Abstract
A 46-year-old female who lost her native kidney function due a biopsy proven collapsing type of FSGS, had a living kidney transplant after which, she developed acute renal allograft dysfunction, thrombocytopenia and microangiopathichemolytic anemia five days post-transplant. Renal biopsy revealed acute antibody-mediated rejection (AMR). Genetic studies showed that this patient has a homozygous mutation of complement factor B (CFB) gene and heterozygous variant of C 3 gene consistent with atypical hemolytic uremic syndrome type 4. Intravenous immunoglobulin (IV IG) and plasma exchange did not resolve these abnormalities. Eculizumab and bortezomib, on other hand, were very effective.
Public Interest Statement
Rejection is a common cause of kidney transplant dysfunction or loss. Rejection comes in a number of foams of which one is caused by the presence of circulating antibodies formed against the tissue of the donor tissue and thus attack and damage the kidney from that donor. This form of rejection is called antibody–mediated rejection (AMR).
We describe a case of this type of rejection which was rather unusual in that this AMR uncovered a genetic abnormality in the competent system in this patient. This triggered the expression of a disease called a typical hemolytic uremic syndrome (aHUS) which I turn contributed to the propagation of severe AMR in this patient’s allograft.
The genetic defects of complement in our patients is in factor B and C3. This clinical scenario has not been previously described in these particular genetic mutations.
Competing Interests
Authors declare no conflict of interest.
1. Introduction
Hemolytic uremic syndrome (HUS) is characterized by thrombi in the microvasculature, hemolytic anemia, consumptive thrombocytopenia, and end-organ dysfunction commonly kidneys (Polito & Kirsztajn, Citation2010).
Post renal–transplant hemolytic uremic syndrome is a recognized cause of graft failure (Schwimmer, Nadasdy, Spitalnik, Kaplan, & Zand, Citation2003) and carries significant morbidity and mortality (Bresin et al., Citation2006). It may develop de novo or recur in patients with end stage renal disease secondary to hemolytic uremic syndrome. The incidence of hemolytic uremic syndrome in renal allograft varies between 0.8 and 14% in different studies (Noris & Remuzzi, Citation2010; Ruggenenti, Citation2002; Schwimmer et al., Citation2003) and is associated with graft loss in 22% of cases (Schwimmer et al., Citation2003). Antibody-mediated rejection (AMR), immunosuppressive medications, pregnancy, viral infection and ischemia—reperfusion injury are all recognized triggers of post renal—transplant HUS in susceptible individual (Bresin et al., Citation2006; Murer et al., Citation2000; Noris & Remuzzi, Citation2010; Robson, Cote, Abbs, Koffman, & Goldsmith, Citation2003; Ruggenenti, Citation2002; Satoskar et al., Citation2010; Waiser, Budde, Rudolph, Ortner, & Neumayer, Citation1999). Cyclosporine and tacrolimus are the most common reported triggers of de novo HUS in renal allograft transplant. This is attributed to their endothelial toxicity and vasoconstrictive actions (Pham et al., Citation2000). The high frequency of the genetic complement regulatory proteins mutations among patients with post renal transplant HUS suggest that genetic abnormalities may represent risk factors for de novo HUS after kidney transplantation and raise the question of the best therapeutic strategies (Le Quintrec et al., Citation2008; Noris & Remuzzi, Citation2010; Zuber et al., Citation2012, Citation2013). We present a case of what we believe is the first case of post renal transplant de novo HUS in a patient with complement factor B (CFB) and C3 genetic mutation most probably triggered by AMR which was proven by histology and donor specific antibody (DSA) and which did not respond to IV IG and plasma exchange but did respond to eculizumab and bortezomib.
2. Case presentation
This is a case of 46-year-old married smoker lady. She has body mass index of 36. She was diagnosed with chronic kidney disease (CKD) secondary to focal segmental glomerulosclerosis (FSGS) based on a renal biopsy finding which did not respond to steroids, cyclosporine and mycophenolate mofetil and progressed to CKD stage 5 requiring dialysis for eight months dialysis before receiving a living donor transplant. She had a history of hypertension, hypothyroidism and bronchial asthma. She is para 3+0 with no recent blood transfusion.
