 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
We investigated the effects of familiarization on the reliability of gross efficiency (GE) and lower-limb electromyography (EMG-rms) of adults and adolescents. We also evaluated the relationship between inter-test differences in GE and EMG-rms. Nine adult and nine adolescent cyclists performed three 10 min cycling tests at 50% of peak power output, separated by 48 h. Forty-five minutes familiarization visits were performed 24 h after each test. No differences were found across the tests for adult cyclists’ GE or EMG-rms, with Coefficient of Variation (CV%) ranging from 2.6 to 2.9% (GE) and 4.3 to 7.4%. Among adolescents, there was an increase in GE between tests 1 and 2 (p < 0.001) but not 2–3 (p = 0.438), with CVs decreasing from 6.8 to 2.6%. The adolescents’ EMG-rms decreased (p < 0.05) between tests 1 and 2, with CVs from 8.4 to 12.5%. There were no relationships (p > 0.05) between the inter-test differences of GE and EMG-rms. GE can be reliably determined; however, adolescents require two exposures to cycling. Without familiarization, adolescent EMG-rms is more variable than adults and would require larger samples to establish differences. The weak relationships between inter-test EMG-rms and inter-test GE questions the link between muscle activation and GE changes.
Public Interest Statement
The world’s elite cyclists rely upon efficient performance and have characteristic muscle activation profiles. With training, both cycling efficiency and muscle activation patterns can be improved but this can only be detected using reliable laboratory tests. However, researchers are commonly unaware of the necessary amount of familiarization they should offer to athletes before testing because of “learning effects”, which yield tests unreliable. This is relevant to adolescents, who are usually less familiar with testing, are less experienced and are not fully matured. Our findings show that efficiency and muscle activity can be measured reliably among both adolescents and adult cyclists but that adolescents require three visits to the laboratory to produce reliable results, whereas adults only require two visits. Understanding that efficiency and muscle activity can be acutely improved among adolescent cyclists after one familiarization should change laboratory practice and might prevent erroneous detection of ostensible changes in these parameters.
Competing Interests
The authors declare no competing interest.
1. Introduction
Gross efficiency (GE) of cycling, defined as the ratio of external power output (PO) to metabolic power input (MP) during exercise, is recognized as a primary determinant of endurance performance (Coyle, Citation1999, Citation2005; Horowitz, Sidossis, & Coyle, Citation1994). Gross efficiency can be measured reliably among adults using a cycle ergometer and breath-by-breath analysis (Moseley & Jeukendrup, Citation2001; Noordhof et al., Citation2010). However, there has been no isolated investigation of GE reliability among adolescent cyclists. This is important since systematic changes in GE owing to training interventions cannot be identified until typical measurement errors have been established.
It has been shown that non-athletes produce less reliable power output (coefficient of variation [CV%] ratio = 1.3) compared to athletes in a variety of physical performance tests (Hopkins, Schabort, & Hawley, Citation2001). In the same meta-analysis, the importance of task-familiarization was highlighted, with typical improvements of 1.2% reported between the first two tests, which were reduced to 0.2% between subsequent tests (Hopkins et al., Citation2001). A “habituation” or “learning” effect has also been reported for measurements of peak power in laboratory cycling tests, whereby non-cycle trained men improved their peak power output by 7% across two days of testing (Martin, Diedrich, & Coyle, Citation2000). Indeed, the economy (energy cost for a given workload) of cyclical physical tasks is typically improved across repeated visits among less experienced participants (Durand, Geoffroi, Varray, & Préfaut, Citation1994; Huang, Kram, & Ahmed, Citation2012; Lay, Sparrow, Hughes, & O’Dwyer, Citation2002; Sparrow, Hughes, Russell, & Le Rossignol, Citation1999). Collectively, it appears that both task experience and the training status of athletes influences the degree of reliability that can be achieved in laboratory cycling tests, both of which characterize adolescent athletes. This is important since changes in GE among adolescents, perhaps owing to training effects or biological maturation, cannot be identified until the systematic changes (practice effects) have been controlled.
Whilst tests for GE are often performed at fixed PO and cadences (Hopker, Jobson, Gregson, Coleman, & Passfield, Citation2012), the specific way in which external mechanical PO is achieved by the participant could influence the internal metabolic cost of exercise (Chapman, Vicenzino, Blanch, & Hodges, Citation2009; Huang et al., Citation2012; Hug, Drouet, Champoux, Couturier, & Dorel, Citation2008; Sparrow & Newell, Citation1998), thus affecting the GE of the cyclist. Indeed, motor redundancy is a common human trait and is characterized by the adoption of different, and thus variable, muscle recruitment patterns in order to achieve the same external PO, even during simple steady-state cycling tasks (Huang et al., Citation2012; Hug et al., Citation2008; Lay et al., Citation2002). Since increases in the EMG amplitude of agonist and antagonist muscle groups during cycling is correlated with an increased metabolic cost (Arnaud, Zattara-Hartmann, Toméi, & Jammes, Citation1997; Huang et al., Citation2012; Sparrow & Newell, Citation1998), excessive variability in muscle recruitment patterns between tests might lead to unnecessary increases in the metabolic cost of cycling for the same mechanical power output (GE). Adult to child differences in cycling efficiency have been attributed to an increased internal metabolic power (energy required to move the legs above basal metabolism) among children at high cadences, which in turn were hypothesized to relate to more variable muscle recruitment patterns (Martin, Hautier, & Bedu, Citation2002). Based on this reasoning, the level of muscle recruitment variability, measured via electromyography (EMG), between visits could provide some explanation for GE variation between tests. While muscle activation patterns are similar among adult cyclists between repeated visits (Jobson, Hopker, Arkesteijn, & Passfield, Citation2013; Laplaud, Hug, & Grélot, Citation2006), their variability between days have not been explored in adolescent cyclists.
