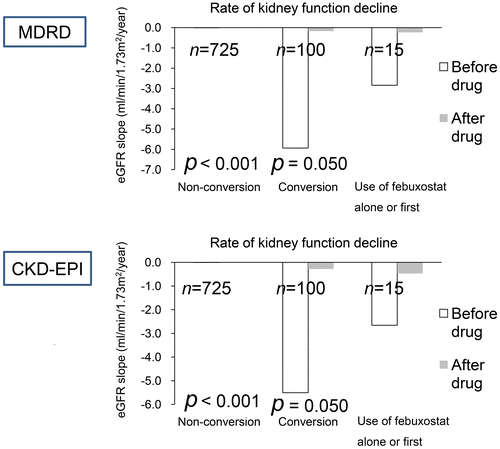
For eGFR decline (), a reduction in eGFR decline was noted in the conversion group (n = 100, eGFR decline in MDRD equation, p = 0.050; eGFR decline in CKD-EPI equation, p = 0.050) and the febuxostat first group (n = 15). There was limited change in eGFR decline in the non-conversion group (n = 725). Furthermore, due to the small sample size of the febuxostat group (n = 15), the statistical power was only 12.7%. Therefore, for the significance to be meaningful, a sample size of at least 161 would be needed to obtain a statistical power of 80% in the febuxostat group.
Figure 3. Decline of estimated glomerular filtration rate before and after urate-lowering agents.
Notes: Our data were mostly calculated by the Wilcoxon sing rank test using values for start drug and after drug. The Friedman test was not used for repetitive three tests in one person. The p-value of the febuxostat group is not shown because of the low power of analysis.
3.4. Variables associated with death and development of end-stage renal disease (ESRD)
Factors affecting the development of end-stage kidney disease () were analyzed and results showed that a beneficial effect was found in the conversion group [adjusted hazard ratio (HR) = 0.51, 95% confidence interval (CI) = 0.39–0.69] in terms of reduced CKD progression to ESRD when compared with the other group (non-conversion group). It was not possible to demonstrate a beneficial effect in terms of reduced ESRD in the febuxostat group owing to the small number of cases and short-term follow-up. Some older patients did not progress to dialysis as they had already died. Among those with initial high eGFR, few of them developed ESRD. Other traditional risk factors for progression to ESRD were diabetes mellitus, cardiovascular disease, hyperlipidemia, anemia, gouty attack, and abnormal BMI.
Table 3. Association of baseline variables with end-stage renal disease using multivariable Cox proportional Hazard analysis
In regard to overall survival (), we found that although the conversion group had low HR = 0.04 and 95% CI = 0.00–0.75 (p = 0.031), the usage of any ULAs had no significant effect. All-cause mortality was clearly related to traditional risk factors, such as old age, diabetes mellitus, malignancy, high hematocrit, and low serum albumin ().
Table 4. Association of baseline variables with death using multivariable Cox proportional Hazard analysis
4. Discussion
In this study, we have demonstrated for the first time that conversion prescription and use of febuxostat alone or first had a beneficial effect in terms of reducing CKD progression in patients with hyperuricemia. We not only demonstrated for attributing the effects on the urate lowering agent used, but also focusing on the serum uric acid achieved. The febuxostat and conversion groups had a beneficial effect in terms of reducing CKD progression in patients with hyperuricemia. It is noteworthy that the aforementioned groups had greatly improved SUA level. The febuxostat and conversion groups exhibited reduced eGFR decline regardless of the method of calculation (MDRD or CKD-EPI equation). Both the use of febuxostat alone or first group and the conversion group showed reduced high serum uric acid level. In addition, the conversion group showed retarded CKD progression to ESRD after adjusting for multiple variables (adjusted HR = 0.51, 95% CI = 0.39–0.69). However, the death rate was not changed, irrespective of prescriptive pattern, because of the short-term follow-up.