She was admitted for living unrelated renal transplant from her 24-year-old stepson. Flow cytomtery cross matching was negative for B and T cells. There were six mismatches and class I DSA against B65 (4,335 mean fluorescence intensity (MFI)) and B63 (2,735 MFI). Prior to her transplant, she received 2 g of intravenous immunoglobulin (IVIG) and four sessions of plasmapheresis which brought down her B63 DSA to 1,442 MFI and B65 DSA to 2,161 MFI. She was induced with ATG, (cumulative dose of 7 mg/kg), methyl prednisone 500 mg, mycophenolate mofetil 1 g twice a day and tacrolimus 2 mg twice a day at the time of transplantation. The immediate post-transplant course was smooth with the serum creatinine level dropping to 72 μmol/L by the fourth day. Following that, the serum creatinine rose rapidly to reach 500 μmol/L by the ninth day ().
Table 1. Biochemical hematological and immunological changes from day of transplant onwards
Repeat cross match, at that time was negative for B cell and positive for T cell. The titer for B63 DSA rose to 12,592 MFI and for t B65 DSA increased to 5,043 MFI (the cutoff value of the assay was 500 MFI).
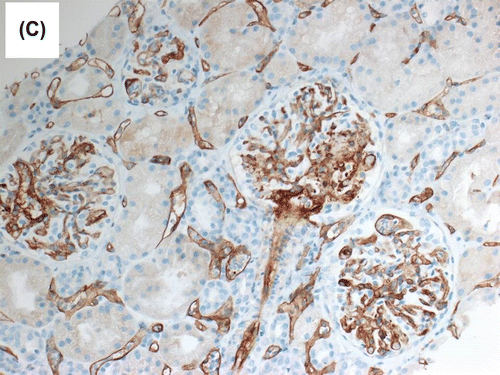
During the same period, the platelet started to fall and LDH started to rise () with the presence of schistocytes in the blood film, a hematological picture consistent with HUS. The patient was given IVIG, tacrolimus was switched to cyclosporine and plasmapheresis commenced with no response. Eculizimab was, therefore, given with good and marked response in platelet count, LDH level, and plateauing and subsequent decrease of serum creatinine. Histological examination showed morphological features of acute AMR. The glomeruli showed mild increase in mesangial matrix with no significant increase in mesangial cellularity. Segmental glomerular scarring in the form of obliteration of the glomerular capillaries and adhesion of the glomerular tufts to the Bowman’s capsule was identified in three glomeruli. The glomerular basement membranes (GBM) showed segmental mild increase in thickness on periodic acid Schiff (PAS) and methenamine silver special stains. No spike reaction or definite double contouring of the GBM was seen. Mild transplant glomerulitis was noted. Segmental glomerular fibrinoid necrosis with karyorrhexis was evident in one glomerulus (Figure ), and arteriolar intimal fibrinoid necrosis was seen at the hilum of one glomerulus. There was no endocapillary hypercellularity, crescent formation or glomerular microthrombi. Moderate interstitial inflammation that involved approximately 30% of renal cortical parenchyma was present, and consisted mainly of lymphocytes and neutrophils (Figure ). This was associated with focal mild lymphocytic tubulitis. Dilatation of the peritubular capillaries and severe peritubular capillaritis (PTC3) were evident; the inflammatory cells in the peritubular capillaries were mixed of mononuclear and polymorph nuclear cells. There was mild interstitial fibrosis and tubular atrophy. Focal mild endarteritis was identified, along with focal interstitial arteriolar fibrinoid necrosis. Immunohistochemical staining for C4d (dilution 1:40, polyclonal Abcam, Cambridge, UK) showed diffuse and strong linear staining in the peritubular capillaries (C4d 3+) in the renal cortex and medulla (Figure ).
Figure 1a. Mild transplant glomerulitis was noted. Segmental glomerular fibrinoid necrosis with karyorrhexis was evident in one glomerulus (periodic acid Schiff (PAS) stain).
