Abstract
Reports about relationship on renal function and vancomycin exposure are conflicted and limited. Goals: To identify if high serum vancomycin levels precede changes in serum creatinine or if it is secondary to reduced glomerular filtration and to analyze associated clinical conditions. Methods: retrospective cohort study, initially of 56.555 measurements of vancomycin levels from 511 patients admitted from December 2011 to June 2012 was analyzed. Patients with uncompleted dates were excluded and the correlation analysis was performed in 127 patients that were divided into four groups based on vancomycin levels (20 mg/mL) and creatinine (1.4 mg/dL) levels. After that, 80 medical charts of these patients was reviewed for the presence of comorbidities, sepsis, acute kidney injury and use of other nephrotoxic drugs. Results: there was a significant increase in vancomycin levels, when creatinine > 1.4 mg/dL and vancomycin ≤ 20 mg/mL (Group 3) on the first measurement. It was identified there was a significant association between creatinine > 1.4 mg/mL and vancomycin > 20 mg/mL, n = 7 (Group 4) in patients aged 50–59 years. Acute kidney injury was present in patients with previous higher vancomycin levels. Conclusions: Impaired renal function occurred before vancomycin levels measurements were taken, influencing the serum levels of vancomycin in the following days. In these patients the elevation of vancomycin did not lead to a worsening in renal function. There was a positive correlation between elevated creatinine and vancomycin levels in elderly, male and septic. Patients who need more attention were those with acute kidney injury who have previous higher vancomycin levels.
Public Interest Statement
Acute kidney injury is a serious complication of hospitalized patients that increases mortality and costs in the hospital. This terrible complication has many causes, including using of antibiotics to treat hospital infections. This article discusses the use of vancomycin, a broad spectrum antibiotic often using in hospital and their relation with acute kidney injury. This complication can be evited if vancomycin levels had be controlled but maybe other clinical factors can be associated, as the presence of another concomitant disease. For this purpose a retrospective study in a large hospital was done and found that the presence of a chronic kidney disease before increased the vancomycin levels was more important than only monitoring of vancomycin level, which costs and value needs more evaluated with controlled studies.
Competing Interests
The authors declare no competing interest.
1. Introduction
Drug-induced nephrotoxicity causes one third of intra-hospital AKI cases. Traditionally, drug-induced nephrotoxicity has been defined in literature as an increase of 0.3 mg/dL in the creatinine level (50%) above the base level or 50% decrease in creatinine clearance from the base level on two consecutive days, in the absence of an alternative explanation. Risk factors for the development of nephrotoxicity include: old age, female, having nephrotic syndrome, underlying chronic kidney disease (CKD), intravascular volume depletion, exposure to multiple nephrotoxic drugs and/or for an extended period of time, metabolic alterations such as hypocalcemia, hypokalemia and hypomagnesemia (Minejima et al., Citation2011; Perazella, Citation2009).
Vancomycin is excreted almost exclusively by the kidneys. Between 80 and 90% of the drug is excreted through urine within 24 h of its administration in patients with normal kidney function. About 5–8.5% of vancomycin’s depuration is extra-renal, possibly by hepatic conjugation. Vancomycin’s renal depuration decreases in a linear matter with the glomerular filtration rate, which represents a half-life of 100–200 h in anuric patients (Marinho et al., Citation2011; Pea et al., Citation2009; Rybak et al., Citation2009a). The use of serum levels as a guide for administration intervals resulted in a safer usage of vancomycin, avoiding complications related to nephrotoxicity. Latest data suggest a higher nephrotoxicity rate with higher vancomycin levels than 15–20 μg/mL recommend before the next dose (Carreno, Kenney, & Lomaestro, Citation2014; Gupta, Biyani, & Khaira, Citation2011; Hal, Paterson, & Lodise, Citation2013; Levine, Citation2006; Lodise, Patel, Lomaestro, Rodvold, & Drusano, Citation2009; Tattevin, Cremieux, Rabaud, & Gauzit, Citation2014; Tattevin, Saleh-Mghir, Davido, et al., Citation2013; Vandecasteele & De Vriese, Citation2010, Citation2011). As regards what was found, the results and conclusions of this study will help in the assessment of the incidence of vancomycin-induced nephrotoxicity in hospitalized patients and to identify whether high concentrations of vancomycin precede the increase in creatinine or if they are secondary to the reduction in the glomerular filtration rate, which may be useful since there is an increasing need for higher doses of the drug. Furthmore, there were few reports of a direct causal relationship between toxicity and serum concentrations of vancomycin, with existing conflicts regarding which is the preceding factor, as mentioned above.