Hyperuricemia could be the result of multifactor interactions including gender, age, renal function, genetic factors, and environmental factors (Liu et al., Citation2011; Vázquez-Mellado, Alvarez Hernández, & Burgos-Vargas, Citation2004). Several well-known factors, such as male gender, racial difference (in Asia, the overall prevalence ranges from 0.1% in Vietnamese and Japanese populations to 11.7% in the Taiwanese aboriginal population), immunosuppressant drugs (cyclosporine therapy), use of diuretics, and the high prevalence of metabolic syndrome were reported (Smith et al., Citation2010). Although several studies (Akimoto et al., Citation2014; Doghramji & Wortmann, Citation2012; Jalal, Chonchol, Chen, & Targher, Citation2013) reported the efficacy of treatment strategy, treatment of hyperuricemia in patients with CKD or non-CKD and asymptomatic hyperuricemia or intermittent gouty attack was often suboptimal (Doghramji & Wortmann, Citation2012; Jalal et al., Citation2013). This may be explained at least in part in the general population and in early or advanced CKD patients by allopurinol-related skin rash or lethal Steven-Johnson syndrome (Somkrua, Eickman, Saokaew, Lohitnavy, & Chaiyakunapruk, Citation2011; Tassaneeyakul et al., Citation2009). Furthermore, many patients with asymptomatic hyperuricemia never have gout attack or renal stone (Alvarez-Lario & Macarron-Vicente, Citation2010). Another reason is that uricosuric agent, benzbromarone, was withdrawn from the market in Japan and the United States’ Food Drug Administration (FDA) withdrew approval due to the elevated risk of hepatic dysfunction. There is also recent concern that uricosurics might worsen disease due to uricosuria, such as in the recent lesinurad trials (Kenneth et al., Citation2014).
An observational study assessed the effectiveness of febuxostat for reducing progression of CKD in conversion to febuxostat or first use of febuxostat cases. Uric acid is associated with metabolic syndrome and traditional risk factors, and is strongly associated with two major outcomes, namely, death and ESRD. The febuxostat and conversion groups are associated with amelioration of eGFR decline, and even the conversion group showed a decrease in the incidence of ESRD to 49% in CKD patients. Thus, the data demonstrate that febuxostat has a lowering effect on serum uric acid level. Death rate was not reduced after febuxostat alone or conversion to febuxostat due to the short-term period of study. The long-term effects on rates of death and ESRD require further study.
Previous studies claimed that febuxostat could be used without dose change in patients with eGFR 5–59 ml/min per 1.73 m2 and serum uric acid ≥8.0 mg/dL. If no improvement was found within two weeks, doctors could change 40 to 80 mg/day (Garcia-Valladares, Khan, & Espinoza, Citation2011). The possible mechanism of amelioration of eGFR decline may be due to a secondary effect such as improved endothelial function (Mazzali et al., Citation2002). From the pathological view of hyperuricemia, renal insufficiency predisposes individuals to a higher SUA concentration, as demonstrated in hyperuricemic rats with remnant kidney (Garcia-Valladares et al., Citation2011). Interestingly, the hyperuricemic animals developed hypertension and microvascular disease, according to the pathological finding of arteriosclerotic lesions (Kalantar, Khalili, Hossieni, Rostami, & Einollahi, Citation2011; Kang et al., Citation2002; Nakagawa et al., Citation2006). Several large interventional trials were designed for the phase II long-term extension study (FOCUS) of normal renal function or CKD patients (Schumacher et al., Citation2009). Other prospective cohort studies assessed the effectiveness of febuxostat, the new non-purine based-xanthine oxidase inhibitor, for treatment of advanced CKD patients and high SUA (Hosoya et al., Citation2014). The 2012 American College of Rheumatology gout guidelines recommended that the target of therapeutic approaches for hyperuricemia is SUA <6 mg/dL, and both allopurinol and febuxostat could be considered as equivalent first-line drugs (Khanna et al., Citation2012). Phase III studies in APEX, FACT, and CONFIRMS were used to evaluate the proportion of subjects with SUA <6.0 mg/dL at the final visit based on an analysis of renal function (Becker et al., Citation2005; Schumacher et al., Citation2008). Although limited clinical interventional trials for hyperuricemia have been conducted, our study revealed that conversion to febuxostat and febuxostat only had beneficial effects. There were also some traditional risk factors for CKD progression, including diabetes mellitus, cardiovascular disease, hyperlipidemia, anemia, gouty attack, and abnormal BMI.