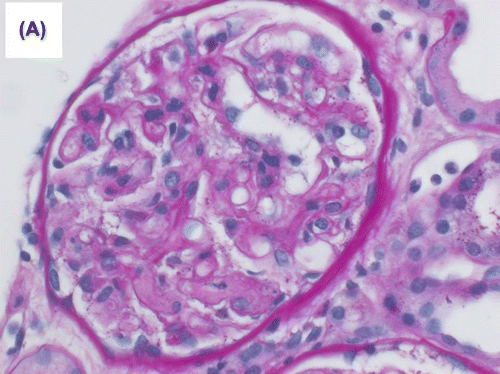
Figure 1b. Moderate interstitial inflammation that involved approximately 30% of renal cortical parenchyma was present, and consisted mainly of lymphocytes and neutrophils.
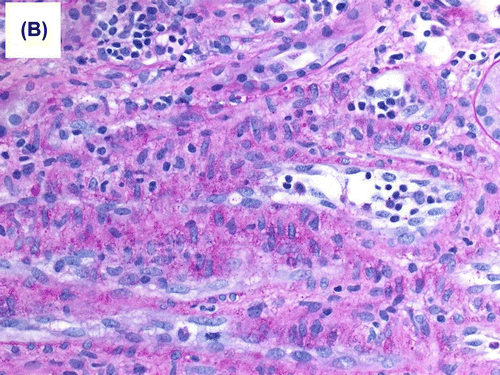
Figure 1c. Immunohistochemical staining for C4d (dilution 1:40, polyclonal Abcam, Cambridge, UK) showed diffuse and strong linear staining in the peritubular capillaries.
Accordingly, four doses of bortezomib (2.5 mg subcut) were given as rescue therapy for severe AMR. Genetic screening for mutations for atypical HUS revealed type 4 mutational abnormality (homozygous mutation CFB and heterozygous mutation C3). The patient was maintained on eculizimab, tacrolimus and mycophenolate mofetil. Her latest laboratory investigations, three months after transplant showed serum creatinine of 148 μmol/L, FK level of 5.6 ng/ml, LDH level of 251 u/L and a platelet count of 193 × 1,000/μl. No protein was detected in the urine.
3. Discussion
Post renal transplant thrombotic microangiopathy may be localized within the kidney transplant or be associated with a full-blown hemolytic uremic syndrome (Noris & Remuzzi, Citation2010; Schwimmer et al., Citation2003). It may be recurrent or be de novo (Noris & Remuzzi, Citation2010; Ruggenenti, Citation2002; Satoskar et al., Citation2010; Zuber et al., Citation2011). All known causes of HUS that are seen in general population may also affect the renal transplant recipient. However, HUS in renal transplant recipient may be specially associated with calcineurin inhibitors (CNI), mTOR inhibitors, infections (including parvovirus, CMV) and AMR (Bresin et al., Citation2006; Murer et al., Citation2000; Noris & Remuzzi, Citation2010; Robson et al., Citation2003; Satoskar et al., Citation2010; Waiser et al., Citation1999). Both AMR and HUS can trigger the development and propagation of each other, affect the renal microvasculature and cause endothelial damage (Artz, Steenbergen, Hoitsma, Monnens, & Wetzels, Citation2003; Bresin et al., Citation2006; Noris & Remuzzi, Citation2010; Satoskar et al., Citation2010; Vergoulas, Citation2006; Zuber et al., Citation2011). This may explain why patients who have acute vascular rejection with underlying HUS as the primary disease present with more severe rejection episodes compared to the patients without HUS (Artz et al., Citation2003; Vergoulas, Citation2006). De novo HUS can affect 3–14% of kidney transplant (Ruggenenti, Citation2002). Thirty percent of renal transplant recipients who develop de novo HUS post renal transplantation have genetic abnormalities in complement regulators (Zuber et al., Citation2011). Usually post-renal transplant de novo HUS occurs in the early post-transplant period but it may also occur at latter stage (Karthikeyan, Parasuraman, Shah, Vera, & Venkat, Citation2003; Noris & Remuzzi, Citation2010; Satoskar et al., Citation2010; Zarifian, Meleg-Smith, O’Donovan, Tesi, & Batuman, Citation1999). In our case, the finding of positive C4d staining along the cortical peritubular capillaries and presence of circulating donor –specific antibodies (DSA) are in keeping with diagnosis of AMR. In addition, the presence of schistocytes in blood smear, hemolytic anemia, high LDH level, negative direct coomb’s test and thrombocytopenia with complement factors mutations are typical of aHUS. These clinicopathological findings are compatible concurrent with complement mediated de novo HUD and AMR.