Exposure to a motor task enables an individual to develop experience of the exercise demands, whilst also becoming more accustomed to the laboratory equipment. Studies have shown that practice of a movement improves ones internal representation for that specific movement which, in turn, improves motor control and reduces both the level of muscle activity and the variability of muscle activation patterns (Osu et al., Citation2002). Therefore, greater familiarization might logically attenuate the effect of the hypothesized mechanisms, such as increased muscle activation, suggested to cause poor efficiency among younger cyclists (Martin et al., Citation2002). Young, maturing cyclists might also be experiencing so-called “adolescent awkwardness”, whereby delays or regressions in limb coordination and sensorimotor skills have been observed (Quatman-Yates, Quatman, Meszaros, Paterno, & Hewett, Citation2012). It has been suggested that differences in cycling performance between novice and expert cyclists can be partly explained by deficits in skilled muscle recruitment patterns (Chapman et al., Citation2009). Therefore, it is feasible that adolescent cyclists possess a similar skill deficit and, for this reason, produce less consistent muscle recruitment patterns and GE, each of which are determinants of cycling performance.
We aimed to establish the inter-test reliability of GE and muscle activation level (via EMG) of adult and adolescent cyclists, across three tests, interspersed with “familiarization” cycling sessions. It was hypothesized that both groups (adults and adolescents) would require task familiarization, improving the reliability of GE and muscle activation level across consecutive exercise tests; however, the adult group were hypothesized to demonstrate superior reliability in both of these variables across all of the tests. A secondary aim was to evaluate the relationship between inter-test differences in EMG activity and inter-test differences in GE. It was hypothesized that there would be a positive relationship between inter-test differences of EMG and GE among both groups, with higher associations among the adolescent cyclists, owing to a learning effect.
2. Methods
2.1. Design
The participants visited the laboratory at the same time (08:00–11:00 am) on a total of six separate occasions. An initial incremental exercise test was performed, followed 24 h later by three steady-state cycling tests of GE. Each GE test was separated by 48 h, interspersed by a familiarization visit (exactly 24 h after the test). Tests comprised one 10 min bout of steady-state exercise at an external power output (PO) on the ergometer equivalent to 50% of external power at .
was established as the mean value recorded over 30 s of a prior maximal incremental test and the corresponding external PO during this time period was deemed to be the peak value (POpeak). The intensity of all visits was thereafter set at 50% of POpeak (50% POpeak). All visits were performed at the participants’ preferred cadence (established in the incremental test). Myoelectric activity of two lower extremity muscles was estimated via EMG during each cycling test. During each familiarization visit, 45 min of cycling was performed by each participant at the same intensity (50% POpeak). No testing or measurements were performed on familiarization visits. The test re-test reliability of both GE and muscle activation level of the lower extremities was subsequently determined between tests 1 and 2, 2 and 3, & 1 and 3. The relationship between the inter-test differences of EMG activity and GE was also assessed to investigate the relationship between the reliability of both muscle activation levels and GE. Consistent with familiarization or “practice” studies that adopt time series designs, a control group was not used (Lay et al., Citation2002; Moir, Sanders, Button, & Glaister, Citation2005).
2.2. Participants
Nine adult (age = 25.7 ± 4.1 y; body mass = 72.4 ± 3.1 kg; stature = 183.1 ± 7.4 cm; = 60.2 ± 4.0 ml kg−1 min−1) and nine adolescent cyclists (age = 14.0 ± 0.5 y; body mass = 56.7 ± 3.1 kg; stature = 163.1 ± 3.3 cm;
= 51.9 ± 7.2 ml kg−1 min−1) regularly competing for their amateur road cycling clubs, provided written informed consent to take part in this study. Parental consent was also provided for the adolescent cyclists to take part in the study. The participants were training up to 25 h per week (mean hours per week; adults = 18 ± 5 h and adolescents = 9 ± 3 h) on the road, using a mixture of training methods. The cyclists were all tested during a pre-planned rest-period. While all of the cyclists had previously experienced the testing protocol, neither group had been in the laboratory for testing in the 6 months prior to this study. The adult cyclists were accustomed to the laboratory conditions during the previous 3–4 years, performing two testing sessions per year, while the adolescent group had only one year of previous testing in the laboratory (two visits in the previous season). Instructions were given to rest and avoid consumption of caffeine, concentrated nitrate or alcohol in the 24 h prior to each test. The participants were also advised to avoid eating food in the 2 h before the testing sessions and completed a 24 h food diary that was replicated before each subsequent visit. This study was granted institutional ethical approval.
2.3. Incremental test
The participants performed an incremental exercise test on an electronically-braked cycle ergometer (Lode Excalibur, Groningen, Netherlands) to determine peak oxygen consumption () and POpeak. After a 5 min, self-paced warm-up at an external workload of 100 W, the test was started at 150 W and increased by 25 W every minute until a cadence of 50 rev min−1 could not be maintained at the power output or volitional fatigue. During the test, minute ventilation (
) and gas fractions of expired oxygen (FEO2) and carbon dioxide (FECO2) were measured breath-by-breath using a mask connected to a gas analysis system (Jaeger Oxycon Pro, Viasys Healthcare, Hoechberg, Germany). The gas analyser and flow turbine were calibrated before each test using a known gas mixture (15% O2 and 5% CO2) and a 3 L syringe, respectively (Hans Rudolph, Kansas City, KS). As previously described, both
and POpeak were determined as the highest mean value of oxygen uptake or power output, respectively, recorded over 30 s of the test. Half of the POpeak value (50% POpeak) was used to determine the individualised intensities of the GE visits for adult and adolescent cyclists. The preferred cadence (rev min−1) of each participant and their exact seating position was also recorded during the incremental test.