The limitations in this study were the study design, which employed a non-randomized control approach, and the relatively short duration of follow-up. However, the aim of the present study was to investigate the improvement in treatment with respect to the reduction in CKD progression and severe hyperuricemia in CKD patients. There is also some critical problems in this study. First, few numbers were enrolled in febuxostat group and only 15 patients had laboratory results such as creatinine and uric acid level. Secondly, the increase of uric acid before febuxostat use in febuxostat start group was accompanied with increase of creatinine or eGFR resulting in restoring baseline level after febuxostat. This might be associated with acute renal injury instead of CKD progression at the time of initiation of febuxostat. Thirdly, we do not adjust the duration of drug exposure in our Cox proportional Hazard analysis due to great difference of individual medication (urate-lowering agents) interval. If those duration of drug exposure were the same, the change of uric acid, creatinine and eGFR might be similar in the conversion and febuxostat first groups. Therefore, the change of eGFR and creatinine in conversion group might be useful to evaluate the effectiveness of febuxostat on renal function by decreasing uric acid level, compared with febuxostat start group. Furthermore, eGFR change in CKD patients is variable according to underling etiology to provoke CKD. There was no detail of underlying disease. Further studies with a large population and longer duration will be helpful for providing further evidence of the beneficial effects of febuxostat in early or advanced CKD patients and even in non-CKD patients. The long-term effects of febuxostat on death and ESRD need to be clarified.
In conclusion, our study provides evidence that the urate-lowering effect of febuxostat was associated with significant improvement of eGFR in CKD patients. These results support the aggressive treatment of hyperuricemia by febuxostat in CKD patients.
Acknowledgements
The authors sincerely appreciate the assistance of the Center for Translational Medicine of Taichung Veterans General Hospital, Taichung, Taiwan. The authors thank the Clinical Informatics Research and Development Center and the Biostatistics Task Force of Taichung Veterans General Hospital (TCVGH), Taichung, Taiwan, R.O.C., for their help with the statistical analysis. We are also very grateful for the study grants provided by TCVGH, Taiwan, R.O.C. (TCVGH-1003606A, TCVGH-1013602A, TCVGH- 1023601A, TCVGH-1033604B, TCVGH-1043602B, TCVGH-YM1040103, TCVGH-YM1050101, CGG-TCVGH1020101-4.4, CGG-TCVGH1030101), Taipei Veterans General Hospital (V101E4-001, V102E4-001, V103E4-001), the National Science Council (NSC 99-2314-B-010-004-MY3; NSC 102-2314-B-010-004-MY3), and the Ministry of Education, Taiwan, Aim for the Top University Plan.
Additional information
Funding
Notes on contributors
Shuo-Chun Weng
Shuo-chun Weng is A Consultant Physician of Center for Geriatrics and Gerontology and Division of Nephrology, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung, Taiwan. He received his PhD training in Institute of Clinical Medicine, National Yang-Ming University, Taipei, Taiwan. His current research interests are the clinical nephrology, basic science, internal medicine, kidney transplantation, geriatrics and gerontology.