The clinical and laboratory manifestations in our patient which poorly responded to treatment with daily plasma exchange and intravenous immunoglobulin, responded effectively to eculizumab and boretzomib. Eculizumab, a monoclonal C5 inhibitor antibody prevents induction of the terminal complement cascade (TCC) and has recently emerged as a therapeutic option for AMR (Locke et al., Citation2009; Lucas, Co, Nwaogwugwu, Dosani, & Sureshkumar, Citation2011). Very few cases of AMR induced by, or manifested as de novo HUS have been reported and some were associated with mutation in complement factor H-related protein (Broeders, Stordeur, Rorive, & Dahan, Citation2014; Noone et al., Citation2012). Noone et al. (Citation2012) reported a case of a highly sensitized 13-year-old female with end-stage kidney disease secondary to spina bifida-associated reflux nephropathy, who developed severe steroid, ATG and plasmapheresis-resistant AMR with HUS one week after her second kidney transplant, bur responded well to eculizumab therapy. Their patient was proven to have genetic abnormality of complement factor H-related protein 3/1 (CFHR3/1). Chandran, Baxter-Lowe, Olson, Tomlanovich, and Webber (Citation2011) reported a case of a 34-year-old female who underwent simultaneous pancreas—kidney transplantation and who developed AMR and de novo HUS seven days post-transplant. She also responded well to eculizumab. Genetic screen for complement abnormality was not performed in this patient. The third case was reported by Broeders et al. (Citation2014). This was a 41-year-old female with CFH abnormality. Noticeably, all reported cases of AMR and HUS were females and the disease presentation was at the early post renal transplant period and all responded effectively to eculizumab. While our case showed CFB and C3 abnormality, the other two cases in which genetic studies were done, showed complement factor H abnormality. Bortezomib, a proteasome inhibitor, was used in our case as rescue therapy for severe AMR and to reduce the high DSA titer (Everly et al., Citation2008; Lucas et al., Citation2011; Noris & Remuzzi, Citation2010). It was not used in the other reported cases. The primary renal disease of the native kidneys in our case was FSGS, which can result in nephrotic range proteinuria induced endothelial injury of the glomerular capillaries and subsequent development of TMA (Manenti et al., Citation2013). On the other hand, acute ischemia induced by TMA may aggravate proteinuria by causing podocyte fusion and dysfunction (Barisoni, Schnaper, & Kopp, Citation2007). The clinical significance of such association between FSGS and HUS remains to be defined further.
Screening for complement mutations should be considered in patients with AMR associated HUS since this has implications on the management, prognosis, immunosuppressant choice, future renal transplant and living related kidney donation.
Eculizumab seems very effective in management of such patients who respond poorly to IV IG and plasmapheresis (Broeders et al., Citation2014; Noone et al., Citation2012; Noris & Remuzzi, Citation2010; Zuber et al., Citation2012). It is not clear, at this stage, whether adding bortezomib has additional prognostic value although it has been recently shown to be of some benefit in AMR (Everly et al., Citation2008; Lucas et al., Citation2011).
To the best of our knowledge, this is the first case report of a patient carries CFB and C3 genetic mutations, who developed de novo HUS induced by AMR after renal transplantation.
Additional information
Funding
Notes on contributors
Abdulla Al Sayyari
Professor Abdulla Al Sayyari is clinical professor of Medicine at King Saud bin Abdulaziz University for Health Sciences and Head, Division of Nephrology & Renal Transplantation, King Abdulaziz Medical City, Riyadh. He qualified with BSc (Honors) in Biochemistry and MB BS (Honors) in Medicine both from University College, London. He then obtained a Doctor of Medicine Degree (MD) from the same university. He is the Chairman, Saudi National Committee of Kidney Transplantation. He authored or co-authored eight books. He published 286 peer reviewed cited articles. His main research interests are in renal transplantation especially in post-transplant Kaposi sarcoma, post-transplant pregnancy, post-transplant infections and Ramadan fasting by post-transplant patients. He also has published papers on the use and outcome of suboptimal and extended criteria kidney donors. He also has a long-standing interest in bioethics particularly in the ethics of kidney donation.