The objectives of this study are: to evaluate the incidence of vancomycin-induced nephrotoxicity in hospitalized patients, to identify if the high concentration of vancomycin levels precede the increase in creatinine or if they are secondary to the reduction in the glomerular filtration rate, to analyze which clinical concomitant clinical factors can influence the nephrotoxicity of vancomycin.
2. Patients and methods
A retrospective cohort study was done, initially with 56.555 measurements of vancomycin levels from 511 patients and their correlation with the creatinine levels in hospitalized patients, admitted between the period of December 2011 and June 2012 at Hospital do Servidor Público Estadual de São Paulo/SP, Brazil (HSPE/SP). Vancomycin levels specimens were drawn during that period and were sent to Biochemical Laboratory of HSPE/SP. Patients with uncompleted dates, under the age of 18 years, with Chronic Kidney Disease (CKD) already on dialysis, those who had less than two serum dosages of vancomycin levels and/or creatinine, since that would render any correlation between doses impossible, were excluded.
After that, we identified 127 patients who had more than three of their vancomycin levels specimens taken and their creatinine levels also analyzed, obtained from the laboratory computerized system and were confronted to identical alterations, that is, the same as the ones of the creatinine serum levels on the day of the vancomycin levels dosage and if it was already altered (Cr > 1.4 mg/dL) prior to the increase of toxic levels present in the vancomycin serum (>20 μg/mL) taken during the valid period established by the institution’s protocol.
Patients with kidney lesion were identified in the laboratory data base when the creatinine serum level was over 1.4 mg/dL and with high vancomycin levels over 20 μg/mL.
For the correlation analysis, 127 patients were divided into 4 groups: Group 1 (Cr ≤ 1.4 mg/dL and Vanco ≤ 20 μg/mL); Group 2 (Cr ≤ 1.4 mg/dL and Vanco > 20 μg/mL); Group 3 (Cr > 1.4 mg/dL and Vanco ≤ 20 μg/mL); Group 4 (Cr > 1.4 mg/dL and Vanco > 20 μg/mL), as described in .
Figure 1. Division of the four groups in order to correlate the vancomycin (Vanco) and creatinine dosages (Cr), n = 127.
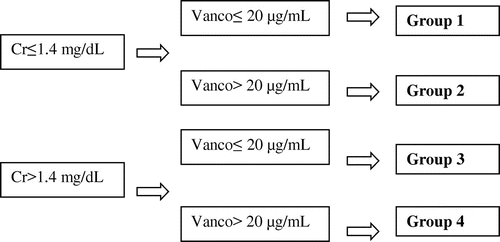
All patients used 1 g 12/12 h of vancomycin IV, before the dosage of vancomycin levels. Correlation between the vancomycin levels and creatinine levels was done, the denouement involving the recovery or absence of renal function and mortality through the information chart system.
Patients’ renal function was evaluated using the serum creatinine biomarker, taken using the enzymatic whitemetric method (Jaffè without desproteinization), using the hardware ADVIA (SIEMENS, Berlin, Germany, 2003).
The vancomycin levels were established in the Biochemistry Laboratory at HSPE/SP, through chemiluminescence, using the hardware AU 5800 (Backman Coulter). The therapeutic monitoring of vancomycin process involves determining serum levels at minimum levels (vale) as established by the institution’s protocol (Rybak et al., Citation2009a).
Before we started the data collection, our work was submitted to the Ethics Committee of the HSPE/SP After its approval, the data collection was requested and authorized as was the evaluation of the patients’ charts.