2.4. Gross efficiency
External power, oxygen uptake () and respiratory exchange ratio (RER) were recorded during the GE tests using the same equipment as the incremental test. The position of each participant on the cycle ergometer replicated that at the baseline incremental test and was fixed across the remainder of the study. During a 10 min exercise bout, breath-by-breath
and RER were used to determine the metabolic power (MP) in the final 2 min of the test, calculated from the
and its energetic equivalent according to the table of nonprotein respiratory quotient (Peronnet & Massicotte, Citation1991). The ratio of the individualised PO and MP in the final 2 min of each exercise test was used to determine GE. After a 30 s countdown, the participants cycled at 50% of POpeak at their preferred cadence (previously determined during the incremental test). Each participant was provided with continuous feedback of cadence to facilitate the control of cadence throughout all of their tests and familiarization visits. No information relating to performance was provided to the participants between visits of the study.
2.5. Electromyography
During each 10 min steady-state cycling test, surface EMG recordings were taken from the vastus lateralis (VL) and biceps femoris (BF) muscles of the participants’ right leg with a telemetric EMG system (Telemyo 900, Noraxon, USA, Inc., Arizona, USA). Two surface electrodes were positioned in a bipolar configuration (2 cm apart) onto the belly of the two muscles, parallel to the direction of muscle fibers (Hermens, Freriks, Disselhorst-Klug, & Rau, Citation2000). A permanent marker pen was used to outline the position of the electrodes, which was re-marked each session. These muscles were selected because of their antagonistic involvement in pedalling. Reference electrodes were placed onto the right tibia of the participants. Prior to electrode placement, the skin was shaved, lightly abraded and cleaned with alcohol. The electrodes and wires were securely attached using hyperfix tape to reduce movement artifact. A telemetric signal was sent to an antenna, which was connected to a computer, capturing at 1,000 Hz. The raw EMG signal was processed using Noraxon software (Myoresearch, V 2.11). The raw data were band-pass filtered from 15 to 500 Hz and amplified. The root mean square (EMG-rms) was computed every 15 s and later averaged across the final 2 min of the steady-state cycling, thus aligning with the GE measurement. The EMG-rms data were normalized to the maximal value obtained by that individual during the three steady-state cycling visits and expressed as VL-EMG-rms (%) and BF-EMG-rms (%) for the VL and BF, respectively (Lucia et al., Citation2004).
2.6. Statistical analyses
After the appropriate checks for normality and heteroscedasticity, 95% Limits of Agreement (LoA) was performed (Atkinson & Nevill, Citation1998). Systematic error in GE, VL-EMG-rms and BF-EMG-rms between consecutive tests was established, firstly, using analysis of variance (ANOVA), with Greenhouse-Geissser corrections corrections when the assumption of sphericity was violated. Post-hoc paired t-tests (p < 0.05) were sued to establish pairwise differences and the degree of random error was quantified by multiplying the standard deviation (SD) of the differences in GE (diff) between visits by 1.96 (Atkinson & Nevill, Citation1998). The coefficient of variation (CV%) and the associated 95% confidence intervals (±95% CI) were also determined; (SD diff/√2)/(grand mean) × 100 (Atkinson & Nevill, Citation1998). We used the CV% as the primary measure of variability on the recommendation of others (see Hopkins, Citation2000). To directly test for differences in the reliability of adults and adolescents, independent t-tests were conducted on the CV% values for GE, EMG-rms for VL and BF obtained by each group between tests (i.e. CV% of adults vs. CV% of adolescents between tests 1 and 2; CV% of adults vs. CV% of adolescents between tests 2 and 3; CV% of adults vs. CV% of adolescents between tests 1 and 3). The relationships between the inter-EMG-rms for VL and BF differences of GE and both EMG-rms for VL and BF were evaluated using Pearson’s correlation coefficient (r). The strength of the correlation was interpreted based on the suggestion of Hopkins, Marshall, Batterham, and Hanin (Citation2009), where; <0.1 = trivial; 0.1–0.3 = small; 0.3–0.5 = moderate; 0.5–0.7 = large; 0.7–0.9 = very large and 0.9–1.0 = almost perfect. Data were expressed as means ± SD unless otherwise stated and all statistical procedures were performed on SPSS version 19. Statistical significance was set at p < 0.05 for all tests.
3. Results
The mean (SD) values of MP, PO, GE, cadence, RER, VL-EMG-rms (%) and BF-EMG-rms (%) during all tests are presented in Figures –. There were no systematic differences (F = (2,16) 0.136, p = 0.874) in GE between tests for the adult cyclists ( and ). In the adult group, the CV% for GE ranged from 2.6 to 2.9% between tests (). In the adolescent group, there were differences in GE between tests (F (2,16) = 19.123, p < 0.001), with pairwise differences between tests 1 and 2 (t (8) = 0.724, p < 0.001), 1 and 3 (t (8)=−4.781, p < 0.001) but not 2–3 (t (8) = 0.816, p = 0.438). The CV% ranged from 2.6 to 8.3% across all tests ().
Figure 1. Metabolic power (MP; W) at an external power output of 50% POpeak across tests 1, 2 and 3 among adult (n = 9) and adolescent cyclists (n = 9).