References
- Artz, M. A., Steenbergen, E. J., Hoitsma, A. J., Monnens, L. A., & Wetzels, J. F. (2003). Renal transplantation in patients with hemolytic uremic syndrome: High rate of recurrence and increased incidence of acute rejections. Transplantation, 76, 821–826.10.1097/01.TP.0000085083.74065.1B
- Barisoni, L., Schnaper, H. W., & Kopp, J. B. (2007). A proposed taxonomy for the podocytopathies: A reassessment of the primary nephrotic diseases. Clinical Journal of the American Society of Nephrology, 2, 529–542.10.2215/CJN.04121206
- Bresin, E., Daina, E., Noris, M., Castelletti, F., Stefanov, R., Hill, P., ... Remuzzi, G. (2006). Outcome of renal transplantation in patients with non-shiga-toxin associated hemolytic syndrome: prognostic significance of genetic background. Clinical Journal of the American Society of Nephrology, 1, 88–99.
- Broeders, E. N., Stordeur, P., Rorive, S., & Dahan, K. (2014). A ‘silent’, new polymorphism of factor H and apparent de novo atypical haemolytic uraemic syndrome after kidney transplantation. BMJ Case Rep, 2014, 23.
- Chandran, S., Baxter-Lowe, L., Olson, J. L., Tomlanovich, S. J., & Webber, A. (2011). Eculizumab for the treatment of de novo thrombotic microangiopathy post simultaneous pancreas-kidney transplantation—A case report. Transplantation Proceedings, 43, 2097–2101.10.1016/j.transproceed.2011.02.064
- Everly, M. J., Everly, J. J., Susskind, B., Brailey, P., Arend, L. J., Alloway, R. R., ... Woodle, E. S. (2008). Bortezomib provides effective therapy for antibody- and cell-mediated acute rejection. Transplantation, 86, 1754–1761.10.1097/TP.0b013e318190af83
- Karthikeyan, V., Parasuraman, R., Shah, V., Vera, E., & Venkat, K. K. (2003). Outcome of plasma exchange therapy in thrombotic microangiopathy after renal transplantation. American Journal of Transplantation, 3, 1289–1294.10.1046/j.1600-6143.2003.00222.x
- Le Quintrec, M., Lionet, A., Kamar, N., Karras, A., Barbier, S., Buchler, M., ... Frémeaux-Bacchi, V. (2008). Complement mutation-associated de novo thrombotic microangiopathy following kidney transplantation. American Journal of Transplantation, 8, 1694–1701.10.1111/ajt.2008.8.issue-8
- Locke, J. E., Magro, C. M., Singer, A. L., Segev, D. L., Haas, M., Hillel, A. T., ... Montgomery, R. A. (2009). The use of antibody to complement protein C5 for salvage treatment of severe antibody-mediated rejection. American Journal of Transplantation, 9, 231–235.
- Lucas, J. G., Co, J. P., Nwaogwugwu, U. T., Dosani, I., & Sureshkumar, K. K. (2011). Antibody-mediated rejection in kidney transplantation: An update. Expert Opinion on Pharmacotherapy, 12, 579–592.10.1517/14656566.2011.525219
- Manenti, L., Gnappi, E., Vaglio, A., Allegri, L., Noris, M., Bresin, E., ... Buzio, C. (2013). Atypical haemolytic uraemic syndrome with underlying glomerulopathies. A case series and a review of the literature. Nephrology Dialysis Transplantation, 28, 2246–2259.10.1093/ndt/gft220
- Murer, L., Zacchello, G., Bianchi, D., Dall’Amico, R., Montini, G., Andreetta, B., ... Zacchello, F. (2000). Thrombotic microangiopathy associated with parvovirus B19 infection after renal transplantation. Journal of the American Society of Nephrology, 11, 1132–1137.