References
- Akimoto, T., Morishita, Y., Ito, C., Iimura, O., Tsunematsu, S., Watanabe, Y., ... Nagata, D. (2014). Febuxostat for hyperuricemia in patients with advanced chronic kidney disease. Drug Target Insights, 8, 39–43. doi:10.4137/DTI.S16524
- Al-Aly, Z., Zeringue, A., Fu, J., Rauchman, M. I., McDonald, J. R., El-Achkar, T. M., ... Eisen, S. (2010). Rate of kidney function decline associates with mortality. Journal of the American Society of Nephrology, 21, 1961–1969. doi:10.1681/ASN.2009121210
- Alderman, M. H., Cohen, H., Madhavan, S., & Kivlighn, S. (1999). Serum uric acid and cardiovascular events in successfully treated hypertensive patients. Hypertension, 34, 144–150. Retrieved from http://hyper.ahajournals.org/content/34/1/144.full.pdf+html10.1161/01.HYP.34.1.144
- Alvarez-Lario, B., & Macarron-Vicente, J. (2010). Uric acid and evolution. Rheumatology, 49, 2010–2015. doi:10.1093/rheumatology/keq204
- Becker, M. A., Schumacher, H. R., MacDonald, P. A., Lloyd, E., & Lademacher, C. (2009). Clinical efficacy and safety of successful longterm urate lowering with febuxostat or allopurinol in subjects with gout. The Journal of Rheumatology, 36, 1273–1282. doi:10.3899/jrheum.080814
- Becker, M. A., Schumacher, Jr., H. R., Wortmann, R. L., MacDonald, P. A., Eustace, D., Palo, W. A., ... Joseph-Ridge, N. (2005). Febuxostat compared with allopurinol in patients with hyperuricemia and gout. New England Journal of Medicine, 353, 2450–2461. doi:10.1056/NEJMoa050373
- Bos, M. J., Koudstaal, P. J., Hofman, A., Witteman, J. C., & Breteler, M. M. (2006). Uric acid is a risk factor for myocardial infarction and stroke: The Rotterdam study. Stroke, 37, 1503–1507. doi:10.1161/01.STR.0000221716.55088.d4
- Brand, F. N., McGee, D. L., Kannel, W. B., Stokes, J., 3rd, & Castelli, W. P. (1985). Hyperuricemia as a risk factor of coronary heart disease: The Framingham study. American Journal of Epidemiology, 121, 11–18. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3964986
- Coresh, J., Turin, T. C., Matsushita, K., Sang, Y., Ballew, S. H., Appel, L. J., ... Levey, A. S. (2014). Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA, 311, 2518–2531. doi:10.1001/jama.2014.6634
- Doghramji, P. P., & Wortmann, R. L. (2012). Hyperuricemia and gout: New concepts in diagnosis and management. Postgraduate Medicine, 124, 98–109. doi:10.3810/pgm.2012.11.2616
- Faruque, L. I., Ehteshami-Afshar, A., Wiebe, N., Tjosvold, L., Homik, J., & Tonelli, M. (2013). A systematic review and meta-analysis on the safety and efficacy of febuxostat versus allopurinol in chronic gout. Seminars in Arthritis and Rheumatism, 43, 367–375. doi:10.1016/j.semarthrit.2013.05.004
- Garcia-Valladares, I., Khan, T., & Espinoza, L. R. (2011). Efficacy and safety of febuxostat in patients with hyperuricemia and gout. Therapeutic Advances in Musculoskeletal Disease, 3, 245–253. doi:10.1177/1759720X11416405
- Gibson, T. (2002). Hyperuricemia, gout and the kidney. Current Opinion in Rheumatology, 24, 127–131. doi:10.1097/BOR.0b013e32834f049f
- Haririan, A., Metireddy, M., Cangro, C., Nogueira, J. M., Rasetto, F., Cooper, M., ... Weir, M. R. (2011). Association of serum uric acid with graft survival after kidney transplantation: A time-varying analysis. American Journal of Transplantation, 11, 1943–1950. doi:10.1111/j.1600-6143.2011.03613.x
- Hayden, M. R., & Tyagi, S. C. (2004). Uric acid: A new look at an old risk marker for cardiovascular disease, metabolic syndrome, and type 2 diabetes mellitus: The urate redox shuttle. Nutrition & Metabolism, 1, 10. doi:10.1186/1743-7075-1-10
