Abstract
Rheumatoid arthritis an antigen-driven autoimmune disease and the eliciting antigens are unknown. They should be present in immune complexes of synovial fluids. The immune complexes were isolated from synovial fluids of patients with rheumatoid arthritis and analyzed by polyacrylamide gel electrophoresis and Western blotting. Immune complexes contained IgG as major protein. The antibodies dissociated at pH 2.5 and self-aggregated upon neutralization. In ELISA assays, they had the highest specificity to homologous IgG, less to normal IgG and lowest of heterologous arthritic IgG confirming earlier results that altered IgG structures were antigenic. Sequencing of the Fc-regions revealed mutations in all patients analyzed. Therefore, mutations within the Fc-regions of IgG could be antigens in rheumatoid arthritis. We propose that methods should be developed to prove this hypothesis.
Public interest statement
Rheumatoid arthritis is a desastrous progressive autoimmune disease for which no causative cure is available, simply because the eliciting antigens are unknown despite intesive research efforts. Most patients have also Rheumatiod factor activity where antibodies bind to their own structures within the constant region. Here we considered, wether mutations in the constant regions of immunoglobulins could represent the eliciting antigens.
Competing Interests
The authors declare no competing interest.
1. Introduction
The chronic inflammation of rheumatoid arthritis mainly affects the synovial membranes of multiple joints and potentially involves vasculitis and pulmonary, ocular and cardiovascular systems. After the onset of the inflammation, the synovium changes dramatically (Edwards, Citation1998). The synovial intima is filled with B-lymphocytes engaged in antibody production against unknown antigens (Bläß, Engel, & Burmester, Citation1999). Infiltrations of plasma cells into the synovia are highly associated with inflammation of rheumatoid arthritis (Dong, Li, Liu, & Zhu, Citation2009; Reparon-Schuijt et al., Citation1998). The resulting immune complexes activate macrophages and complement and drive a T-cell dependent antibody production in the synovial tissue. The immune complexes are mainly rheumatoid factors that are defined as auto-antibodies against Fc-fragments of IgG (Tighe & Carson, Citation2001) and occur in about 90% of rheumatoid arthritis patients (Dörner, Egerer, Feist, & Burmester, Citation2004). Normally rheumatoid factors bind to an antibody-antigen complex and facilitate clearance by binding to Fc-receptors, fixation of complement and antigen processing by B-lymphocytes (Carson, Chen, & Kipps, Citation1991). The rheumatoid factor binding site resides in CH2-CH3 domain of Fc (Artandi, Calame, Morrison, & Bonagura, Citation1992; Bonagura et al., Citation1998; Sutton et al., Citation1998). However, rheumatoid factors are also found in other conditions of B-cell hyperreactivity.
The driving force for autoimmune diseases are self-reactive antibodies directed against “altered self” which can be modified proteins (Trouw, Huizinga, & Toes, Citation2013). So far posttranslational modifications have been detected in citrullinated antigens that are highly specific for rheumatoid arthritis. Citrulline residues arise from arginine by peptidyl arginine deiminase. However, this posttranslational modification cannot fully explain the pathogenesis of rheumatoid arthritis (Klareskog, Amara, & Malmström, Citation2014).
Changes of IgG glycosylation in the IgG were also thought to be involved in rheumatoid arthritis (Parekh et al., Citation1985), but recent studies showed that the glycosylation loci are not associated with rheumatoid arthritis (Yarwood et al., Citation2016).
Other modifications include oxidized IgG that are recognized by circulating lymphocytes leading to a proliferative response and secrete IL-2 (Grinnell, Yoshida, & Jasin, Citation2005). IgG is also covalently cross linked by reactive oxygen and nitric oxide products secreted by inflammatory cells (Uesugi, Hayashi, & Jasin, Citation1998).
