Abstract
Objective: Ten percent of adults presenting with iron deficiency anaemia (IDA) have underlying cancer. This study was undertaken to prospectively validate the observation in a previous retrospective study that three simple clinical parameters can usefully predict the likelihood of gastrointestinal (GI) malignancy on investigation of patients with IDA, and to screen for other potential clinical predictors of risk. Method: Observational study of a cohort of 643 subjects attending an IDA clinic at a District General Hospital between 2012 and 2015, with multivariable analysis of the predictive value of a series of clinical variables including sex, age and haemoglobin concentration ([Hb]) for underlying GI malignancy. Results: Analysis of the validation cohort data confirmed the original observation that sex, age, and Hb were associated with the risk of GI malignancy—the parsimonious model including only these variables yielded odds ratios of 1.9 (95% confidence interval (CI): 1.1, 3.3) for males vs. females; 1.6 (95% CI: 0.9, 2.9) for age >70 vs. ≤70 years; and 2.9 (95% CI: 1.2, 6.9) for [Hb] <90.6 g/l vs. >112 g/l. Combining data from the observation and validation cohorts (total n = 1,363) identified sub-groups with cancer risks ranging from 0% to over 20%. No other predictive clinical variables were identified. Conclusions: Three simple and objective clinical parameters can be combined to provide a clinically useful cancer risk stratification model for subjects with IDA. This may assist with patient counselling and the prioritisation of investigational resources.
Public Interest Statement
Iron deficiency anaemia (IDA) is a common medical condition. In about 10% of cases it is caused by an underlying cancer of the stomach or bowel, and it is often the first indication of this problem. Current guidelines therefore recommend that everyone with IDA should undergo endoscopy to examine these areas.
This study reports a detailed analysis of 643 patients with IDA. It confirms that the risk of underlying cancer can be predicted by three simple clinical criteria—age, sex and severity of anaemia.
The importance of this is that it identifies a sub-group of patients at high risk of cancer, who therefore warrant urgent investigation. It also identifies a sub-group at low risk of cancer, who may not require invasive investigation at all.
Competing Interests
The authors declare no competing interest.
1. Introduction
Iron deficiency anaemia (IDA) is a common clinical problem, with an overall incidence in excess of one case per 1,000 pa. Published case series have consistently shown that about 30% of males and post-menopausal females with IDA have significant underlying gastrointestinal (GI) pathology, with malignancy accounting for about a third of these, often in the absence of clear clinical pointers to the diagnosis (Camaschella, Citation2015; Liu & Kaffes, Citation2012; Pasricha et al., Citation2010; Rockey, Citation1999; Surgenor, Kirkham, Parry, Williams, & Snook, Citation2014).
The IDA clinic at Poole Hospital has been operational since 2004, and is the point of referral for the many patients with IDA who have minimal or no symptoms to indicate the nature or location of the underlying cause of iron deficiency (Surgenor et al., Citation2014). Basic patient data has been collected since inception for the purpose of clinical care, audit and service evaluation. The referral rate to the IDA clinic approaches 300 new patients per annum.
It is current standard practice to advise urgent investigation of IDA, on the grounds that there might be an underlying cancer (Goddard, James, McIntyre, & Scott, Citation2011). A simple but reliable pre-test predictor of risk would therefore help considerably with patient counselling. It could also improve the use of resources, with prioritisation of high-risk subjects for fast-track investigation, and perhaps avoidance of invasive investigation altogether in particularly low-risk individuals.
Previous work by our group and others (James, Chen, Goddard, Scott, & Goddard, Citation2005; Silva et al., Citation2014) has demonstrated that three simple and objective clinical variables—age, sex and haemoglobin concentration—appear to be strong and independent predictors of underlying GI malignancy in IDA. In the IDIOM study (Silva et al., Citation2014), the combination of these variables was used to derive a score corresponding to the percentage probability of underlying GI malignancy—which ranged from less than 2% in low-risk subgroups to more than 20% in high-risk subgroups. These studies do however have the shortcomings that both were retrospective in design and lacked an a priori hypothesis—simply because there was insufficient evidence to base such a hypothesis on.
The aims of this study were twofold. Firstly to provide prospective validation of the independent variables identified in the original IDIOM study as predictors of underlying GI malignancy. Secondly to determine whether other clinical variables might be additional predictors of GI malignancy risk in IDA.
2. Method
Anonymised clinical data were collected prospectively for all 643 new patients added to the Poole Hospital IDA register between 2012 and 2015, for whom information on the final diagnosis was available—the validation cohort. This data included age, sex, haemoglobin concentration [Hb], mean red cell volume (MCV), mean cell haemoglobin (MCH), erythrocyte sedimentation rate (ESR), family history (FH) of GI cancer, recent unexplained weight loss (>3 kg), use of regular aspirin and/or anti-inflammatory medications (ASA/NSAIDs) and final diagnosis.
