?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The Clavulanic acid (CLV) is a suicide inhibitor of bacterial beta-lactamase enzymes from Streptomyces clavuligerus. In the present study, a reliable drug delivery system using poly (ε-caprolactone)-poly (ethylene glycol)-poly (ε-caprolactone) (PCL-PEG-PCL) was synthesized and the release profile of the CLV from the drug-loaded polymersomes was evaluated. In this study, CLV was encapsulated within PCL-PEG-PCL nanoparticles by a double emulsion technique (w/o/w), leading to creation of CLV-loaded PCL-PEG-PCL (CLV/PCL-PEG-PCL) polymersomes. Characterization, stability of polymersomes, the particle size was determined by DLS. The release profile of the CLV from the polymersomes which ready by the drug-loaded copolymer, was evaluated. Our studies resulted in a successful establishment of uniformity and spherical CLV-loaded PCL-PEG-PCL polymersomes. The loading efficiency of CLV was 16.00 ± 1.45%. The results of DLS show that the polymersomes have spherical shapes with size of 113 nm. In vitro release of CLV from CLV-entrapped polymersomes was remarkably sustained. The results indicate the successful formulation of curcumin loaded PCL-PEG-PCL polymersomes.
Public Interest Statement
Clavulanic acid (CLV) and its salts and esters. The acid is a suicide inhibitor of bacterial beta-lactamase enzymes from Streptomyces clavuligerus. In the present study, a reliable drug delivery system using poly (ε-caprolactone)-poly (ethylene glycol)-poly (ε-caprolactone) (PCL-PEG-PCL) was established. In this study, CLV was encapsulated within PCL-PEG-PCL nanoparticles by a double emulsion technique (w/o/w), leading to creation of CLV-loaded PCL-PEG-PCL (CLV/PCL-PEG-PCL) polymersomes. Characterization, stability of polymersomes, the particle size was determined by DLS. Our studies resulted in a successful establishment of uniformity and spherical clavulanic acid-loaded PCL-PEG-PCL polymersomes. The in vitro release of CLV from CLV-entrapped polymersomes was remarkably sustained. Our study showed that this nano-carrier provided a suitable and appropriate system for delivery of CLV. This developed nano-drug delivery system can also be used as a promising base to design further nanoparticle systems to improve the therapeutic effect of CLV in the future.
Competing Interests
The authors declare no competing interest.
1. Introduction
Polymersomes are hollow spheres, made of polymers that contain an aqueous solution in the core surrounded by a bi-layer membrane. The bi-layer membrane is composed of hydrated hydrophilic coronas both at the inside and outside of the hydrophobic middle part of the membrane separating and protecting the fluidic core from the outside medium. In drug delivery context, the aqueous core can be utilized for the encapsulation of hydrophilic therapeutic molecules such as small-molecule drugs, enzymes, proteins, peptides, DNA and RNA fragments (Christian et al., Citation2009; Kim, Meeuwissen, Nolte, & Hest, Citation2010; Lomas et al., Citation2007) while the membrane can incorporate hydrophobic drugs within its hydrophobic core (Ahmed et al., Citation2006; Chen, Meng, Cheng, & Zhong, Citation2010; Kale & Torchilin, Citation2007; Khurana, Patel, Agrahari, Pal, & Mitra, Citation2015; Nie, Citation2010). Based on their multidrug loading capacity, stability, long blood circulation times, membrane robustness and stealth properties, polymersomes are highly attractive for drug delivery of highly toxic therapeutics with tuned pharmacokinetics in order to greatly increase therapeutic efficacy. Clavulanic acid (CLV) and its salts and esters. The acid is a suicide inhibitor of bacterial beta-lactamase enzymes from Streptomyces clavuligerus. Administered alone, it has only weak antibacterial activity against most organisms, but given in combination with beta-lactam antibiotics prevents antibiotic inactivation by microbial lactamase. To achieve drug controlled release, biodegradable polymeric nanoparticles are often used in advanced anticancer drug delivery systems (Al-Musawi et al., Citation2014; Allen & Cullis, Citation2004; Danafar, Davaran, Rostamizadeh, Valizadeh, & Hamidi, Citation2014; Danafar, Rostamizadeh, Davaran, & Hamidi, Citation2014, Citation2015; Jackson & Singletary, Citation2004; Kumari, Yadav, & Yadav, Citation2010; Manjili et al., Citation2016). A number of drug delivery systems using recyclable polymer such as nanoparticles delivering antitumor agents are commercially accessible. Poly (caprolactone)-poly (ethylene glycol) (PCL-PEG) copolymers are co-friendly, easy to produce, amphiphilic, and have a sturdy probable function in drug delivery system (Danafar, Citation2016b; Danafar, Davaran, et al., Citation2014; Khurana et al., Citation2015; Lin et al., Citation2012). Tian et al. evaluated albumin based polymeric delivery system was successful and has the probable to enhance the therapeutic effect and anti-tumor activity of sulforaphane (Patel, Vaishya, Yang, Pal, & Mitra, Citation2014). For this reason, it seems that encapsulating of CLV in PCL-PEG-PCL polymersomes may be a talented method for developing an improved CLV formulation (Cheng et al., Citation2016; Danafar, Citation2016c; Danafar, Manjili, & Najafi, Citation2016; Danafar, Sharafi, Kheiri, Manjili, & Andalib, Citation2016). In the current study, a polymersome delivery system with PCL-PEG-PCL was prepared. In this study, an effective polymersomes delivery system by PCL-PEG-PCL was obtained. We present an efficient nano-drug delivery system to improve the combination and therapeutic level of CLV. It was presumed that the PCL-PEG-PCL could be a good candidate for the delivery CLV. In this contribution, we are aimed to encapsulate CLV in PCL-PEG-PCL polymersomes as a promising carrier with sustained release characteristics. In this way, a novel drug delivery system with PCL-PEG-PCL was synthesized and the release profile of the CLV from the polymersomes prepared using the drug-loaded copolymer was evaluated.
2. Materials and methods
2.1. Materials
All used chemicals were of analytical grade and purchased from commercial sources. Poly (ethylene glycol) PEG (Mn = 6,000 Da) (Aldrich, St. Louis, USA, CAS.81323), ε-caprolactone (98% purity) (Acros, New Jersy, USA, CAS.502443), clavulanate potassium (CLV) (Zahravi Company, Iran), stannous 2-ethyl-hexanoate (Sn (Oct)2) (Aldrich, St. Louis, USA, CAS. 301100), phosphate buffer (20 mM, pH 7.8), poly (vinyl alcohol), M W = 145,000 (Aldrich, St. Louis, USA) and corresponding salts which were used throughout this research was obtained from Sigma-Aldrich or Merck.
2.2. Synthesis of PCL-PEG-PCL copolymer
The synthesis of PCL-PEG–PCL copolymers was prepared according to our previous report (Manjili et al., Citation2016). That were synthesized by a ring opening polymerization of ε-caprolactone with PEG as initial molecule and Sn (Oct) 2 as catalyst was performed. Briefly, ε-caprolactone (4 g), PEG (2 g), and Sn (Oct)2 (0.01 mmol) were heated to 120°C to start polymerization. After 12 h, the resulting polymer was cooled to room temperature (23°C), dissolved in chloroform, and precipitated in cold diethyl ether. The copolymer was dried under vacuum at room temperature for 24 h.
The obtained copolymers was characterized by Fourier Trans form Infrared spectroscopy (FT-IR) (Bruker, Tensor 27) were used for characterization of the chemical structure of the PCL-PEG-PCL copolymer and Proton Nuclear Magnetic Resonance Spectroscopy (1H NMR) in CDCl3 at 400 MHz (Bruker, Evans, 400). Thermal analysis of tri block copolymers was determined by differential scanning calorimetry (DSC) (Mettler Toledo, model Star SW 9.30) and the data were recorded from 0 to 250°C. Molecular weight and distribution of the PCL-PEG-PCL copolymers were calculated by gel permeation chromatography (GPC) (Knaure, Berlin, Germany) composed of with an ultrastyragel column and differential refract metric detector (4.6 × 30 mm) (Waters, Milford, USA, model HR 4E). Tetrahydrofuran (THF) as a mobile phase was with a flow-rate of 1 ml/min and the injection volume was 100 μl of stock solutions (0.1–0.5 w/v%). The standards of polystyrene mono-disperse in the range of 4,500-29,500 DA (Varian Palo Alto, CA) obtained before measurements for use as the calibration curve for determination of average molecular weight of the PCL-PEG-PCL copolymers(Manjili et al., Citation2016).