After carrying out the first correlations between serum vancomycin and creatinine dosage, 80 medical charts were reviewed to check the influence of clinical data in the alterations, such as comorbidities, sepsis, exams using contrast, and the use of known nephrotoxic drugs such as aminoglycosides, polymyxin, amphotericin B and Nonsteroidal anti-inflammatory drugs (NSAIDs). And the age groups were characterized to better analyze the influence of age in the correlation between increased creatinine and vancomycin levels. The age groups were categorized the following way: up to 49 years; 50–59 years, 60–69 years, 70–79 years and above 80 years.
For statistical analysis, non-parametric data were expressed as median upper and lower quartiles and compared using the Mann-Whitney test, for two samples and two moments the Wilconxon test was used. Continuous data and semi-continuous data were analyzed using the Pearson or Spearman correlation test. The χ2 test was performed crossing variables such as: gender, age, comorbidities (such as hypertension, diabetes), presence of sepsis, use of aminoglycosides, polymyxin and the diagnosis found in the chart of CKD and AKI with the correlations between Cr ( ≤ or > 1.4) and vancomycin levels ( ≤ or > 20) levels. The significance level was set to 0.05 for all of the tests; the analyses were performed using SPSS version 15.0 for Windows (Statistical Package for the Social Sciences, SPSS Inc., Chicago, IL, USA).
3. Results
The laboratory correlation analysis was performed in 127 patients. From those cases, patients mean age was of 64.7 ± 13 years, 59.6% were male and 40.4% were female. The average level of Cr in patients at 1st dosing was 2.38 ± 1.95 mg/dL. The average level of the first Vanco measured in the group was 22.9 ± 18.5 mg/mL. The number of deaths that occurred during the study period in which vancomycin was used was 21.6% ().
Table 1. Characteristics of the 127 patients using vancomycin
After applying the correlation test between the first dosages of Cr and Vanco and on the subsequent dosages, it was observed that the correlation was significant when the Cr > 1.4 mg/dL and ≤ Vanco 20 ug/mL in the first measurement. In the 2nd, 3rd and 4th days following dosages high levels of Cr and vancomycin were maintained and after the 5th dose there was significant correlation loss.
This results show that 31% of patients presented no change in renal function (creatinine ≤ 1.4 mg/dL) and no change in serum vancomycin (Vanco ≤ 20 mg/mL) levels. Whereas 27% had no change in renal function, but showed high levels of vancomycin levels (Vanco > 20 mg/mL). It can also be observed that 20% of patients presented changes in renal function (creatinine > 1.4 mg/dL), but did not present elevated serum levels of vancomycin. Finally, 22% of the studied patients presented alteration in renal function and high levels of vancomycin levels after the first dosage measurement. It was shown that 42% (Groups 3 and 4) of the patients presented a change in renal function (creatinine > 1.4 mg/dL) after the first dose, which no longer occurred after the 5th dose.
From the medical records we found that other nephrotoxic drugs were used, the most prevalent being: aminoglycosides (74.3%) and polymyxin B (21.4%), yet none of those drugs were correlated with the increased levels of Cr or Vanco levels. We found a significant association between Group 4 (Cr > 1.4 + Vanco > 20, n = 7) with patients in the age group 50–59 years—despite those in the age group between 60 and 69 years are more often those without high Cr—with Group 1 which presented a low Vanco (Cr ≤ 1.4 and Vanco ≤ 20, n = 11) as shown in .
Table 2. Correlation between the patients clinical characteristics while on vancomycin and the laboratory correlation (n = 80)
Males were more associated with Group 2 (Cr ≤ 1.4 + Vanco > 20, n = 17), as men had higher vancomycin levels without presenting changes in Cr levels. Patients with sepsis had the same profile CR ≤ 1.4 + Vanco > 20, as in males. Evolution to death was not significant with the laboratory correlation of higher Cr and increased vancomycin levels. All the data between laboratory and clinical correlations are shown in .
After reviewing the 80 charts, we observed that AKI was diagnosed in 65.7% (n = 46) of cases, as shown in , most being in cases with high vancomycin levels. A binary logistic regression of the analyzed variables was then performed and proved to be significant with the Q-square test (gender, age, presence of sepsis and AKI). It was observed that only medical diagnosis of AKI present on the chart was significant with the correlation between Cr > 1.4 + Vanco > 20, which means Group 4 (CR > 1.4 + Vanco > 20), was the group that had actually been diagnosed with AKI (3-fold) as shown in .