- Hosoya, T., Kimura, K., Itoh, S., Inaba, M., Uchida, S., Tomino, Y., ... Hayakawa, H. (2014). The effect of febuxostat to prevent a further reduction in renal function of patients with hyperuricemia who have never had gout and are complicated by chronic kidney disease stage 3: Study protocol for a multicenter randomized controlled study. Trials, 15, 26. doi:10.1186/1745-6215-15-26
- Jalal, D. I., Chonchol, M., Chen, W., & Targher, G. (2013). Uric acid as a target of therapy in CKD. American Journal of Kidney Diseases, 61, 134–146. doi:10.1053/j.ajkd.2012.07.021
- Johnson, R. J., Nakagawa, T., Jalal, D., Sanchez-Lozada, L. G., Kang, D. H., & Ritz, E. (2013). Uric acid and chronic kidney disease: which is chasing which? Nephrology Dialysis Transplantation, 28, 2221–2228. doi:10.1093/ndt/gft029
- Kalantar, E., Khalili, N., Hossieni, M. S., Rostami, Z., & Einollahi, B. (2011). Hyperuricemia after renal transplantation. Transplantation Proceedings, 43, 584–585. doi:10.1016/j.transproceed.2011.01.062
- Kang, D. H., Nakagawa, T., Feng, L., Watanabe, S., Han, L., Mazzali, M., ... Johnson, R.J. (2002). A role for uric acid in the progression of renal disease. Journal of the American Society of Nephrology, 13, 2888–2897. Retrieved from http://jasn.asnjournals.org/content/13/12/2888.full.pdf+html10.1097/01.ASN.0000034910.58454.FD
- Kenneth, G.S., Scott, A., Nihar, B., Maple, F., Jeff, K., Chris, S., ... Thomas, B. (2014). Lesinurad, a novel selective uric acid reabsorption inhibitor, in two phase III clinical trials: Combination study of lesinurad in allopurinol standard of care inadequate responders (CLEAR 1 and 2). ACR/ARHP Annual Meeting, Boston MA. http://acrabstracts.org/abstract/lesinurad-a-novel-selective-uric-acid-reabsorption-inhibitor-in-two-phase-iii-clinical-trials-combination-study-of-lesinurad-in-allopurinol-standard-of-care-inadequate-responders-clear-1-and-2/
- Khanna, D., Fitzgerald, J. D., Khanna, P. P., Bae, S., Singh, M. K., Neogi, T., ... Terkeltaub, R. (2012). 2012 American College of Rheumatology guidelines for management of gout. Part 1: Systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care & Research, 64, 1431–1446. doi:10.1002/acr.21772
- Levey, A. S., Stevens, L. A., Schmid, C. H., Zhang, Y. L., Castro, A. F., 3rd, Feldman, H. I., ... Coresh, J. (2009). A new equation to estimate glomerular filtration rate. Annals of Internal Medicine, 150, 604–612. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2763564/pdf/nihms132246.pdf10.7326/0003-4819-150-9-200905050-00006
- Levin, A., Djurdjev, O., Beaulieu, M., & Er, L. (2008). Variability and risk factors for kidney disease progression and death following attainment of stage 4 CKD in a referred cohort. American Journal of Kidney Diseases, 52, 661–671. doi:10.1053/j.ajkd.2008.06.023
- Little, R. J. A., & Rubin, D. B. (2002). Missing data in experiments. In N. J. Hoboken (Ed.), Statistical analysis with missing data (2nd ed., pp. 24–40). Hoboken, NJ: Wiley. doi:10.1002/9781119013563
- Liu, B., Wang, T., Zhao, H. N., Yue, W. W., Yu, H. P., Liu, C. X., ... Nie, H.W. (2011). The prevalence of hyperuricemia in China: A meta-analysis. BMC Public Health, 11, 832. doi:10.1186/1471-2458-11-832
- Liu, W. C., Hung, C. C., Chen, S. C., Yeh, S. M., Lin, M. Y., Chiu, Y. W., ... Chen, H. C. (2012). Association of hyperuricemia with renal outcomes, cardiovascular disease, and mortality. Clinical Journal of the American Society of Nephrology, 7, 541–548. doi:10.2215/CJN.09420911