2.3. Preparation of CLV-loaded polymersomes
CLV loaded polymersomes were prepared by a double emulsion technique (w/o/w). In essence, 1 ml aqueous solution of CLV with known concentration 6 mg/ml was first poured into the PCL-PEG-PCL copolymer solution in 2 ml chloroform (25 mg/ml) to form a w/o emulsion. The resulting emulsion was then injected drop-wise through a syringe (G = 22) into 20 ml of distillated water containing 0.4 wt% of poly (vinyl alcohol) under certain mixing rates. Then, the mixture stirring was continued magnetically at room temperature until complete evaporation of the organic solvent. Subsequently, evaporation of the organic solvent in aqueous environment leads the amphiphilic copolymers to self-associate and form the nanoparticles. Finally, all of the samples were centrifuged at 20,000 g, and freeze-dried (at pressure of 14 Pa and −78°C) to remove all of the residual solvents and to produce the final nanospowder form.
2.4. Characterization of the polymersomes
2.4.1. Determination of particle size
The particle size distribution of the prepared polymersomes was determined by dynamic light scattering (DLS) using a nano/zetasizer (Malvern Instruments, Worcestershire, UK, model Nano ZS).
2.4.2. Stability of polymersomes
The physical stability of polymersomes was evaluated by monitoring the particle size distribution of the polymersomes while suspended in phosphate-buffer saline(PBS, pH 7.4) and kept in in room temperature at times 0, 15, and 30 days after preparation using the method described in 2.3.
2.4.3. Determination of loading efficiency
To determine the loading efficiency of the drugs in the polymersomes, two parameters including the drug loading ratio and efficiency of entrapment were evaluated. Drug loading ratio was determined as:
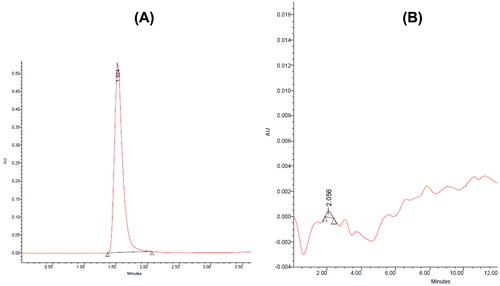
where W drug in polymersomes and W polymersomes show weight of the encapsulated drug and the total weight of the corresponding drug-encapsulated polymersomes, respectively. DL% is the drug loading ratio (%). For determination of the drug loading ratio, 1 mg of the final freeze-dried Nano dispersion was dissolved in 1 ml of chloroform, and the drug content was measured by high performance liquid chromatography (HPLC) (Danafar, Citation2016a, Citation2016b; Danafar & Hamidi, Citation2013, Citation2016). The mobile phase consisted of a mixture of buffer phosphate (pH 4.4) and methanol (90:10, v/v), and was delivered at a flow rate of 1.0 ml/min using a double-reciprocation pump (Waters, model Breeze, USA). The sample was injected through a 20 μl sample loop. The column effluent at 1.52 min was detected at 220 nm with a variable wavelength detector (Waters, model Breeze, USA). A C8 analytical column (150 × 4.6 mm, particle size 5 μm; Perfectsill, MZ-Analysen technik, Germany) equipped by a guard column of the same packing was used.
Entrapment efficiency was determined in reference to loading ratio and total dried nanodispersion weight obtained using the following equation:
where EE% is the efficiency of entrapment (%), weight drug in polymersomes and W feed drug stand for the total mass of powders obtained after freeze-drying and the drug fed initially in the polymersomes preparation step, respectively.