Table 3. Binary logistic regression of clinical factors associated with higher levels of creatinine (Cr) and vancomycin (Vanco), n = 80
4. Discussion
This study analyzes the direct causal relationship between the nephrotoxicity and serum concentrations of vancomycin and examines the temporal sequence of events involving changes in secondary renal function after being exposed to vancomycin (Rybak et al., Citation2009b). These results show that deterioration in renal function is a risk factor in elevated vancomycin levels. Past studies have argued that pre-existing renal dysfunction is associated with an increased risk of nephrotoxicity in patients treated with vancomycin (Carreno et al., Citation2014; Minejima et al., Citation2011; Perazella, Citation2009) but no studies that we are aware of mentioned the possibility of high levels of vancomycin levels being directly related to previous renal injury, not just with the nephrotoxic effect of vancomycin.
Our study initially showed that previous renal injury only increased the vancomycin levels with no association with its nephrotoxicity. However, high levels of vancomycin in patients with previous renal injuries ended up exposing them to greater amounts of vancomycin, thus generating greater susceptibility and also nephrotoxicity. It eventually turns into a vicious cycle where we have a history of renal injury, an accumulation of vancomycin due to this injury, with a slower elimination, raising vancomycin levels, increasing exposure to nephrotoxicity, worsening the renal injury. These two factors combined, prior renal injury and nephrotoxicity have the potential to further increase the vancomycin levels. So for a more efficient control of those patients who have an increased risk of toxicity due to pre-existing kidney damage, doctors need to consider reinforced monitoring strategies, such as daily creatinine levels, strict control of diuresis, and more frequent analysis of vancomycin levels (Carreno et al., Citation2014).
The correlation of elevated vancomycin levels, described in the results as being positive for those who already had change of Cr at the first dosage, appeared after the 5th day of dosing measurements, suggesting that the drug dosage adjustment according to renal function alteration or its suspension could have occurred and impacted the levels of vancomycin levels.
Most hospitalized patients who used vancomycin were male (59.6%), but that does not mean that they are more susceptible to nephrotoxicity, but that they were suffering from other conditions which required this antibiotic. According to Perazella (Citation2009), women are more susceptible to nephrotoxicity, thus being a risk factor however this was not shown in this study.
The large majority of the patients were elderly, with 60 of the 80 subjects analyzed in the study aged ≥60 years, which shows a greater susceptibility of this age group to conditions that require the use of vancomycin, meaning infections caused by more resistant microorganisms. During the analysis according to age group, there was not significant association with older age (), which means that just being elderly makes you at greater risk.
Our study showed that 21.6% of the deaths occurred while patients were on vancomycin, which represents a significant percentage, even though they were not directly correlated with cases of AKI. Another study noted that the development of AKI was significantly associated with a higher mortality rate within 28 days when related to nephrotoxicity by vancomycin (19%) than those who did not develop nephrotoxicity (5%), as said in the definition AKIN (Minejima et al., Citation2011).
Since this is an age group with much comorbidity or even due to the possibility of associations with other causes of nephrotoxicity, medical charts were also evaluated, as was the presence of other comorbidities, which were also not correlated to a higher nephrotoxicity risk factor as described in .
According to the literature, risk factors for nephrotoxicity associated with vancomycin can be categorized as being related to vancomycin’s formulation, the patient characteristics and nephrotoxic drugs administered concurrently (Carreno et al., Citation2014). They are also associated with prolonged periods of hospitalization, increase in mortality and the need for renal replacement therapy (Carreno et al., Citation2014).
As a strategy to limit the toxicity, prospect research can be used to improve understanding between the duration of vancomycin therapy and nephrotoxicity. In cases in which a prolonged exposure to the drug cannot be avoided, such as cases of endocarditis and osteomyelitis, aggressive monitoring schemes can be used to facilitate early detection of acute renal injury (Carreno et al., Citation2014; Gupta et al., Citation2011; Hal et al., Citation2013; Jeffres, Isakow, Doherty, Micek, & Kollef, Citation2007; Lodise, Lomaestro, Graves, & Drusano, Citation2008; Rybak et al., Citation2009b; Vandecasteele & De Vriese, Citation2010, Citation2011).