IgG has long been implicated in the pathogenesis of rheumatoid arthritis. When immune complexes from synovial fluids of patients with rheumatoid arthritis were analyzed for their constituents, mainly IgG and IgM antibodies were found (Male & Roitt, Citation1981). They did not contain antibodies with rheumatoid factor specificity and a structural alteration of the IgG was considered as a cause for antigenicity (Carter, Makh, Ponsford, & Elson, Citation1989). Sutton, Corper, Bonagura, and Taussig (Citation2000) suggested that rheumatoid factors bind Fc-region and foreign antigen antigens simultaneously and the affinity is potentiated by somatic mutation. Indeed, Fc-binding antibodies from rheumatoid arthritis synovial fluids show imprints of an antigen-dependent process of somatic hypermutation and clonal selection in the variable regions of the L- and H-chains (Van Esch et al., Citation2003). It is clear that the synovium of patients with rheumatoid arthritis is prone to mutations (Firestein, Citation2010) and several multi-evidence genes in genome wide studies have been identified (Whitaker et al., Citation2015). All data suggest that synovial lymphocytes are selectively activated and clonally expanded by local antigens, whose nature remains enigmatic (Klareskog et al., Citation2014; Trouw et al., Citation2013; Yarwood et al., Citation2016). In this study of the CH3 domain we considered the possibility that the Fc-region might be mutated.
2. Patients and methods
2.1. Patients
Synovial fluids were aspirated from patients with rheumatoid arthritis that fulfilled the criteria for rheumatoid arthritis (Arnett, Edworthy, & Bloch, Citation1988). All patients signed an informed consent that the remainder of biological samples obtained for diagnostic purposes may be used anonymously for research. The patient′s identity was blurred by number codes. The study was approved by the local Medical Ethics Board. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
2.2. Materials
Chemicals were from Sigma Chemical Co. unless otherwise stated.
2.3. Isolation of immune complexes with active antigens
Synovial fluids were frozen immediately after aspiration at −20°C. After thawing at 0°C, debris was removed by centrifugation for 2 min at 10,000 g. The supernatant (15 ml) was mixed with 135 ml of cold phosphate buffered saline (PBS), and 15 ml of 30% polyethylene glycol in PBS was added while stirring the solution. It was kept for 15 min at 0°C and ultracentrifuged for 20 min at 100,000 g and 4°C. The sediment was dissolved in 2 ml of 150 mM NaCl, 50 mM sodium phosphate buffer pH 2.5 and mixed with 5 ml of a 1:1 suspension of Protein-A-Sepharose in cold PBS. The suspension was neutralized to pH 7.0 by addition of 1 M Tris-HCl pH 9.0 and poured into a small column. The beads were washed with 30 ml of cold PBS and eluted with cold 150 mM NaCl, 50 mM sodium phosphate buffer pH 2.5. The eluate was monitored by absorbance at 280 nm, the protein containing fractions were collected and the concentration of IgG was calculated using the specific absorption of OD280 = 1.0 at 1.4 mg/ml. The eluate was immediately frozen to −70°C or used for further analysis.
2.4. ELISA-assays
An aliquot of antibodies isolated above was labelled with biotin as described (Harlow & Lane, Citation1988). The wells of microtiter plates (Immuno from NUNC) were coated with 100 μl of the above Protein A-Sepharose eluate at a concentration of 10 μg/ml in ice-cold 50 mM sodium phosphate buffer, 0.9% sodium chloride pH 2.5 overnight at 4°C. Controls were the above buffer solution alone or normal human immunoglobulins (from Sigma) under identical conditions. Free binding sites were blocked by 400 μl of 2% bovine serum albumin in PBS. Dilutions (100 μl) of the biotin labelled antibodies in a solution of 2% bovine serum albumin in PBS were applied to the wells and allowed to adsorb for 1 h at 37°C. Unadsorbed material was washed off four times with PBS. An avidin-peroxidase solution in PBS (100 μl) was added to the wells and incubated for 30 min at room temperature. Unadsorbed material was washed off four times with PBS. For color development, the wells were filled with 100 μl of 2,2-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (0.5 mg/ml) and 0.03% H2O2 in 0.1 M citrate phosphate buffer pH 4.5. The color was read in an ELISA reader at 405 nm. The data were recorded by subtraction of the buffer control values from the sample values.