2.5. Drug release study
This test was performed to evaluate the release behavior of CLV from polymersomed copolymer. The solubility of CLV in PBS, pH 7.4 is approximately 10 mg/ml. Briefly, 5 mg of freeze-dried drug-loaded carriers were dispersed in 2 ml phosphate-buffered saline (PBS) and the resulting suspension was placed within a dialysis sac (Mw 12 kDa) and incubated at 37°C while immersed in 15 ml of PBS. Then, at predetermined time intervals, 2 ml of the dialysate was taken out and replaced by 2 ml fresh PBS. The concentration of CLV in the dialysate was measured at 220 nm using HPLC. All the release studies were carried out in triplicate. In order to study the pH-dependency of the drug release, the experiments were performed using PBS at a pH of 5.5. As controls, the release of free CLV was studied in PBS with pH 7.4 and pH 5.5.
3. Results and discussion
3.1. Synthesis and characterization of PCL-PEG-PCL copolymers
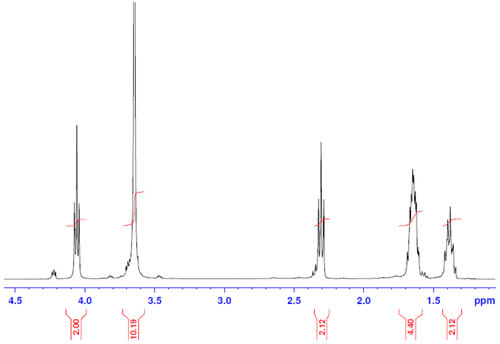
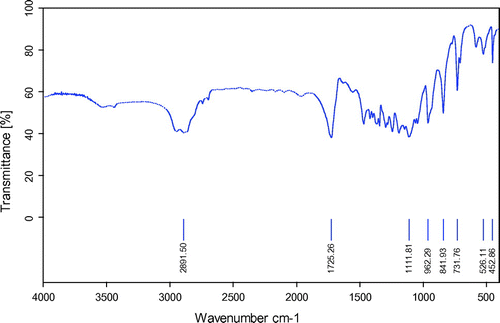
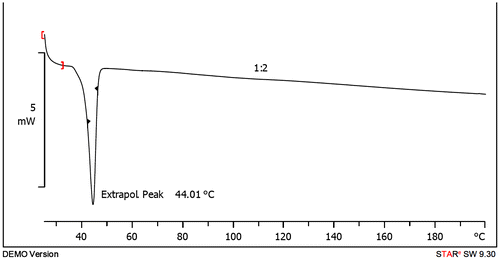
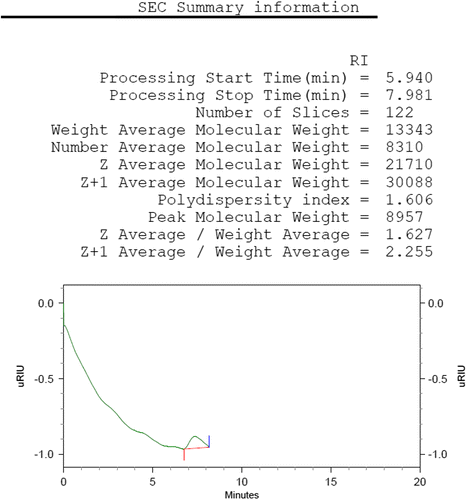
PCL-PEG-PCL tri-block copolymers was synthesized using the ring-opening polymerization of caprolactone in presence of PEG, whose hydroxyl end group initiated the ring opening and explain to pervious work (Manjili et al., Citation2016). The structure and composition of the synthesized PCL-PEG-PCL tri-block copolymers was determined by H NMR spectroscopy in CDCl3 (). The presence of ethylene’s (CH2) in PCL was observed around 1.35, 1.69, 2.43 and 4.06 ppm, the methylene (CH2) groups of PEG were around 3.72 ppm. shows the characteristics of synthesized copolymers. FT-IR spectrum of PCL-PEG-PCL copolymer show the sharp and intense bands at 1,725 and 1,111 cm−1 were awardable to the presence of carboxylic ester (C=O) and ether (C–O) groups, thereby indicating that the formation of PCL-PEG-PCL copolymer has occurred successfully (). To DSC thermo grams copolymers the endotermic peak (44.01°C) including two merged peaks of PEG and PCL which presented in . GPC results showed that the weight- and number-based average molecular weights of copolymer were 13.3 and 8.3 KDa, respectively ().