Body weight, preexisting renal dysfunction and severe diseases are the three most important factors associated with nephrotoxicity by vancomycin therapy. In the assessment of some risk factors for AKI, such as sepsis and aminoglycosides and polymyxin B, the presence of sepsis was a factor for differential diagnosis for AKI, being more significantly identified than with the use of other nephrotoxic drugs ().
Pre-existing renal dysfunction is associated with an increased risk of nephrotoxicity in patients treated with vancomycin (Carreno et al., Citation2014). As discussed above in our study, the presence of previous changes of serum creatinine was a factor correlated with increased serum levels of Vanco and thus increased even more the risk of nephrotoxicity in the studied patients (Cano et al., Citation2012; Carreno et al., Citation2014; Hall et al., Citation2013; Hidayat, Hsu, Quist, Shriner, & Wong-Beringer, Citation2006; Horey, Mergenhagen, & Mattappallil, Citation2012; Pritchard et al., Citation2010; Satoskar, Nadasdy, Plaza, et al., Citation2006).
In the study of the association between sepsis and nephrotoxicity induced by vancomycin, the fact of being in the intensive care unit (ICU) has been used as a marker by some authors (Davis, Scheetz, Bosso, Goff, & Rybak, Citation2013; van Hal & Fowler, Citation2013; Kullar, Davis, Taylor, Kaye, & Rybak, Citation2012; Roberts et al., Citation2014). This association was initially found in our patients, however in the binary regression analysis, it was not a factor correlated to deteriorating renal function with increased vancomycin levels ().
Studies of combined clinical and observational trials (Cosgrove et al., Citation2009; Jeffres et al., Citation2007; Rybak, Albrecht, Boike, & Chandrasekar, Citation1990), suggest that the concomitant use of aminoglycosides with vancomycin is associated with increased risk of nephrotoxicity. As we mentioned above, in our patients the usage of aminoglycosides was not correlated with the increase in Cr not with increased vancomycin levels. The vancomycin and other agents ratio, such as diuretics, anti-inflammatories, iodinated contrast agents amphotericin B, had inconclusive results. In our study, a positive correlation was found with the use of other agents evaluated, such as polymyxin B, amphotericin B, iodinated contrast media and NSAIDs. In any given case, the propensity for an increased risk of nephrotoxicity when there is a combination of vancomycin with other nephrotoxic drugs, the simultaneous use of those drugs should be avoided. When these associative therapies can’t be avoided, patients should be closely monitored on a daily basis so as to minimize the length of use of these nephrotoxic drugs (Carreno et al., Citation2014).
Based on the literature, the direction of any future research can help improve the safety of vancomycin usage, including: the use of alternative drugs to replace vancomycin, risk stratification, alternative dosage use and monitoring strategies (Carreno et al., Citation2014).
5. Conclusions
In this study renal dysfunction occurred before the increase in vancomycin levels, which influenced the serum levels measured the following days. After the 5th day of dosing there was no such correlation, suggesting that the drug dosage adjustment according to renal dysfunction or suspension could have occurred and impacted of vancomycin levels. The laboratory evaluation of these patients, the elevation of vancomycin did not cause deterioration in renal function not after the toxic levels were identified.
Although AKI diagnosis was higher in patients with renal dysfunction prior to the use of the drug, a positive correlation was found between increased Cr levels and vancomycin levels in the elderly, male and septic patients. These were the factors which increased vancomycin levels the most and need greater attention.
There was no positive association of mortality with the presence of other comorbidities and use of other nephrotoxic drugs in this study, although regular monitoring of renal function is suggested with these known risk factors.
Additional information
Funding
Notes on contributors
Benedito Jorge Pereira
Benedito Jorge Pereira, PhD in Sciences from the University of Sao Paulo (USP), nephrologist doctor at Nephrology Service of Hospital do Servidor Publico Estadual (HSPE) and University Nove de Julho. Participates of the group of Clinical Research of acute renal failure at HSPE.