- Noone, D., Al-Matrafi, J., Tinckam, K., Zipfel, P. F., Herzenberg, A. M., Thorner, P. S., ... Licht, C. (2012). Antibody mediated rejection associated with complement factor H-related protein 3/1 deficiency successfully treated with eculizumab. American Journal of Transplantation, 12, 2546–2553.10.1111/ajt.2012.12.issue-9
- Noris, M., & Remuzzi, G. (2010). Thrombotic microangiopathy after kidney transplantation. American Journal of Transplantation, 10, 1517–1523.10.1111/ajt.2010.10.issue-7
- Pham, P. T., Peng, A., Wilkinson, A. H., Gritsch, H. A., Lassman, C., Pham, P. C., & Danovitch, G. M. (2000). Cyclosporine and tacrolimus associated thrombotic microangiopathy. American Journal of Kidney Diseases, 36, 844–850.10.1053/ajkd.2000.17690
- Polito, M. G., & Kirsztajn, G. M. (2010). Thrombotic microangiopathies: Thrombotic thrombocytopenia purpura / hemolytic uremic syndrome. Brazilian Journal of Nephrology, 32, 303–315.10.1590/S0101-28002010000300013
- Robson, M., Cote, I., Abbs, I., Koffman, G., & Goldsmith, D. (2003). Thrombotic micro-angiopathy with sirolimus-based immunosuppression: potentiation of calcineurin-inhibitor-induced endothelial damage? American Journal of Transplantation, 3, 324–327.10.1034/j.1600-6143.2003.00051.x
- Ruggenenti, P. (2002). Post-transplant hemolytic–uremic syndrome. Kidney International, 62, 1093–1104.10.1046/j.1523-1755.2002.00543.x
- Satoskar, A. A., Pelletier, R., Adams, P., Nadasdy, G. M., Brodsky, S., Pesavento, T., ... Nadasdy, T. (2010). De novo thrombotic microangiopathy in renal allograft biopsies-role of antibody-mediated rejection. American Journal of Transplantation, 10, 1804–1811.10.1111/ajt.2010.10.issue-8
- Schwimmer, J., Nadasdy, T. A., Spitalnik, P. F., Kaplan, K. L., & Zand, M. S. (2003). De novo thrombotic microangiopathy in renal transplant recipients: A comparison of hemolytic uremic syndrome with localized renal thrombotic microangiopathy. American Journal of Kidney Diseases, 41, 471–479.10.1053/ajkd.2003.50058
- Vergoulas, G. V. (2006). Hemolytic uremic syndrome after renal transplantation. Hippokratia, 10, 99–104.
- Waiser, J., Budde, K., Rudolph, B., Ortner, M. A., & Neumayer, H. H. (1999). De novo hemolytic uremic syndrome postrenal transplant after cytomegalovirus infection. American Journal of Kidney Diseases, 34, 556–559.10.1016/S0272-6386(99)70085-5
- Zarifian, A., Meleg-Smith, S., O’Donovan, R., Tesi, R. J., & Batuman, V. (1999). Cyclosporine-associated thrombotic microangiopathy in renal allografts. Kidney International, 55, 2457–2466.10.1046/j.1523-1755.1999.00492.x
- Zuber, J., Le Quintrec, M., Krid, S., Bertoye, C., Gueutin, V., Lahoche, A., ... French Study Group for Atypical HUS. (2012). Eculizumab for atypical hemolytic uremic syndrome recurrence in renal transplantation. American Journal of Transplantation, 12, 3337–3354.10.1111/ajt.2012.12.issue-12
- Zuber, J., Le Quintrec, M., Morris, H., Frémeaux-Bacchi, V., Loirat, C., & Legendre, C. (2013). Targeted strategies in the prevention and management of atypical HUS recurrence after kidney transplantation. Transplantation Reviews, 27, 117–125.10.1016/j.trre.2013.07.003
- Zuber, J., Le Quintrec, M., Sberro-Soussan, R., Loirat, C., Frémeaux-Bacchi, V., & Legendre, C. (2011). New insights into postrenal transplant hemolytic uremic syndrome. Nature Reviews Nephrology, 7, 23–35.10.1038/nrneph.2010.155