2.5. Measurement of self-aggregation
For turbimetric measurements, the acidic eluate from the Protein A-Sepharose column was mixed with 1/10 volume of 30% solution polyethylene glycol in water. This solution (100 μl) was transferred into wells of a microtiter plate and neutralized with 5 μl of 1 M Tris-HCl pH 9.0. The turbidity was measured in a microtiter plate reader at 630 nm.
The acidic eluate from the Protein A-Sepharose column was neutralized and analyzed with a Coulter N4plus nephelometer at 20°C at various time periods.
2.6. Preparation of synovial fluid mononuclear cells
The synovial fluid was diluted 1:1 with PBS and subjected to a Ficoll-Hypaque gradient centrifugation at 1,000 g for 20 min at 20°C. The low-density fraction (p = 1.077) was enriched for lymphocytes and mononuclear phagocytes. The resuspended cells were directly processed for PCR.
2.7. DNA amplification and sequencing
Genomic DNA was extracted from synovial cells using QIAamp® DNA Mini Kit. Amplification of Fc-region was accomplished in a two-step procedure by PCR. Sequences of the first primers used for amplification were:
IgG1-for CCACCTCCATCTCTTCTTCAGCACCCTGA
IgG1-rev GCGCGACCCCGAGAGCCCTGG
The PCR products were separated on a 1% agarose gel and recovered using the Zymoclean Gel DNA recovery kit (Zymo research). PCR amplifications were followed by seminested PCR reactions using the following primers:
IgG-seq_rev CTGAGAGTGACCGCTGTAACC
IgG1-rev GCGCGACCCCGAGAGCCTGG
The products were again isolated on an agarose gel and cloned directly in to the Zero BluntTopo PCR cloning kit (Invitrogen). Ten minipreps were prepared from each patient using the QIAPREP 8 miniprep kit (Qiagen) and sequenced on an ABI prism 310 Genetic Analyzer. The accession numbers of the mutated nucleotide sequences listed in are KT375074–KT375086.
Table 1. Mutationen in der Fc-region von IgG
3. Results
3.1. The rheumatoid antigens reside in IgG
Since rheumatoid arthritis is an antigen-driven autoimmune disease, the driving antigens should be found in immune complexes in almost equimolar amounts to the antibodies. Immune complexes were isolated from rheumatoid synovial fluid by precipitation with polyethylene glycol and further purified by affinity chromatography on Protein-A-Sepharose. The yields of immune complexes were about 0.1 mg/ml of synovial fluid from patients with rheumatoid arthritis and less than 0.01 mg/ml from patients with osteoarthritis that served as non-inflammatory controls. The eluted proteins were analyzed by SDS-polyacrylamide gel electrophoresis under reducing conditions with an overloaded amount of protein to identify possible antigens other than the expected IgG polypeptides, but the protein pattern showed only the heavy and light chains of IgG as the major constituents ((A)).
Figure 1. Analysis of immune complexes from synovial fluid of a patient with rheumatoid arthritis. (A) 10% polyacrylamide gel stained with Commassie Blue. (B) Western blot with homologous biotinylated IgG.
Note: The results were representative of 5 preparations.
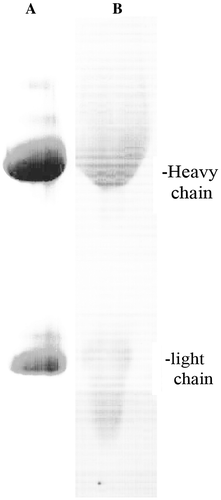
An aliquot of immune complexes eluted from the Protein A-Sepharose column was labelled with biotin and used in Western blotting of the same eluate. (B) shows that it reacted mainly with the heavy chains.
Immune complex formation was studied by turbimetry and nephelometry. (A) shows the kinetics of aggregation of 2.6 mg/ml IgG from immune complexes of patient #198 (IC198) in the presence of 3% polyethylene glycol after neutralization of the acidic Protein-A-Sepharose eluate. After 25 min flaky precipitates became visible.