Essentially the methodology and investigation algorithm used was as detailed in our previous paper (Silva et al., Citation2014). IDA was confirmed on the basis of iron studies. All patients were investigated by means of a gastroscopy and colonic examination—colonoscopy in most cases, CT colonography in a minority. The follow-up period concluded once these investigations had been undertaken. About 10% of subjects were unwilling, unable or not fit enough to complete these investigations, and were excluded from the analysis.
The predictive value of those variables shown to be independent risk factors for GI malignancy in the original IDIOM study (Silva et al., Citation2014) were compared for the original observation cohort of 720 subjects seen prior to 2012 reported in that study, and for this validation cohort. Subjects from the validation cohort with a complete data-set were used to screen for additional clinical variables that might be independent predictors of GI malignancy risk in IDA.
Least square regression analysis was used to determine which of the different patient characteristics and clinical variables predicted the occurrence of GI cancer. As the dependent variable was a dichotomous categorical variable, models used logistic regression. All analyses were undertaken using STATA (version 13) software, the aim being to identify a parsimonious model that provided the most appropriate explanation of variations in the occurrence of GI malignancy. As the study design was entirely observational and anonymous, the National Research Ethics Service deemed that formal Research Ethics Committee approval was not required.
3. Results
The validation cohort comprised 643 subjects, of whom 423 (65.8%) were female. The median age of this group was 71.3 years (IQR : 60.1–79.0), and the median [Hb] 103 g/l (IQR : 90.5–112.0). A total of 61 (9.5%) proved on investigation to have GI cancer.
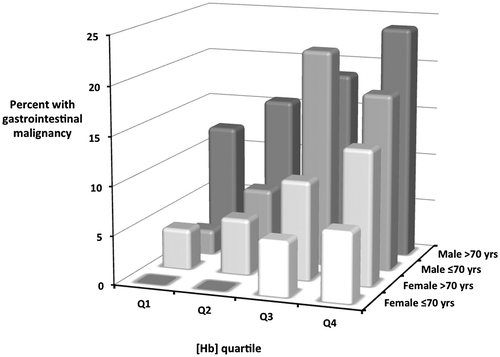
Multivariate analysis revealed that age, sex and [Hb] were independent predictors of the risk of GI malignancy in the validation cohort. The odd ratios tended to be marginally closer to unity than in the observation cohort, and the figure for age didn’t quite reach statistical significance (). A sub-group analysis for the 16 combinations of these three variables for the pooled data is shown in , with the relationship summarised in a 3 dimensional bar chart (). There was a wide spectrum of absolute GI malignancy risk, ranging from 0% in females under 70 with mild anaemia to 24% in males over 70 with more severe anaemia.
Table 1. Odds ratios (95% confidence intervals) for parsimonious multiple logistic regression models of the clinical variables associated with GI malignancy for the observation and validation cohorts (Poole Hospital 2004–2015)
Table 2. The percentage prevalence (with 95% CIs) of GI malignancy in 16 subgroups by sex, age and haemoglobin quartile of 1,363 subjects with IDA (Poole Hospital 2004–2015). Absolute numbers for each sub-group are shown immediately below.
Figure 1. The percentage prevalence of GI malignancy in 16 subgroups by sex, age and haemoglobin quartile of 1,363 subjects with IDA (Poole General 2004–2015). Haemoglobin quartile ranges are as in .
Of the 643 subjects in the validation cohort, 315 had a complete data-set for all variables—and the characteristics of this cohort are shown in . Multivariate analysis revealed no additional independent predictors of GI cancer risk amongst the variables analysed. The absence of an ESR result was the commonest reason for an incomplete data-set, and there were no major differences in the characteristics of those subjects excluded from the analysis due to missing data.
Table 3. Univariate odds ratios (95% CIs) and significance levels for the associations between other clinical characteristics and GI malignancy in 315 subjects (Poole Hospital 2012–2015)
4. Discussion
Malignancy is the most important GI pathology underlying IDA. Several retrospective multivariate analyses have attempted to identify clinical risk factors predictive of GI malignancy in subjects with IDA, with generally rather discordant conclusions (Capurso et al., Citation2004; Ho et al., Citation2005; James et al., Citation2005; Silva et al., Citation2014). However, some of these studies were relatively small and therefore likely to be underpowered, and none had a validation cohort. The combined data-set of well over 1,000 subjects with IDA presented in this paper is far larger than any published study on this topic.