Table 1. Molecular characterics of the synthesized copolymers
3.2. Preparation and characterization of copolymeric polymersomes
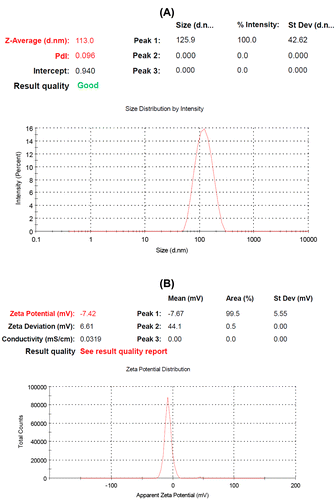
The size of polymersomes was measured by dynamic light scattering technique (DLS). The Z-average and Zeta potential of CLV loaded PCL-PEG-PCL polymersomes were found to be about 113 nm and −7.42 mv, with their corresponding PDI being 0.096 (). The loading ratio and encapsulation efficiencies of CLV loaded to PCL-PEG-PCL polymersomes were determined by HPLC to be 16.00 ± 1.45% and 69.33 ± 1.12%, respectively. The chromatogram of CLV loaded to PCL-PEG-PCL polymersomes was showed to .
3.3. In vitro release of CLV
3.3.1. The impact of different release media on drug release from polymersomes
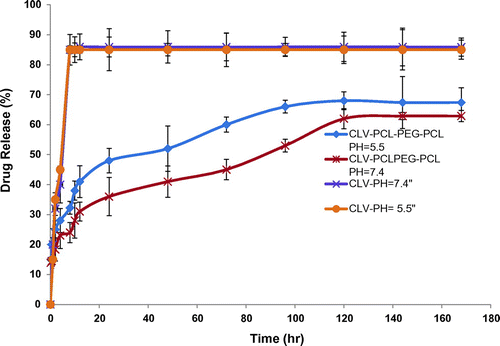
In order to examine the influence of the chemical and biochemical factors on the release of CLV from polymersomes, the release study was performed on drug-loaded polymersomes in neutral (pH 7.4) and acidified PBS solution (pH 5.5). As controls, the release of free CLV was studied to verify that the diffusion of drug molecules across the dialysis membrane was not a rate-limiting step during the release process. Free CLV was observed to be rapidly released and reached its peak of 85.15 and 86.03% of the total in the first 8 h at 7.4 and 5.5 pH respectively. shows the release profiles of CLV from the drug-loaded polymersomes, at 7.4 and 5.5 pH. As expected, no considerable initial burst CLV release was observed from the polymersomes. As shown in , the percentage of CLV released from the polymersomes increased as the pH value decreased from 7.4 to 5.5. For example, after 96 h incubation, the amounts of CLV released in the media with pH values of 7.4 and 5.5 were about 66.15, and 53.11%, respectively. This fact may be due to the physical loading of CLV in to the polymersomes. The results revealed that the maximum drug releases were 68.34, and 62.87% respectively for PBS pH 7.4 and pH 5.5 after a period of 120 h. The sustained release of CLV can be attributed to the entrapment of CLV in core of polymersomes. Therefore, the obtained copolymeric polymersomes can be regarded as highly attractive nano-carriers for time-controlled drug delivery for hydrophobic drugs to achievement of different therapeutic objectives.
3.4. Physical stability of polymersomes
In the clinical administration of nanoparticle dispersions, the stability of the sizes and volume of the nano-carriers is of great importance both as a measure of the particle structure integrity and as an indicator of the possible inter-particular associations (aggregation). For this purpose, the particle size stability was monitored in this study over a 30-days course. The size of all polymersomes was increased slightly throughout the measurement period. This observation cannot be a sign of aggregation, which usually leads to several fold increases. Probably, some kind of copolymer swelling and/or hydration (as a result of presence of the hydrophilic PEG portions in polymersomes surfaces) can be responsible for this event.