- Matsushita, K., Mahmoodi, B. K., Woodward, M., Emberson, J. R., Jafar, T. H., Jee, S. H., ... Levey, A. S. (2012). Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA, 307, 1941–1951. doi:10.1001/jama.2012.3954
- Matsushita, K., Selvin, E., Bash, L. D., Franceschini, N., Astor, B. C., & Coresh, J. (2009). Change in estimated GFR associates with coronary heart disease and mortality. Journal of the American Society of Nephrology, 20, 2617–2624. doi:10.1681/ASN.2009010025
- Mazzali, M., Kanellis, J., Han, L., Feng, L., Xia, Y. Y., Chen, Q., ... Johnson, R. J. (2002). Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. American Journal of Physiology - Renal Physiology, 282, F991–F997. doi:10.1152/ajprenal.00283.2001
- Nakagawa, T., Mazzali, M., Kang, D. H., Sánchez-Lozada, L. G., Herrera-Acosta, J., & Johnson, R. J. (2006). Uric acid – a uremic toxin? Blood Purif, 24, 67–70. doi:10.1159/000089440
- Park, S. H., Shin, W. Y., Lee, E. Y., Gil, H. W., Lee, S. W., Lee, S. J., ... Hong, S. Y. (2011). The impact of hyperuricemia on in-hospital mortality and incidence of acute kidney injury in patients undergoing percutaneous coronary intervention. Circulation Journal, 75, 692–697. Retrieved from https://www.jstage.jst.go.jp/article/circj/75/3/75_CJ-10-0631/_pdf10.1253/circj.CJ-10-0631
- Schumacher, H. R., Jr, Becker, M. A., Lloyd, E., MacDonald, P. A., & Lademacher, C. (2009). Febuxostat in the treatment of gout: 5-yr findings of the FOCUS efficacy and safety study. Rheumatology (Oxford), 48, 188–194. doi:10.1093/rheumatology/ken457
- Schumacher, H. R., Jr, Becker, M. A., Wortmann, R. L., MacDonald, P. A., Hunt, B., Streit, J., ... Joseph-Ridge, N. (2008). Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: A 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis & Rheumatism, 59, 1540–1548. doi:10.1002/art.24209
- Smith, E. U., Díaz-Torné, C., Perez-Ruiz, F., & March, L. M. (2010). Epidemiology of gout: An update. Best Practice & Research Clinical Rheumatology, 24, 811–827. doi:10.1016/j.berh.2010.10.004
- Somkrua, R., Eickman, E. E., Saokaew, S., Lohitnavy, M., & Chaiyakunapruk, N. (2011). Association of HLA-B*5801 allele and allopurinol-induced stevens johnson syndrome and toxic epidermal necrolysis: A systematic review and meta-analysis. BMC Medical Genetics, 12, 43–119. doi:10.1186/1471-2350-12-118
- Tassaneeyakul, W., Jantararoungtong, T., Chen, P., Lin, P. Y., Tiamkao, S., Khunarkornsiri, U., ... Tassaneeyakul, W. (2009). Strong association between HLA-B*5801 and allopurinol-induced Stevens–Johnson syndrome and toxic epidermal necrolysis in a Thai population. Pharmacogenetics and Genomics, 19, 704–709. doi:10.1097/FPC.0b013e328330a3b8
- Vázquez-Mellado, J., Alvarez Hernández, E., & Burgos-Vargas, R. (2004). Primary prevention in rheumatology: The importance of hyperuricemia. Best Practice & Research Clinical Rheumatology, 18, 111–124. doi:10.1016/j.berh.2004.01.001
- Weng, S. C., Shu, K. H., Tarng, D. C., Cheng, C. H., Chen, C. H., Yu, T. M., ... Wu, M. J. (2014). Uric acid is highly associated with kidney allograft survival in a time-varying analysis. Transplantation Proceedings, 46, 505–510. doi:10.1016/j.transproceed.2013.09.038
- Weng, S. C., Tarng, D. C., Chen, C. M., Cheng, C. H., Wu, M. J., Chen, C. H., ... Shu, K. H. (2014). Estimated glomerular filtration rate decline is a better risk factor for outcomes of systemic disease-related nephropathy than for outcomes of primary renal diseases. PLoS ONE, 9, e92881. doi:10.1371/journal.pone.0092881