The IDIOM (Iron Deficiency as an Indicator Of Malignancy) study demonstrated that age, sex and [Hb] are moderately strong independent predictors of underlying GI malignancy in patients with IDA (Silva et al., Citation2014)—in agreement with the largest previous analysis from Nottingham (James et al., Citation2005). Whilst the odds ratios tend to be smaller and the relationship with age is only borderline significant, the current study now provides prospective validation of this observation. It also demonstrates that other clinical variables do not appear to add usefully to cancer risk prediction.
Because these three clinical predictors are independent, they collectively provide a powerful predictor tool for cancer risk, as shown by the sub-group breakdown of the combined data-set presented in and , providing IDIOM scores equivalent to the percentage prevalence of GI malignancy. The risk broadly rises with worsening anaemia in each age/sex sub-group. At one extreme, none of 186 females under the age of 70 with mild anaemia proved to have underlying cancer, even though the majority of this group were post-menopausal. At the other end of the spectrum, GI malignancy was identified in over 20% of 217 males with more severe anaemia.
Why is risk stratification in IDA important? Many patients with unexplained IDA have minimal or no symptoms, and in view of the well-recognised cancer risk, fast-track referral for bidirectional endoscopy (BDE) is considered best practice regardless of age, sex or degree of anaemia (Goddard et al., Citation2011). However, BDE carries a small but significant risk of major complications—especially in older patients and those with major co-morbidities. It is also a labour-intensive process that places a significant burden on Endoscopy Departments—each BDE can take up to an hour to complete thoroughly, and we estimate that 5–10% of all GI endoscopy procedures are for the investigation of IDA. Yet the overall yield of this approach is relatively poor, with only 1 in 10 patients proving to have malignant pathology.
Validation of a simple risk stratification system is important because it is a tool that would help clinicians to counsel patients regarding the risk/benefit ratio of invasive investigation, and to target those investigations more appropriately (Kaminski et al., Citation2014; Snook, Citation2014; Wong et al., Citation2014). In particular, a case could be made for avoiding invasive investigation in low risk sub-groups as long as they exhibit a sustained haematological response to iron replacement therapy. It is worth highlighting that several of these low-risk IDIOM sub-groups have a cancer risk that is less than the arbitrary threshold of 3% currently advocated for the justification of fast-track referrals from primary care in the UK. At the other end of the spectrum, subjects in high-risk IDIOM sub-groups clearly warrant swift investigation.
How good a risk stratifcation system is the IDIOM score? The perfect stratification system would allocate all subjects to a low-risk or high-risk sub-group with 0% or 100% risk respectively, but this is rarely achievable in clinical practice. A more pragmatic assessment might be to consider the proportion of patients in stratification sub-groups with a risk that is more than double or less than half of the overall mean population risk. The figures of 7% and 23% respectively for our combined data-set compare favourably with those from other recently published cancer risk stratification systems (Kaminski et al., Citation2014; Wong et al., Citation2014).
Can the IDIOM risk stratification system be improved further? This remains to be established, but we believe that it might be. Faecal occult blood (FOB) testing is a simple and non-invasive investigation that has already been shown to be of some value in identifying patients with IDA due to underlying GI malignancy (Chowdhury, Longcroft-Wheaton, Davis, Massey, & Goggin, Citation2014; Cilona, Zullo, Hassan, Ridola, & Annese, Citation2011; Majid, Salih, Wasaya, & Jafri, Citation2008; Nakama, Zhang, Fattah, & Zhang, Citation2011). We are currently exploring the possibility of combining FOB testing with the IDIOM score to assess whether this further enhances risk stratification.
In conclusion, the original IDIOM study derived a model to predict the risk of underlying GI malignancy in patients with IDA based on their age, sex and [Hb]. This has been prospectively validated in the present study, which has not revealed any additional significant clinical predictors of risk. The findings provide a simple yet valuable method of clinical risk stratification for individual patients with IDA.
Additional information
Funding
Notes on contributors
Clare M. Wijayasekara
Poole Hospital is a busy district general hospital in the south of England. The Gastroenterology Unit has a long track record of clinical research, notably in the areas of coeliac disease and inflammatory bowel disease. We have also developed a particular interest in clinical aspects of iron deficiency anaemia (IDA), and set up the first dedicated IDA clinic in the UK in 2004.
The current paper is one of a series of publications resulting from data generated by this clinic. It provides prospective validation of the previous observation that subjects with IDA can be stratified according to cancer risk by the use of three simple clinical criteria – age, sex and severity of anaemia. The risk in sub-groups ranges from less than 2% to over 20%, and so can usefully inform clinical decision-making.