4. Conclusion
A PCL-PEG-PCL copolymer was synthesized and characterized by H NMR, FTIR, DSC and GPC techniques. The copolymer PCL-PEG-PCL was self-assembled into polymersomes in aqueous solution in presence of CLV. The obtained polymersomes were characterized by DLS. The encapsulation efficiency of CLV was 69.33 ± 1.12%. The results revealed that the polymersomes formed had spherical structure with size of 113 nm. In vitro release of CLV from CLV-entrapped polymersomes was clearly sustained in all the media tested for this purpose, with the apparent release plateau reached late at about 120 h. Our study showed that this nano-carrier provided a suitable and appropriate system for delivery of CLV. This developed nano-drug delivery system can also be used as a promising base to design further nanoparticle systems to improve the therapeutic effect of CLV in the future.
Funding
The author received no direct funding for this research.
Additional information
Notes on contributors
Hossein Danafar
Hossein Danafar is Assistant Professor of Medicinal Chemistry and Dean of Department of Medicinal Chemistry at the School of Pharmacy, Zanjan University of Medical Sciences, Zanjan, Iran. He received his PhD (2013) degree in Medicinal Chemistry from Tabriz University of Medical Sciences, Tabriz, Iran. His current research interests are the analytical chemistry, pharmaceutical analysis, design, synthesis and characterization of polymeric drug delivery systems, including micelles, nanogels and molecularly imprinted carriers, bioconjugates and magnetic targeted drug delivery systems. The research study titled is (Preparation and characterization of PCL-PEG-PCL polymersomes for delivery of clavulanic acid). The future study is application of the other block copolymers in drug delivery systems.
References
- Ahmed, F., Pakunlu, R., Brannan, A., Bates, F., Minko, T., & Discher, D. E. (2006). Biodegradable polymersomes loaded with both paclitaxel and doxorubicin permeate and shrink tumors, inducing apoptosis in proportion to accumulated drug. Journal of Controlled Release, 116, 150–158.10.1016/j.jconrel.2006.07.012
- Allen, T. M., & Cullis, P. R. (2004). Drug delivery systems: Entering the mainstream. Science, 303, 1818–1822.10.1126/science.1095833
- Al-Musawi, S., Naderi-Manesh, H., Mohammad Hassan, Z., Yeganeh, H., Nikzad, S., & Kheiri Manjili, H. (2014). Construction of polyurethane polymeric-based nano-carriers for curcumin in cancer therapy. Modares Journal of Medical Sciences: Pathobiology, 17, 25–39.
- Chen, W., Meng, F. H., Cheng, R., & Zhong, Z. Y. (2010). pH-Sensitive degradable polymersomes for triggered release of anticancer drugs: A comparative study with micelles. Journal of Controlled Release, 142, 40–46.10.1016/j.jconrel.2009.09.023
- Cheng, C. C., Lin, I. H., Chen, J. K., Liao, Z. S., Huang, J. J., Lee, D. J., & Xin, Z. (2016). Nucleobase-functionalized supramolecular micelles with tunable physical properties for efficient controlled drug release. Macromolecular Bioscience, 4. doi:10.1002/mabi.201600189
- Christian, D. A., Cai, S., Bowen, D. M., Kim, Y., Pajerowski, J. D., & Discher, D. E. (2009). Polymersome carriers: From self-assembly to siRNA and protein therapeutics. European Journal of Pharmaceutics and Biopharmaceutics, 71, 463–474.10.1016/j.ejpb.2008.09.025
- Danafar, H. (2016a). MPEG–PCL copolymeric nanoparticles in drug delivery systems. Cogent Medicine, 3, 1142411.
- Danafar, H. (2016b). Simple and sensitive high performance liquid chromatographic (HPLC) method for the determination of the selegiline in human plasma. Cogent Medicine, 3, 1–10. 1179244. doi:10.1080/2331205X.2016.1179244
- Danafar, H. (2016c). Applications of copolymeric nanoparticles in drug delivery systems. Drug Research. doi:10.1055/s-0042-109865
- Danafar, H., & Hamidi, M. (2013). A rapid and sensitive LC–MS method for determination of Ezetimibe concentration in human plasma: Application to a bioequivalence study. Chromatographia, 76, 1667–1675.10.1007/s10337-013-2548-x
- Danafar, H., & Hamidi, M. (2016). Method validation of amlodipine and atorvastatinby liquid chromatography–mass spectrometry(LC–MS) method in human plasma. Cogent Medicine, 3, 1129790.