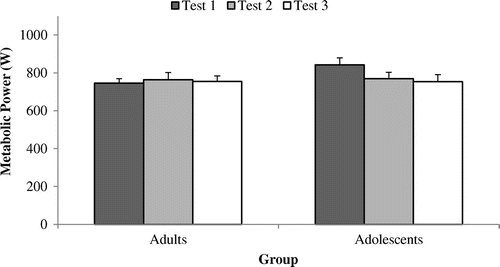
Figure 2. External power output (PO; W) at 50% POpeak across tests 1, 2 and 3 among adult (n = 9) and adolescent cyclists (n = 9).
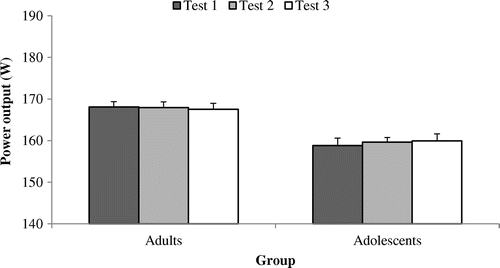
Figure 3. Gross efficiency (GE%) at a power output of 50% POpeak across tests 1, 2 and 3 among adult (n = 9) and adolescent cyclists (n = 9).
Note: † = Significantly different to test l for that group.
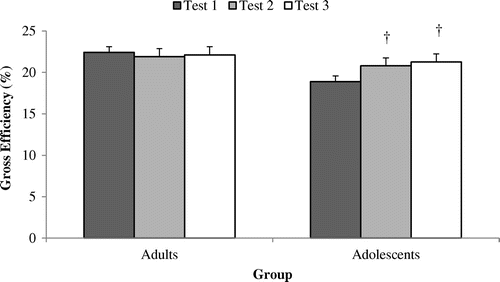
Figure 4. Cadence (rev·min−1) at an external power output of 50% POpeak across tests 1, 2 and 3 among adult (n = 9) and adolescent cyclists (n = 9).
Figure 5. Respiratory exchange ratio (RER) at an external power output of 50% POpeak across tests 1, 2 and 3 among adult (n = 9) and adolescent cyclists (n = 9).
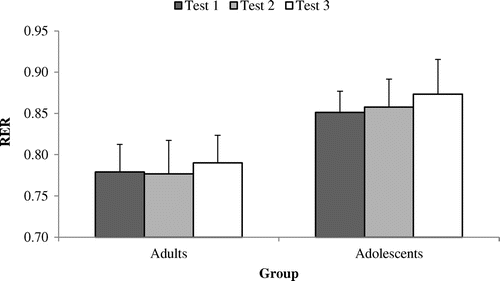
Figure 6. EMG root mean square of the vastus lateralis (VL-EMG-rms [%]) at a power output of 50% POpeak across tests 1, 2 and 3 among adult (n = 9) and adolescent cyclists (n = 9).
Note: † = Significantly different to test l for that group.
![Figure 6. EMG root mean square of the vastus lateralis (VL-EMG-rms [%]) at a power output of 50% POpeak across tests 1, 2 and 3 among adult (n = 9) and adolescent cyclists (n = 9).Note: † = Significantly different to test l for that group.](/cms/asset/abf91614-fd06-4967-93cf-146246edbacb/oamd_a_1237606_f0006_b.gif)
Figure 7. EMG root mean square of the biceps femoris (BF-EMG-rms [%]) at a power output of 50% POpeak across tests 1, 2 and 3 among adult (n = 9) and adolescent cyclists (n = 9).
Note: † = Significantly different to test l for that group.
![Figure 7. EMG root mean square of the biceps femoris (BF-EMG-rms [%]) at a power output of 50% POpeak across tests 1, 2 and 3 among adult (n = 9) and adolescent cyclists (n = 9).Note: † = Significantly different to test l for that group.](/cms/asset/872386a7-2816-4e8e-941b-475b069cd4c8/oamd_a_1237606_f0007_b.gif)
Table 1. Reliability of GE, VL-EMG-rms (%), BF-EMG-rms (%) among adult (n = 9) and adolescent cyclists (n = 9)
The VL-EMG-rms was different across tests (F (2,16) = 10.098, p = 0.001) among the adolescents, with pairwise differences between tests 1 and 2 (t (8) = 3.368, p = 0.010) and 1 and 3 (t (8) = 4.439, p = 0.002) ( and ). The CV% between tests ranged from 8.4 to 12.2% for VL-EMG-rms among adolescents (). Among the adolescents, the BF-EMG-rms were different between tests (F (2,16) = 6.066, p = 0.011), with differences between tests 1 and 2 (t (8) = 3.753, p = 0.006) and 1 and 3 (t (8) = 2.950, p = 0.018). The CV% between tests ranged from 11.5 to 12.5% for BF-EMG-rms among adolescents ( and ). There were no differences (p < 0.05) in EMG-rms for any muscle group between any of the tests for adult cyclists (Figures and ). The CV% ranged from 4.3 to 7.4% for all muscle groups across all tests for adult cyclists ().
The CV% for were larger among the adolescent group compared to the adults for GE between tests 1 and 2 (p < 0.001) and 1 and 3 (p = 0.017). CV% between tests 1 and 3 were also larger among adolescents for VL-EMG-rms (p = 0.036) and between tests 1 and 2 for BF-EMG-rms (p = 0.043) ().
There were no significant relationships found (p > 0.05) between any of the inter-test differences in GE and EMG-rms measurements ().