Figure 2. Kinetics of immune complex formation. (A) IgG was isolated from synovial immune complexes of a patient with rheumatoid arthritis by Protein A-Sepharose affinity chromatography. Polyethylene glycol was added to a final concentration of 5%, the acid eluate was neutralized and the turbidity at 630 nm was recorded at room temperature. (B) Size of immune complexes. Particle diameters were determined by nephelometry after neutralization of the Protein A-Sepharose eluate. The weight percents are recorded for the indicated diameters.
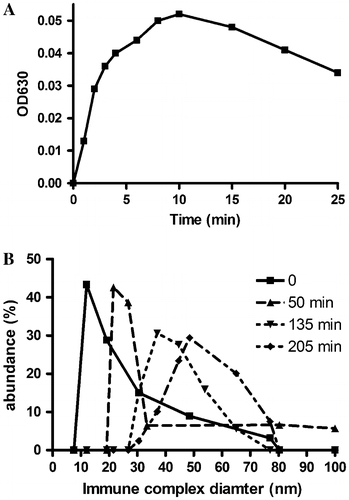
Aggregation was also observed in the absence of polyethylene glycol by nephelometric measurements ((B)). While the mean diameter of normal IgG was 7.6 nm (data no shown), the diameter of 2.5 mg/ml immune complexes from patient #156 increased steadily after neutralization of the Protein A-Sepharose eluate. These results indicated that aggregation of IgG to immune complexes may be caused by epitopes and paratopes present in these molecules.
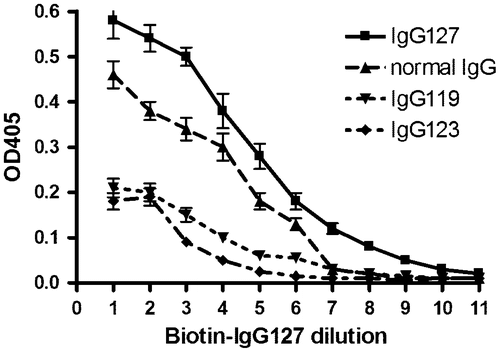
In order to determine whether the self-reactivity of the biotinylated antibodies was of rheumatoid factor specificity directed to normal IgG, an ELISA assay was performed to compare the reactivity towards equal amounts of normal IgG with the Protein A-Sepharose eluted immune complexes. shows that biotinylated IgG from immune complexes of patient #127 (IgG127) had the highest specificity towards its own IgG. It reacted lesser with normal IgG. This reaction is defined as IgG rheumatoid factor activity. The reactivity towards IgG from immune complexes of other patients (IgG119 and IgG123) was much lower. Taken together, these results suggested that each patient had different antigenic structures that were not represented in normal IgG.
Figure 3. Specificity of rheumatoid antibodies. Immune complexes were isolated from synovial fluid of patients with rheumatoid arthritis.
Notes: One portion of the immune complexes from patient #127 was biotinylated and used as paratope. Another portion from the same patient #127 immune complexes from two other patients #119 and #123 and normal IgG were used as epitope and adsorbed at pH 2.5 to microtiter plate. Biotinylated IgG127 was serially diluted 1:2 and the binding affinity was determined in an ELISA assay. The error bars indicate the range of two determinations.
3.2. Mutations in the Fc-region of IgG
For detection of structural alterations within the heavy chain of IgG, cells were isolated from synovial fluids of patients with rheumatoid arthritis, the genomic DNA was prepared and the constant region of heavy chains were amplified by PCR. The PCR products were subjected to sequence analysis. Mutations were detected in all preparations from rheumatoid arthritis patients.
The sequences were compared to the corresponding sequences of IgG1–4. lists only those mutations that differ from the four sequences of IgG isotypes. It is apparent that at least one or multiple mutations was present in all patients. As a control, leukocytes isolated from peripheral blood of a newborn child were analyzed similarly. This sample did not contain any mutations (data not shown). In another control, blood leukocytes from a healthy 58 year old person were analyzed, and a mutation was identified in one of ten sequences. The same substitution was also present in SF201 in addition to a second mutation.