- Danafar, H., Davaran, S., Rostamizadeh, K., Valizadeh, H., & Hamidi, M. (2014). Biodegradable m-PEG/PCL core-shell polymersomes: Preparation and characterization as a sustained release formulation for curcumin. Advanced Pharmaceutical Bulletin, 4, 501–510.
- Danafar, H., Rostamizadeh, K., Davaran, S., & Hamidi, M. (2014). PLA-PEG-PLA copolymer-based polymersomes as nano-carriers for delivery of hydrophilic and hydrophobic drugs: preparation and evaluation with atorvastatin and lisinopril. Drug Development and Industrial Pharmacy, 40, 1411–1420.10.3109/03639045.2013.828223
- Danafar, H., Rostamizadeh, K., Davaran, S., & Hamidi, M. (2015). Drug-conjugated PLA–PEG–PLA copolymers: A novel approach for controlled delivery of hydrophilic drugs by micelle formation. Pharmaceutical Development and Technology, 1–11.
- Danafar, H., Sharafi, A., Kheiri Manjili, H., & Andalib, S. (2016). Sulforaphane delivery using mPEG–PCL co-polymer nanoparticles to breast cancer cells. Pharmaceutical Development and Technology, 1–10.
- Danafar, H., Manjili, H. K., & Najafi. M. (2016). Study of copolymer composition on drug loading efficiency of enalapril in polymersomes and cytotoxicity of drug loaded nanoparticles, 2016, 1–10. doi:10.1055/s-0042-109865
- Jackson, S. J., & Singletary, K. W. (2004). Sulforaphane inhibits human MCF-7 mammary cancer cell mitotic progression and tubulin polymerization. The Journal of nutrition, 134, 2229–2236.
- Kale, A. A., & Torchilin, V. P. (2007). “Smart” drug carriers: PEGylated TATp-modified pH-sensitive liposomes. Journal of Liposome Research, 17, 197–203.10.1080/08982100701525035
- Khurana, V., Patel, S. P., Agrahari, V., Pal, D., & Mitra, A. K. (2015). Novel pentablock copolymer based nanoparticles containing pazopanib: A potential therapy for ocular neovascularization. Nanomedicine, 5, 57–68.
- Kim, K. T., Meeuwissen, S. A., Nolte, R. J. M., & Hest, J. C. M. (2010). Smart nanocontainers and nanoreactors. Nanoscale, 2, 844–858.
- Kumari, A., Yadav, S. K., & Yadav, S. C. (2010). Biodegradable polymeric nanoparticles based drug delivery systems. Colloids and Surfaces B: Biointerfaces, 75, 1–18.10.1016/j.colsurfb.2009.09.001
- Lin, L.-C., Yeh, C.-T., Kuo, C.-C., Lee, C.-M., Yen, G.-C., Wang, L.-S., … Wu, A. T. (2012). Sulforaphane potentiates the efficacy of imatinib against chronic leukemia cancer stem cells through enhanced abrogation of Wnt/β-Catenin function. Journal of Agricultural and Food Chemistry, 60, 7031–7039.10.1021/jf301981n
- Lomas, H., Canton, I., MacNeil, S., Du, J., Armes, S. P., Ryan, A. J. … Battaglia, G. (2007). Biomimetic pH sensitive polymersomes for efficient DNA encapsulation and delivery. Advanced Materials, 19, 4238–4243.10.1002/(ISSN)1521-4095
- Manjili, H. K., Sharafi, A., Danafar, H., Hosseini, M., Ramazani, A., & Ghasemi, M. H. (2016). Poly(caprolactone)–poly(ethylene glycol)–poly(caprolactone) (PCL–PEG–PCL) nanoparticles: A valuable and efficient system for in vitro and in vivo delivery of curcumin. RSC Adv, 6, 14403–14415.10.1039/C5RA24942B
- Nie, S. (2010). Understanding and overcoming major barriers in cancer nanomedicine. Nanomedicine, 5, 523–528.10.2217/nnm.10.23
- Patel, S. P., Vaishya, R., Yang, X., Pal, D., & Mitra, A. K. (2014). Novel thermosensitive pentablock copolymers for sustained delivery of proteins in the treatment of posterior segment diseases. Protein & Peptide Letters, 21, 1185–1200.10.2174/092986652111141001122054