Table 2. The relationship (r-values) between the inter-test differences of GE and inter-test differences EMG-rms of the vastus lateralis and biceps femoris among adults (n = 9) and adolescents (n = 9)
4. Discussion
The first important finding of this study was that the reliability of GE among trained adults and adolescent cyclists can reach a similar level (CV; ~2–3%), reflecting values previously reported in the literature among adults (CV; 1.3–4.4%) (Hopker et al., Citation2012; Moseley & Jeukendrup, Citation2001; Noordhof et al., Citation2010). However, whilst the GE of adult cyclists was stable across the three tests, the adolescent group demonstrated a learning effect between tests 1 and 2. The improvement in GE was reflected by a significant reduction in MP, for the same external PO, between the first two tests among the adolescent, but not the adult participants. These findings are in partial agreement with the first hypothesis of this study and are similar to the findings reported among non-trained adult participants (Martin et al., Citation2000). In agreement with previous studies, these findings infer the presence of a “familiarization” effect, which might be explained by a change in the technique of the younger cyclists and familiarity with the testing equipment (Hopkins et al., Citation2001; Martin et al., Citation2000). Our findings are comparable to the 9% improvement in movement economy (reduced O2 cost) reported across consecutive days of fixed-power rowing performance among non-experienced participants (Lay et al., Citation2002). A key difference in the current study was the addition of familiarization sessions between tests, offering the participants further time to develop their cycling technique. This is relevant to research in cycling as many studies require participants to perform five or more testing sessions and should anticipate variability in GE of the magnitude presented herein. It should be noted that the adolescent group were not entirely unfamiliar with the testing equipment and protocols but were generally less experienced cyclists, with a lower training volume and exposure to the laboratory conditions in the years preceding the study.
The EMG-rms of the VL and BF changed (p < 0.05) between test 1 and 2 among the adolescents, thus also exhibiting familiarization effects. Systematic changes were not apparent for the adults. This provides evidence of the capacity to systematically reduce EMG activity over a short period of time, acutely decreasing the degree of neural output, whilst using constant cadences and work-rate on a cycle ergometer. That both GE and EMG-rms-VL and EMG-rms-BF changed between tests provides preliminary support for our second hypothesis. Others have reported practice-related reductions in the integrated EMG (i-EMG) of non-athletes whilst performing fixed power outputs (with variable cadence) on a rowing ergometer (Lay et al., Citation2002). However, Lay and colleagues provided 10 familiarization sessions across an average period of 40 days. Unfortunately, the authors only reported changes from baseline in i-EMG and other aspects of performance after 10 days of practice, thus the time-course of change in i-EMG was not evaluated. We observed differences in muscle recruitment among the adolescent cyclists between their second and third test, after only two exposures to cycling activity. This finding provides a preliminary indication that familiarization to very specific mode of exercise is related to an acute (48 h) response (reduction) in muscle recruitment patterns of at least two muscle of the lower-limb and an associated increase GE among adolescent cyclists. Moreover, given that the cadence of the current participants was fixed across tests, in contrast to previous reports (Lay et al., Citation2002), our findings suggest that such changes are attributable to factors other than pedalling rate. Whilst we speculate on these reasons for these changes hereafter, the exact reasons for this require further investigation and, in particular, a more comprehensive evaluation of the changes in muscle recruitment patterns across the lower limb musculature.
Across all of the comparisons, the variability (CV% and 95% LoA) of the EMG-rms measurement was greater than for GE; however, the highest variability was observed for the adolescents (CV = 8.4–12.5%). This was anticipated as inter-test CVs between 15 and 25% have been reported for the VL-i-EMG of mixed ability adults during steady-state cycling between consecutive days (Jobson et al., Citation2013). The relatively larger variation reported by Jobson et al. (Citation2013) could be explained by the treatment of EMG data, since the current study used EMG-rms rather than i-EMG. Others have reported an EMG-rms inter-day bias of 1.5% with 21% random variation (95% LoA) for steady-state cycling between days, using adult participants (Laplaud et al., Citation2006). These findings are remarkably similar to that of the current study for the adult cyclists (). For the first time, we have shown that adolescent cyclists are capable of achieving equivalent levels of reliability for lower-limb muscle activation during cycling. However, adolescents required familiarization sessions in order to do so, with a 12.7% mean bias (± 20%) between test 1 and 2, reduced to 1.0% bias (± 24.6%) by their fifth visit to the laboratory. Regardless of the improvements in EMG-rms bias between sessions, the consistently large random variation should be treated with caution by researchers when attempting to interpret changes in EMG-rms between tests. There are various well-established threats to the reliability of EMG measurements, such as electrode placement, signal crosstalk from adjacent muscles and motion artifacts (Hug & Dorel, Citation2009). However, even when the appropriate methodological steps are taken to account for such factors, the random variation of EMG-rms level between consecutive testing sessions remains large. In agreement with others (Jobson et al., Citation2013), the level of muscle recruitment during steady-state cycling performance is inherently variable and cannot be maintained across consecutive days, or more specifically, over the course of a five-visit study.