Follow-on studies are underway aiming to further enhance this risk stratification system.
References
- Camaschella, C. (2015). Iron deficiency anaemia. The New England Journal of Medicine, 372, 1832–1843.
- Capurso, G., Baccini, F., Osborn, J., Panzuto, F., Di Giulio, E., Delle Fave, G., & Annibale, B. (2004). Can patient characteristics predict the outcome of endoscopic evaluation of iron deficiency anemia: A multiple logistic regression analysis. Gastrointestinal Endoscopy, 59, 766–771.10.1016/S0016-5107(04)00348-7
- Chowdhury, A. T. D. M., Longcroft-Wheaton, G., Davis, A., Massey, D., & Goggin, P. (2014). Role of faecal occult bloods in the diagnosis of iron deficiency anaemia. Frontline Gastroenterology, 5, 231–236.10.1136/flgastro-2013-100425
- Cilona, A., Zullo, A., Hassan, C., Ridola, L., & Annese, M. (2011). Is faecal-immunochemical test useful in patients with iron deficiency anaemia and without overt bleeding? Digestive & Liver Disease, 43, 1022–1024.
- Goddard, A. F., James, M. W., McIntyre, A. S., & Scott, B. B. (2011). Guidelines for the management of iron deficiency anaemia. Gut, 60, 1309–1316.10.1136/gut.2010.228874
- Ho, C. H., Chau, W. K., Hsu, H. C., Gau, J. P., You, J. Y., & Chen, C. C. (2005). Predictive risk factors and prevalence of malignancy in patients with iron deficiency anemia in Taiwan. American Journal of Hematology, 78, 108–112.10.1002/(ISSN)1096-8652
- James, M. W., Chen, C. M., Goddard, W. P., Scott, B. B., & Goddard, A. F. (2005). Risk factors for gastrointestinal malignancy in patients with iron deficiency anaemia. European Journal of Gastroenterology & Hepatology, 17, 1197–1203.
- Kaminski, M. F., Polkowski, M., Kraszewska, E., Rupinski, M., Butruk, E., & Regula, J. (2014). A score to estimate the likelihood of detecting advanced colorectal neoplasia at colonoscopy. Gut, 63, 1112–1119.10.1136/gutjnl-2013-304965
- Liu, K., & Kaffes, A. J. (2012). Iron deficiency anaemia: A review of diagnosis, investigation and management. European Journal of Gastroenterology & Hepatology, 24, 109–116.
- Majid, S., Salih, M., Wasaya, R., & Jafri, W. (2008). Predictors of gastrointestinal lesions on endoscopy in iron deficiency anemia without gastrointestinal symptoms. BMC Gastroenterology, 8, 158.10.1186/1471-230X-8-52
- Nakama, H., Zhang, B., Fattah, A. S., & Zhang, X. (2011). Colorectal cancer in iron deficiency anemia with a positive result on immunochemical fecal occult blood. International Journal of Colorectal Disease, 15, 271–274.
- Pasricha, S. R., Flecknoe-Brown, S. C., Allen, K. J., Gibson, P. R., McMahon, L. P., Olynyk, J. K., ... obinson, K. L. (2010). Diagnosis and management of iron deficiency anaemia: A clinical update. Medical Journal of Australia, 193, 525–532.
- Rockey, D. C. (1999). Gastrointestinal tract evaluation in patients with iron deficiency anaemia. Seminars in Gastrointestinal Disease, 10, 53–64.
- Silva, A., Sheppard, Z. A., Surgenor, S. L., Williams, E. J., Thomas, P. W., & Snook, J. A. (2014). Clinical risk factors for underlying gastrointestinal malignancy in iron deficiency anaemia: The IDIOM study. Frontline Gastroenterology, 5, 237–242.10.1136/flgastro-2013-100386
- Snook, J. A. (2014). Investigating for GI malignancy in iron deficiency anaemia – the case for risk stratification. Frontline Gastroenterology, 5, 225–226.
- Surgenor, S. L., Kirkham, S., Parry, S. D., Williams, E. J., & Snook, J. A. (2014). The development of a nurse-led iron deficiency anaemia service in a district general hospital. Frontline Gastroenterology, 5, 219–223.10.1136/flgastro-2013-100385
- Wong, M. C. S., Lam, T. Y. T., Tsoi, K. K. F., Hirai, H. W., Chan, V. C. W., Ching, J. Y. L., ... Sung, J. J. Y. (2014). A validated tool to predict colorectal neoplasia and inform screening choice for asymptomatic subjects. Gut, 63, 1130–1136.10.1136/gutjnl-2013-305639