References
- Cano, E. L., Haque, N. Z., Welch, V. L., Cely, C. M., Peyrani, P., Scerpella, E. G., ... Kett, D. H. (2012). Incidence of nephrotoxicity and association with vancomycin use in intensive care unit patients with pneumonia: Retrospective analysis of the IMPACT-HAP database. Clinical Therapeutics, 34, 149–157.10.1016/j.clinthera.2011.12.013
- Carreno, J. J., Kenney, R. M., & Lomaestro, B. (2014). Vancomycin-associated renal dysfunction: Where are we now? Pharmacotherapy, 34, 1259–1268.
- Cosgrove, S. E., Vigliani, G. A., Campion, M., Fowler, Jr., V. G., Abrutyn, E., Corey, G. R., ... Boucher, H. W. (2009). Initial low‐dose gentamicin for Staphylococcus aureus bacteremia and endocarditis is nephrotoxic. Clinical Infectious Diseases, 48, 713–721.10.1086/598174
- Davis, S. L., Scheetz, M. H., Bosso, J. A., Goff, D. A., & Rybak, M. J. (2013). Adherence to the 2009 consensus guidelines for vancomycin dosing and monitoring practices: A cross-sectional survey of U.S. hospitals. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, 33, 1256–1263.10.1002/phar.1327
- Gupta, A., Biyani, M., & Khaira, A. (2011). Vancomycin nephrotoxicity: Myths and facts. Netherlands Journal of Medicine, 69, 379–383.
- Hal, S. J. V., Paterson, D. L., & Lodise, T. P. (2013). Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother, 57, 734–744.
- Hall, 2nd., R. G., Hazlewood, K. A., Brouse, S. D., Giuliano, C. A., Haase, K. K.Frei, C. R., ... Alvarez, C. A. (2013). Empiric guideline-recommended weight-based vancomycin dosing and nephrotoxicity rates in patients with methicillin-resistant Staphylococcus aureus bacteremia: A retrospective cohort study. BMC Pharmacology and Toxicology, 14, 2138.10.1186/2050-6511-14-12
- Hidayat, L. K., Hsu, D. I., Quist, R., Shriner, K. A., & Wong-Beringer, A. (2006). High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections. Archives of Internal Medicine, 166, 2138–2144.10.1001/archinte.166.19.2138
- Horey, A., Mergenhagen, K. A., & Mattappallil, A. (2012). The relationship of nephrotoxicity to vancomycin trough serum concentrations in a veteran’s population: A retrospective analysis. Annals of Pharmacotherapy, 46, 1477–1483.10.1345/aph.1R158
- Jeffres, M. N., Isakow, W., Doherty, J. A., Micek, S. T., & Kollef, M. H. (2007). A retrospective analysis of possible renal toxicity associated with vancomycin in patients with health care-associated methicillin- resistant Staphylococcus aureus pneumonia. Clinical Therapeutics, 29, 1107–1115.10.1016/j.clinthera.2007.06.014
- Kullar, R., Davis, S. L., Taylor, T. N., Kaye, K. S., & Rybak, M. J. (2012). Effects of targeting higher vancomycin trough levels on clinical outcomes and costs in a matched patient cohort. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, 32, 195–201.10.1002/phar.2012.32.issue-3
- Levine, D. P. (2006). Vancomycin: A history. Clinical Infectious Diseases, 42, S5–S12.10.1086/491709
- Lodise, T. P., Lomaestro, B., Graves, J., & Drusano, G. L. (2008). Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrobial Agents and Chemotherapy, 52, 1330–1336.10.1128/AAC.01602-07
- Lodise, T. P., Patel, N., Lomaestro, B. M., Rodvold, K. A., & Drusano, G. L. (2009). Relationship between initial vancomycin concentration‐time profile and nephrotoxicity among hospitalized patients. Clinical Infectious Diseases, 49, 507–514.10.1086/599188
- Marinho, D. S., Huf, G., Ferreira, B. L. A., Castro, H., Rodrigues, C. R., Sousa, V. P., & Cabral, L. M. (2011). The study of vancomycin use and its adverse reactions associated to patients of a Brazilian university hospital. BMC Research Notes, 4, 236.10.1186/1756-0500-4-236