As suggested by others (Huang et al., Citation2012; Hug et al., Citation2008; Lay et al., Citation2002; Sparrow & Newell, Citation1998; Sparrow et al., Citation1999), an improvement in the control of movement, after the familiarization visit, could have occurred among the adolescent cyclists, thus reducing the internal work required to achieve the same external PO. Indeed, some have suggested that improvements in external movement efficiency with practice, or in this study “familiarization”, might be attributable to the stimuli provided by metabolic feedback processes (Sparrow & Newell, Citation1998). For example, there is an energetic cost associated with overcoming the inertia of the flywheel, propelling the lower-limbs, as well as maintaining a cycling posture (Weinstein, Kamerman, Berry, & Falk, Citation2004). Indeed, it is suggested that the work done by the limbs to overcome gravitational and inertial forces, relative to the centre of mass (i.e. internal work and power) requires energy expenditure that does not necessarily contribute to forward propulsion (Minetti, Pinkerton, & Zamparo, Citation2001). With this in mind, performing a greater amount of internal work for a given external load would result in poorer efficiency, since a greater proportion of the work performed will be internally dissipated for the same ATP consumption (Minetti, Citation2011). In simple terms, the adolescent group may have altered their muscle activation patterns between tests 1 and 2, such that the same external PO was produced for a lower MP. Given that the power output and cadence of participants was fixed across all visits, with insufficient time for muscle adaptation, it is possible that the acute changes observed in muscle activation could explain the improvement in the GE of adolescents. The known association between EMG amplitude and metabolic cost of exercise (Huang et al., Citation2012; Hug et al., Citation2008; Lay et al., Citation2002; Sparrow & Newell, Citation1998) further supports this theory. Indeed, one important factor that has been suggested to affect internal work is concomitant agonist-antagonist muscle activation or co-activation (Arnaud et al., Citation1997), which refers to the coordination and timing of the opposing lower-limb musculature (van Ingen Schenau, Boots, de Groot, Snackers, & van Woensel, Citation1992). Whilst muscle co-activation was not directly assessed in the current study, the general decreases in the muscle activation of agonist and antagonist (VL and BF) muscle groups among the adolescents during steady-state cycling might have supported this theory.
Based on the above reasoning, we hypothesized that the reduction in EMG-rms activity of both the VL and BF between tests would correlate with inter-test changes in GE. Indeed, given that both GE and EMG-rms of the VL and BF changed between test 1 and 2, an association between GE and muscle recruitment might have been anticipated. However, further analysis revealed no significant relationships between the inter-test differences of GE and EMG-rms-VL and EMG-rms-BF. Despite the lack of significant relationships, it is worth noting that there were “small”, non-significant, inverse relationships between inter-test differences of GE and EMG-rms-VL in both adolescents and adults (). An inverse relationship between these variables indicates that a decrease in muscle recruitment relates to an increase in GE, which is consistent with previous reports that have linked the level of muscle activation with metabolic energy expenditure (Lay et al., Citation2002). Further research is required to elucidate these relationships. The large inter-test random variation () of the EMG-rms, particularly for the BF, could partly explain the poorer relationships found. However, based on previous research, this level of variability should be anticipated for surface EMG recordings and is unlikely to be improved. It is also possible that the small number of measured muscle groups limited the strength of these relationships, as many other muscles of the lower-limb segments, such as vastus medialis, rectus femoris, gastrocnemius, soleus and tibialis anterior, are involved in cycling performance (Ryan & Gregor, Citation1992) and may explain more of the variance in GE between visits (Hug & Dorel, Citation2009). Future studies should investigate the relationship between the inter-visit differences in pooled EMG-rms activity across all major cycling muscle groups to the inter-visit GE differences, since this will provide a more comprehensive understanding of the factors that explain acute improvements in the GE of adolescents. Irrespective of the reasons for the variable EMG findings in this study, researchers using small numbers of muscle groups in cycling studies should be aware of the potential for variation in EMG patterns between tests. Of course, it is possible that the changes in muscle recruitment patterns of the lower-limbs do not explain changes in internal power and that other non-propulsive, yet energy demanding process, such as changes in ventilatory muscle efficiency are responsible for changes in GE.
Our findings provide important information for practitioners attempting to evaluate GE among adolescent cyclists, without offering sufficient (at least two sessions of cycling) familiarization. For example, without a familiarization visit, the observed error of 6.8% CV or 95% LoA of 1.90 ± 1.06% would prevent the detection of small changes in cycling efficiency, such as the important 6.6% relative change in GE (21.18–22.66%) reported in a Tour de France champion over a 5-year period of maturity (Coyle, Citation2005). However, following familiarization, the reduced error of 2.6% CV or 95% LoA of 0.45 ± 1.59% would permit the detection of the above changes. Therefore, studies attempting to evaluate changes in GE or related values, such as delta efficiency (DE) during adolescence (Martin et al., Citation2002) should provide at least two familiarization visits between tests and consider that changes in GE smaller than 2.6% are unlikely to be detected. Furthermore, given the reliance of DE on GE, the findings of this study might partly account for the poorer DE values reported among children compared to adults, particularly when no familiarization sessions are provided (i.e. Martin et al., Citation2002). While it was important to establish the effect of familiarization visits on GE, future research might also consider examining the effect of this on related variables such as DE or net efficiency. Since adults appear to be reliable between visits of GE assessment, it is also important to establish if a “washout” or “detraining” period of familiarization exists among adolescents. This would inform practitioners of when to provide familiarization for younger participants.
In practice, researchers evaluating GE among adolescents (i.e. Butte et al., Citation2007; Mccann & Adams, Citation2003; Weinstein et al., Citation2004) should be mindful of the results presented herein, providing at least two familiarization sessions prior to testing GE. Moreover, practitioners should be encouraged to assess the reliability of their sample, adopting a suitable sample size in order to detect the required change in GE. For example, to identify a 5% change in GE among adolescents, with a CV% obtained after two steady-state tests (2.6%), a sample size of nine would be required (Batterham & Atkinson, Citation2005). However, without a familiarization session, the sample size required would be 20, which might be problematic for practitioners working with select teams of athletes or small intervention groups. The EMG-rms measurements of the VL and BF are more variable between visits and would require a sample of between ~50 and 80 (VL and BF) to find a 5% change for adults or adolescents. This sample size requirement would not change appreciably, even with familiarization visits, owing to the degree of random variation across all of the participants.