4. Discussion
Many structural alterations have been identified in proteins of patients with rheumatoid arthritis, but none has been proven to fulfill the criteria of being the driving force for chronic inflammation (Klareskog et al., Citation2014; Trouw et al., Citation2013; Yarwood et al., Citation2016). Since rheumatoid arthritis is an antigen driven autoimmune disease with large amounts of lymphocytes in the synovia engaged in antibody production, the eliciting antigen should be present in immune complexes. Thus we isolated the immune complexes and analyzed them in greater detail. They contained IgG as the sole component that recognized its own heavy chain and self aggregated. This indicated that the antigen resided in IgG itself and made other proteins as source of potential antigens unlikely. This is in agreement with the vast knowledge gathered on the specificity of rheumatoid factors (Sutton et al., Citation2000).
Since biotinylated IgG from one patient had the highest affinity towards its own structure, a lower affinity to natural IgG and the lowest affinity to IgG from immune complexes of other patients (), we hypothesized that the antigen must be different from rheumatoid factors and could arise from mutations. Indeed, when terminal Fc-regions from lymphocytes obtained from synovial fluids were sequenced, we found nucleotide substitutions resulting in new amino acids in all patients. The new amino acids were uncommon in the corresponding sequence of all four known IgG isotypes. It is therefore possible that the mutated sites form antigens in rheumatoid arthritis patients.
The Fc region is considered to be “constant” and thus little attention has been paid to its mutagenesis as a possible cause for antigenic sites of rheumatoid arthritis. To the best of our knowledge, naturally occurring human mutations in the Fc-region have not been described. Interestingly, a mutation in the Fc-region was also detected in a peripheral blood sample from a healthy person indicating that mutations alone are not sufficient to elicit rheumatoid inflammation. The presence of mutations in ten synovial fluids of rheumatoid arthritis patients as well as in the peripheral blood of a healthy individual indicates that our study does not allow any conclusion about their antigenicity. This fundamental problem could be solved by the isolation of antigenic peptides, but not by statistical comparison. Our attempts to identify antigenic peptides from rheumatoid immune complexes failed. It is clear that more investigations are required to extend our observations onto other domains of the Fc-regions and to define the specificity of Fc-mutations for rheumatoid arthritis. New experimental strategies have to be developed to prove our hypothesis.
The genetic impact of germline mutations on rheumatoid arthritis was evaluated to be 12% by monozygotic twin studies on 13,299 patiens, indicating that somatic mutations must have an overwhelming impact (Aho, Koskenvuo, Tuominen, & Kaprio, Citation1986). Genome-wide association studies revealed many potential risk loci, but the causual mutation has not been pinpointed (Diogo, Okada, & Plenge, Citation2014; Plenge, Citation2009). It is well known that somatic mutations occur in the synovium of patients with rheumatoid arthritis, but the investigated targets p53 protein or mitochondrial DNA did not prove to be directly responsible (Firestein, Citation2010). Even the variable region of the IgG heavy chain has been analyzed for mutations and it showed little evidence of mutations (Brown et al., Citation1995). Another study on the variable regions of the heavy chains indicated that the RA synovial microenvironment sustains somatic mutations (Williams, Moyes, & Mageed, Citation1999).
If the Fc-receptor region is mutated or masked by self-antibodies, it could lose the ligand function for the Fc-receptor. This might explain impaired cellular uptake and processing of immune complexes leading to persistence and chronic inflammation, particularly if the self-antibodies themselves have altered Fc-regions. The hypothesis of mutated Fc-regions as a cause of rheumatoid arthritis is in agreement with the proven benefit of B-cell depletion or hematopoietic stem cell transplantation for therapy of rheumatoid arthritis (Bingham & Moore, Citation2004; Calero, Nieto, & Sanz, Citation2010).
5. Conclusion
Frequent mutants occur in the constant region of IgG from patients with rheumatoid arthritis. These mutations could elicit antigenic reactions to drive the autoimmune disease.
Funding
This work supported by the Muenster University Hospital.
Additional information
Notes on contributors
Peter Prehm
Dr Peter Prehm is a retired professor from the Institute of Physiological Chemistry and Pathobiochemistry at the University Hospital Münster, Germany. His research interests are the biochemistry of glycosaminoglycans with particular emphasis on hyaluronan and pathobiochemical disturbances.