- Minejima, E., Choi, J., Beringer, P., Lou, M., Tse, E., & Wong-Beringer, A. (2011). Applying new diagnostic criteria for acute kidney injury to facilitate early identification of nephrotoxicity in vancomycin-treated patients. Antimicrobial Agents and Chemotherapy, 55, 3278–3283.10.1128/AAC.00173-11
- Pea, F., Furlanut, M., Negri, C., Pavan, F., Crapis, M., Cristini, F., & Viale, P. (2009). Prospectively validated dosing nomograms for maximizing the pharmacodynamics of vancomycin administered by continuous infusion in critically ill patients. Antimicrobial Agents and Chemotherapy, 53, 1863–1867.10.1128/AAC.01149-08
- Perazella, M. A. (2009). Renal vulnerability to drug toxicity. Clinical Journal of the American Society of Nephrology, 4, 1275–1283.10.2215/CJN.02050309
- Pritchard, L., Baker, C., Leggett, J., Sehdev, P., Brown, A., & Bayley, K. B. (2010). Increasing vancomycin serum trough concentrations and incidence of nephrotoxicity. The American Journal of Medicine, 123, 1143–1149.10.1016/j.amjmed.2010.07.025
- Roberts, J. A., Abdul-Aziz, M. H., Lipman, J., Mouton, J. W., Vinks, A. A., Felton, T. W., ... Kuti, J. L. (2014). Individualised antibiotic dosing for patients who are critically ill: Challenges and potential solutions. The Lancet Infectious Diseases, 14, 498–509.10.1016/S1473-3099(14)70036-2
- Rybak, M. J., Albrecht, L. M., Boike, S. C., & Chandrasekar, P. H. (1990). Nephrotoxicity of vancomycin, alone and with an aminoglycoside. Journal of Antimicrobial Chemotherapy, 25, 679–687.10.1093/jac/25.4.679
- Rybak, M. J., Lomaestro, B. M., Rotschafer, J. C., Moellering, Jr., R., Craig, W. A., Billeter, M., ... Levine, D. P. (2009a). Therapeutic monitoring of vancomycin in adult patients: A consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. American Journal of Health-System Pharmacy, 66, 82–98.10.2146/ajhp080434
- Rybak, M. J., Lomaestro, B. M., Rotschafer, J. C., Moellering, Jr., R. C., Craig, W. A., Billeter, M., ... Levine, D. P. (2009b Aug). Vancomycin therapeutic guidelines: A summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clinical Infectious Diseases, 49, 325–327.10.1086/599187
- Satoskar, A. A., Nadasdy, G., Plaza, J. A., Sedmak, D., Shidham, G., Hebert, L., & Nadasdy, T. (2006). Staphylococcus infection-associated glomerulonephritis mimicking IgA nephropathy. Clinical Journal of the American Society of Nephrology, 1, 1179–1186.10.2215/CJN.01030306
- Tattevin, P., Saleh-Mghir, A., Davido, B., Ghout, I., Massias, L., Garcia de la Maria, C., ... Cremieux, A. C. (2013). Comparison of six generic vancomycin products for treatment of methicillin-resistant Staphylococcus aureus experimental endocarditis in rabbits. Antimicrobial Agents and Chemotherapy, 57, 1157–1162.10.1128/AAC.01669-12
- Tattevin, P., Cremieux, A. C., Rabaud, C., & Gauzit, R. (2014). Efficacy and quality of antibacterial generic products approved for human use: A systematic review. Clinical Infectious Diseases, 58, 458–469.10.1093/cid/cit769
- Vandecasteele, S. J., & De Vriese, A. S. (2010). Recent changes in vancomycin use in renal failure. Kidney International, 77, 760–764.10.1038/ki.2010.35
- Vandecasteele, S. J., & De Vriese, A. S. (2011). Vancomycin dosing in patients on intermittent hemodialysis. Seminars in Dialysis, 24, 50–55.10.1111/sdi.2011.24.issue-1
- van Hal, S. J., & Fowler, Jr., V. G. (2013). Is it time to replace vancomycin in the treatment of methicillin-resistant Staphylococcus aureus infections? Clinical Infectious Diseases, 56, 1779–1788.10.1093/cid/cit178