5. Conclusion
Gross efficiency can be reliably determined among adult and adolescent cyclists; however, adolescents should be provided with at least two familiarization visits prior to the administration of a test for GE. Further research is required to elucidate the mechanisms responsible for the acute improvement in GE between tests, as the weak relationships between inter-test EMG-rms activity rejected the hypothesis that muscle recruitment patterns of the VL and BF explained changes in GE among the less experienced adolescents. The poorer inter-test reliability of the EMG-rms measurements is likely to have affected this relationship, alongside activation of nearby muscle groups that were not measured in this study. However, further research is required since others have attributed concurrent systematic changes in GE and muscle activity to a discovery-type learning, whereby participants attempt various movement strategies and response modifications to optimise their performance on a task (Huang et al., Citation2012; Hug et al., Citation2008; Lay et al., Citation2002; Sparrow & Newell, Citation1998). Since all external parameters (cadence and PO) were fixed in the current study, it remains possible that the improvements in GE could explained by subtle alterations in the internal synergies between muscle groups, other than the ones investigated herein. Given the training status of the current participants and their habituation to cycling, it is likely that less well-trained participants will exhibit inter-test variation of either the same, or larger, magnitude. It is important that future research investigates a larger pool of muscle groups to confirm these changes and considers the inclusion of a control group, as this will help to establish test-to-test changes in these variables without familiarization visits. Nevertheless, the findings of this study convey an important message to researchers regarding the potential preparation that is necessary prior to measuring, and attempting to identify changes, in GE or muscle activity among adult and adolescent cyclists.
Additional information
Funding
Notes on contributors
Mark Waldron
Dr Mark Waldron is a senior lecturer at St Mary’s University in the UK and an adjunct lecturer at the University of New England in Australia. Mark’s work is concerned with the optimization of human performance among adults and adolescents.
Jamie Highton
Dr Jamie Highton is the area lead for sports physiology at the University of Chester in the UK and has an interest in multiple-sprint sport and interventions that offset fatigue.
Adrian Gray
Dr Adrian Gray is a senior lecturer at the University of New England in Australia and has an interest in the energetics of human performance. The current article supports the continuing collaboration between the authors’ universities, which aims to understand and improve the application of sports science principles to practice among athletes.
References
- Arnaud, S., Zattara-Hartmann, M. C., Toméi, C., & Jammes, Y. (1997). Correlation between muscle metabolism and changes in M-wave and surface electromyogram: Dynamic constant load leg exercise in untrained subjects. Muscle and Nerve, 20, 1197–1199.10.1002/(ISSN)1097-4598
- Atkinson, G., & Nevill, A. M. (1998). Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Medicine, 26, 217–238.10.2165/00007256-199826040-00002
- Batterham, A., & Atkinson, G. (2005). How big does my sample need to be? A primer on the murky world of sample size estimation. Physical Therapy in Sport, 6, 153–163.10.1016/j.ptsp.2005.05.004
- Butte, N. F., Puyau, M. R., Vohra, F. A., Adolph, A. L., Mehta, N. R., & Zakeri, I. (2007). Body size, body composition, and metabolic profile explain higher energy expenditure in overweight children. Journal of Nutrition, 137, 2660–2667.
- Chapman, A., Vicenzino, B., Blanch, P., & Hodges, P. (2009). Do differences in muscle recruitment between novice and elite cyclists reflect different movement patterns or less skilled muscle recruitment? Journal of Science and Medicine in Sport, 12, 31–34.10.1016/j.jsams.2007.08.012
- Coyle, E. F. (1999). Physiological determinants of endurance exercise performance. Journal of Science and Medicine in Sport, 2, 181–189.10.1016/S1440-2440(99)80172-8
- Coyle, E. F. (2005). Improved muscular efficiency displayed as Tour de France champion matures. Journal of Applied Physiology, 98, 2191–2196.10.1152/japplphysiol.00216.2005
- Durand, M., Geoffroi, V., Varray, A., & Préfaut, C. (1994). Study of the energy correlates in the learning of a complex self-paced cyclical skill. Human Movement Science, 13, 785–799.10.1016/0167-9457(94)90018-3
- Hermens, H. J., Freriks, B., Disselhorst-Klug, C., & Rau, G. (2000). Development of recommendations for SEMG sensors and sensor placement procedures. Journal of Electromyography and Kinesiology, 10, 361–374.10.1016/S1050-6411(00)00027-4
- Hopker, J. G., Jobson, S. A., Gregson, H. C., Coleman, D. A., & Passfield, L. (2012). Reliability of cycling gross efficiency using the douglas bag method. Medicine and Science in Sports and Exercise, 44, 290–296.10.1249/MSS.0b013e31822cb0d2
- Hopkins, W. G. (2000). Measures of reliability in sports medicine and science. Sports Medicine, 30, 1–15.10.2165/00007256-200030010-00001
- Hopkins, W. G., Marshall, S. W., Batterham, A. M., & Hanin, J. (2009). Progressive statistics for studies in sports medicine and exercise science. Medicine and Science in Sports and Exercise, 41, 3–13.10.1249/MSS.0b013e31818cb278