References
- Aho, K., Koskenvuo, M., Tuominen, J., & Kaprio, J. (1986). Occurrence of rheumatoid arthritis in a nationwide series of twins. Journal of Rheumatology, 13, 899–902.
- Arnett, F. C., Edworthy, S. M., & Bloch, D. A. (1988). The american rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis and Rheumatism, 31, 315–324.10.1002/(ISSN)1529-0131
- Artandi, S. E., Calame, K. L., Morrison, S. L., & Bonagura, V. R. (1992). Monoclonal IgM rheumatoid factors bind IgG at a discontinuous epitope comprised of amino acid loops from heavy-chain constant-region domains 2 and 3. Proceedings of the National Academy of Sciences, 89, 94–98.10.1073/pnas.89.1.94
- Bingham, S. J., & Moore, J. J. (2004). Rheumatoid arthritis. Best Practice & Research Clinical Haematology, 17, 263–276.10.1016/j.beha.2004.05.002
- Bläß, S., Engel, J. M., & Burmester, G. R. (1999). The immunologic homunculus in rheumatoid arthritis. Arthritis and Rheumatism, 42, 2499–2506.10.1002/(ISSN)1529-0131
- Bonagura, V. R., Agostino, N., Borretzen, M., Thompson, K. M., Natvig, J. B., & Morrison, S. L. (1998). Mapping IgG epitopes bound by rheumatoid factors from immunized controls identifies disease-specific rheumatoid factors produced by patients with rheumatoid arthritis. Journal of Immunology, 160, 2496–2505.
- Brown, C. M., Fitzgerald, K. J., Moyes, S. P., Mageed, R. A., Williams, D. G., & Maini, R. N. (1995). Sequence analysis of immunoglobulin heavy-chain variable region genes from the synovium of a rheumatoid arthritis patient shows little evidence of mutation but diverse CDR3. Immunology, 84, 367–374.
- Calero, I., Nieto, J. A., & Sanz, I. (2010). B cell therapies for rheumatoid arthritis: Beyond B cell depletion. Rheumatic Disease Clinics of North America, 36, 325–343.10.1016/j.rdc.2010.02.003
- Carson, D. A., Chen, P. P., & Kipps, T. J. (1991). New roles for rheumatoid factor. Journal of Clinical Investigation, 87, 379–383.10.1172/JCI115007
- Carter, S. D., Makh, S. R., Ponsford, F. M., & Elson, C. J. (1989). Rheumatoid factor has increased reactivity with IgG from synovial fluids of patients with rheumatoid arthritis and osteoarthritis. Rheumatology, 28, 233–238.10.1093/rheumatology/28.3.233
- Diogo, D., Okada, Y., & Plenge, R. M. (2014). Genome-wide association studies to advance our understanding of critical cell types and pathways in rheumatoid arthritis: recent findings and challenges. Current opinion in rheumatology, 26, 85–92.
- Dong, W., Li, X., Liu, H., & Zhu, P. (2009). Infiltrations of plasma cells in synovium are highly associated with synovial fluid levels of APRIL in inflamed peripheral joints of rheumatoid arthritis. Rheumatology International, 29, 801–806.10.1007/s00296-008-0773-7
- Dörner, T., Egerer, K., Feist, E., & Burmester, G. R. (2004). Rheumatoid factor revisited. Current Opinion in Rheumatology, 16, 246–253.10.1097/00002281-200405000-00013
- Edwards, J. C. W. (1998). The synovium. In J. H. Klippel & P. A. Dieppe (Eds.), Rheumatology (5th ed., pp. 6.1–6.8). London: Mosby.
- Firestein, G. S. (2010). Somatic mutations and anti-mutated citrullinated vimentin antibodies in rheumatoid arthritis: Comment on the editorial by Levesque et al. Arthritis and Rheumatism, 62, 303–304.10.1002/art.v62:1
- Grinnell, S., Yoshida, K., & Jasin, H. E. (2005). Responses of lymphocytes of patients with rheumatoid arthritis to IgG modified by oxygen radicals or peroxynitrite. Arthritis and Rheumatism, 52, 80–83.10.1002/(ISSN)1529-0131
- Harlow, E., & Lane, D. (1988). Antibodies. A laboratory manual. New York, NY: Cold Spring Harbor Laboratory.