- Hopkins, W., Schabort, E. J., & Hawley, J. A. (2001). Reliability of power in physical performance tests. Sports Medicine, 31, 211–234.10.2165/00007256-200131030-00005
- Horowitz, J. F., Sidossis, L. S., & Coyle, E. F. (1994). High efficiency of type I muscle fibers improves performance. International Journal of Sports Medicine, 15, 152–157.10.1055/s-2007-1021038
- Huang, H. J., Kram, R., & Ahmed, A. A. (2012). Reduction of metabolic cost during motor learning of arm reaching dynamics. Journal of Neuroscience, 32, 2182–2190.10.1523/JNEUROSCI.4003-11.2012
- Hug, F., & Dorel, S. (2009). Electromyographic analysis of pedaling: A review. Journal of Electromyography and Kinesiology, 19, 182–198.10.1016/j.jelekin.2007.10.010
- Hug, F., Drouet, J. M., Champoux, Y., Couturier, A., & Dorel, S. (2008). Interindividual variability of electromyographic patterns and pedal force profiles in trained cyclists. European Journal of Applied Physiology, 104, 667–678.10.1007/s00421-008-0810-y
- Jobson, S. A., Hopker, J., Arkesteijn, M., & Passfield, L. (2013). Inter- and intra-session reliability of muscle activity patterns during cycling. Journal of Electromyography and Kinesiology, 23, 230–237.10.1016/j.jelekin.2012.08.013
- Laplaud, D., Hug, F., & Grélot, L. (2006). Reproducibility of eight lower limb muscles activity level in the course of an incremental pedaling exercise. Journal of Electromyography and Kinesiology, 16, 158–166.10.1016/j.jelekin.2005.04.002
- Lay, B. S., Sparrow, W. A., Hughes, K. M., & O’Dwyer, N. J. (2002). Practice effects on coordination and control, metabolic energy expenditure, and muscle activation. Human Movement Science, 21, 807–830.10.1016/S0167-9457(02)00166-5
- Lucia, A., San Juan, A. F., Montilla, M., Canete, S., Santalla, A., Earnest, C., & Perez, M. (2004). In professional road cyclists, low pedaling cadences are less efficient. Medicine and Science in Sports and Exercise, 36, 1048–1054.10.1249/01.MSS.0000128249.10305.8A
- Martin, J. C., Diedrich, D., & Coyle, E. F. (2000). Time course of learning to produce maximum cycling power. International Journal of Sports Medicine, 21, 485–487.10.1055/s-2000-7415
- Martin, R., Hautier, C., & Bedu, M. (2002). Effect of age and pedalling rate on cycling efficiency and internal power in humans. European Journal of Applied Physiology, 86, 245–250.10.1007/s00421-001-0546-4
- Mccann, D. J., & Adams, W. C. (2003). The size-independent oxygen cost of running. Medicine and Science in Sports and Exercise, 35, 1049–1056.10.1249/01.MSS.0000069409.44016.04
- Minetti, A. E. (2011). Bioenergetics and biomechanics of cycling: The role of ‘internal work’. European Journal of Applied Physiology, 111, 323–329.10.1007/s00421-010-1434-6
- Minetti, A. E., Pinkerton, J., & Zamparo, P. (2001). From bipedalism to bicyclism: Evolution in energetics and biomechanics of historic bicycles. Proceedings of the Royal Society B: Biological Sciences, 268, 1351–1360.10.1098/rspb.2001.1662
- Moir, G., Sanders, R., Button, C., & Glaister, M. (2005). The influence of familiarization on the reliability of force variables measured during unloaded and loaded vertical jumps. Journal of Strength and Conditioning Research, 19, 140–145.
- Moseley, L., & Jeukendrup, A. E. (2001). The reliability of cycling efficiency. Medicine and Science in Sports and Exercise, 33, 621–627.10.1097/00005768-200104000-00017
- Noordhof, D. A., de Koning, J. J., van Erp, T., van Keimpema, B., de Ridder, D., Otter, R., & Foster, C. (2010). The between and within day variation in gross efficiency. European Journal of Applied Physiology, 109, 1209–1218.10.1007/s00421-010-1497-4
- Osu, R., Franklin, D. W., Kato, H., Gomi, H., Domen, K., Yoshioka, T., & Kawato, M. (2002). Short- and long-term changes in joint co-contraction associated with motor learning as revealed from surface EMG. Journal of Electromyography and Kinesiology, 88, 991–1004.
- Peronnet, F., & Massicotte, D. (1991). Table of nonprotein respiratory quotient: An update. Canadian Journal of Sports Science, 16, 23–29.
- Quatman-Yates, C., Quatman, C. E., Meszaros, A. J, Paterno, M. V., & Hewett, T. E. (2012). A systematic review of sensorimotor function during adolescence: A developmental stage of increased motor awkwardness? British Journal of Sports Medicine, 46, 649–655.10.1136/bjsm.2010.079616
- Ryan, M. M., & Gregor, R. J. (1992). EMG profiles of lower extremity muscles during cycling at constant workload and cadence. Journal of Electromyography and Kinesiology, 2, 69–80.10.1016/1050-6411(92)90018-E
- Sparrow, W. A., & Newell, K. M. (1998). Metabolic energy expenditure and the regulation of movement economy. Psychonomic Bulletin & Review, 5, 173–196.10.3758/BF03212943
- Sparrow, W. A., Hughes, K. M., Russell, A. P., & Le Rossignol, P. F. (1999). Effects of practice and preferred rate on perceived exertion, metabolic variables and movement control. Human Movement Science, 18, 137–153.10.1016/S0167-9457(99)00005-6
- van Ingen Schenau, G. J., Boots, P. J., de Groot, G., Snackers, R. J., & van Woensel, W. W. (1992). The constrained control of force and position in multi-joint movements. Neuroscience, 46, 197–207.10.1016/0306-4522(92)90019-X
- Weinstein, Y., Kamerman, T., Berry, E., & Falk, B. (2004). Mechanical efficiency of normal-weight prepubertal boys predisposed to obesity. Medicine and Science in Sports and Exercise, 36, 567–573.10.1249/01.MSS.0000121958.99985.A5