- Klareskog, L., Amara, K., & Malmström, V. (2014). Adaptive immunity in rheumatoid arthritis. Current Opinion in Rheumatology, 26, 72–79.10.1097/BOR.0000000000000016
- Male, D. K. & Roitt, I. M. (1981). Molecular analysis of complement-fixing rheumatoid synovial fluid immune complexes. Clinical and experimental immunology, 46, 521–529.
- Parekh, R. B., Dwek, R. A., Sutton, B. J., Fernandes, D. L., Leung, A., Stanworth, D., ... Kobata, A. (1985). Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature, 316, 452–457.10.1038/316452a0
- Plenge, R. M. (2009). Recent progress in rheumatoid arthritis genetics: One step towards improved patient care. Current Opinion in Rheumatology, 21, 262–271.10.1097/BOR.0b013e32832a2e2d
- Reparon-Schuijt, C. C., van Esch, W. J., van Kooten, C., Levarht, E. W., Breedveld, F. C., & Verweij, C. L. (1998). Functional analysis of rheumatoid factor-producing B cells from the synovial fluid of rheumatoid arthritis patients. Arthritis and Rheumatism, 41, 2211–2220.10.1002/(ISSN)1529-0131
- Sutton, B. J., Corper, A. L., Sohi, M. K., Jefferis, R., Beale, D., & Taussig, M. J. (1998). The structure of a human rheumatoid factor bound to IgG Fc. Advances in Experimental Medicine and Biology, 435, 41–50.10.1007/978-1-4615-5383-0
- Sutton, B., Corper, A., Bonagura, V., & Taussig, M. (2000). The structure and origin of rheumatoid factors. Immunology Today, 21, 177–183.10.1016/S0167-5699(00)01589-9
- Tighe, H., & Carson, D. A. (2001). Rheumatoid Factor. In S. Ruddy, E. D. Harris, & C. B. Sledge (Eds.), Kelley’s textbook of rheumatology (pp. 151–158). Philadelphia, PA: W.B. Saunders.
- Trouw, L. A., Huizinga, T. W., & Toes, R. E. (2013). Autoimmunity in rheumatoid arthritis: Different antigens–common principles. Annals of the Rheumatic Diseases, 72, ii132–ii136.10.1136/annrheumdis-2012-202349
- Uesugi, M., Hayashi, T., & Jasin, H. E. (1998). Covalent cross-linking of immune complexes by oxygen radicals and nitrite. Journal of Immunology, 161, 1422–1427.
- Van Esch, W. J., Reparon-Schuijt, C. C., Hamstra, H. J., Van Kooten, C., Logtenberg, T., Breedveld, F. C., & Verweij, C. L. (2003). Human IgG Fc-binding phage antibodies constructed from synovial fluid CD38+ B cells of patients with rheumatoid arthritis show the imprints of an antigen-dependent process of somatic hypermutation and clonal selection. Clinical and Experimental Immunology, 131, 364–376.10.1046/j.1365-2249.2003.02068.x
- Whitaker, J. W., Boyle, D. L., Bartok, B., Ball, S. T., Gay, S., Wang, W., & Firestein, G. S. (2015). Integrative omics analysis of rheumatoid arthritis identifies non-obvious therapeutic targets. PLOS ONE, 10, e0124254.10.1371/journal.pone.0124254
- Williams, D. G., Moyes, S. P., & Mageed, R. A. (1999). Rheumatoid factor isotype switch and somatic mutation variants within rheumatoid arthritis synovium. Immunology, 98, 123–136.10.1046/j.1365-2567.1999.00841.x
- Yarwood, A., Viatte, S., Okada, Y., Plenge, R., Yamamoto, K., BRAGGSS, RACI, ... Eyre, S. (2016). Loci associated with N-glycosylation of human IgG are not associated with rheumatoid arthritis: A Mendelian randomisation study. Annals of the Rheumatic Diseases, 75, 317–320.10.1136/annrheumdis-2014-207210
