Abstract
Peritonitis remains the main complication of peritoneal dialysis (PD). Antimicrobial therapy may be threatened by development of bacterial resistance, demanding continuous surveillance of infectious agents to improve empirical treatment. The aims of this study were to evaluate the bacterial agents causing peritonitis and their antimicrobial susceptibility in patients undergone PD in a Brazilian center, between 1993 and 2013. Strains were recovered from peritoneal fluid, identified by usual methods and submitted to Etest antimicrobial susceptibility. A total of 400 strains were studied, of which 65.8% were Staphylococcus spp.: S. epidermidis, the main species (22.8%), followed by S. aureus (21.3%). Over time, we verified a decrease in overall peritonitis occurrence, and an increase in gram-negative bacteria proportion, attributed, mainly, to a decrease in gram-positive agents. Vancomycin was effective against all Staphylococcus strains, and oxacillin resistance was higher in coagulase-negative Staphylococcus compared to S. aureus (p < 0.01). Regarding gram-negative bacteria, Enterobacteriaceae species accounted for 20.3% of the strains, but P. aeruginosa, Acinetobacter species, S. maltophilia, B. cepacia, P. fluorescens and A. xylosoxidans were also detected (14.3%). Non-fermentative gram-Negative bacilli presented increased antimicrobial resistance compared to Enterobacteriaceae, and imipenem was the most active antimicrobial drug. Over the 20 year period, increasing or decreasing in the antimicrobial susceptibilities were not observed, despite the occurrence of punctual oscillations. In summary, we verified peritonitis occurrence declining over years, gram-positive infections proportion dropping, and, inversely, gram-negative pathogens proportion increasing. Our finding reinforces recommendations regarding retrospective evaluation of antimicrobial susceptibility to define the optimal empirical therapy in each center.
Public Interest Statement
Patient with chronic kidney disease often progress to renal failure. Peritoneal dialysis (PD) represents an option in treating these cases before hemodialysis or renal transplantation. PD uses peritoneum as a filter, being a practical alternative with comparable survival rates to hemodialysis. Peritonitis is the main cause of PD interruption, with known impact on morbidity and mortality. It is an infection that can be caused by several microorganisms, presenting variable pathogenic capacity and antimicrobial resistance. Serious problems for the practice of rational antibiotic therapy can occur. In this study we investigated, over 20 years (1993–2013), the frequency and the antimicrobial sensibility of 400 bacterial strains isolated from peritonitis, in order to analyze the impact of practice changes adopted in one center and to provide data for improving patient care. Our microbiological data are in accordance to the current international guidelines, which recommends retrospective local results to define the optimal empiric therapy.
Competing Interests
The authors declare no competing interest.
1. Introduction
Peritoneal dialysis (PD) represents an option in treating chronic renal diseases. This method presents the same survival rates as hemodialysis in treating end-stage renal disease, but has an important advantage in patient satisfaction and life quality reports (Tokgoz, Citation2009). Advances in this treatment option resulted in reducing peritonitis rates in recent decades, but infective events are still the main adverse cause of technique failure, with significant impacts on morbidity and mortality rates (Pérez Fontan, Rodríguez-Carmona, García-Naveiro, et al., Citation2005). Despite geographic specificities, the main etiological agents of infections are Staphylococcus species (Nishina et al., Citation2014; van Esch, Krediet, & Struijk, Citation2014). We have shown that outcomes of peritonitis due to Staphylococcus are influenced by implicated species (Barretti, Montelli, Batalha, Caramori, & Maria de Lourdes, Citation2009; Camargo et al., Citation2014), virulence factors (Barretti, Montelli, et al., Citation2009; Barretti et al., Citation2012; Cunha et al., Citation2004, Citation2005), antimicrobial resistance (Camargo et al., Citation2014; Oliveira et al., Citation2012) besides some specific host features (Barretti et al., Citation2012). Indeed, peritonitis due to gram-negative rods are milder when caused by Enterobacteriaceae strains compared to non-fermentative gram-negative bacilli (NFGNB) (Oliveira et al., Citation2012); very frequent catheter removal and hospitalization rates are reported for patients with P. aeruginosa infections (Borràs, Citation2009; Siva et al., Citation2009). The International Society for Peritoneal Dialysis (ISPD) recommends that empirical treatment must be initiated based on retrospective data of antimicrobial susceptibility of each center (Li et al., Citation2010; Piraino et al., Citation2005). Changes in the practices, in the agents implicated in peritonitis and in their antimicrobial susceptibility, warrant continuous surveillance of infections in PD (Szeto, Citation2014). In this study, we aimed to evaluate the frequency and the antimicrobial susceptibility of the bacterial agents causing PD-infections in a single Brazilian center over the last two decades (1994–2013), in order to analyze the impact of practice changes adopted in this center and to provide data for improving patient care.
2. Materials and methods
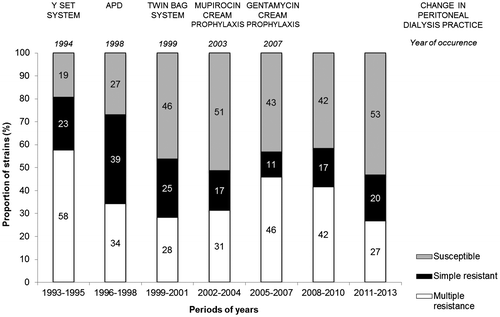
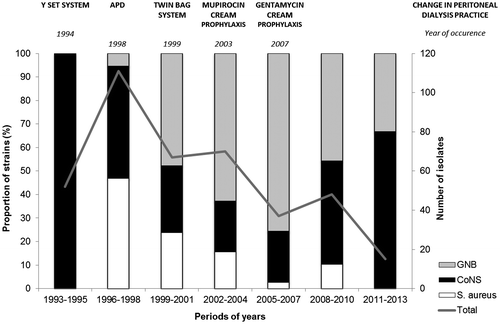
This study evaluated the most prevalent bacterial isolates (Staphylococcus and gram-negative bacilli) recovered from peritoneal fluid of patients attending a single university center in Brazil, continuously, over a period of 20 years (1993–2013). Patients were diagnosed with peritonitis by the presence of at least two of the following criteria: (1) cloudy peritoneal effluent, (2) abdominal pain, (3) a dialysate white-cell count higher than 100/μL with at least 50% polymorphonuclear cells, and (4) positive culture of peritoneal effluent (Li et al., Citation2010; Piraino et al., Citation2005). Dialysis practices changed over the evaluated period (): a Y-set system was introduced in 1994; automated PD (APD) in 1998; a twin bag was introduced in 1999. Daily mupirocin cream application at the exit site has been recommended since 2003 and gentamicin, since 2007.
Figure 1. Temporal distribution of bacterial causative organisms of peritoneal dialysis-related peritonitis in a Brazilian center over two decades. Left axis (columns) represents the proportion (percentage) of each bacterial group, according to the 3-year periods evaluated. Right axis (line) indicates the overall number of isolates in each of the same 3-year periods. Changes in peritoneal dialysis practice are named above the bars, as well as the year of their implementation. APD: automated peritoneal dialysis; CoNS: coagulase-negative Staphylococcus; GNB: gram-negative bacilli.
The initial cultures from centrifuged peritoneal fluid samples were performed with the Bactec® Automated System (Becton Dickinson Company, Sparks, Maryland, USA), and then seeded onto blood agar and MacConkey Agar (Oxoid, Basingstoke, United Kingdom). The isolates were gram stained to confirm purity and determine morphology and specific color. Bacteria identification was performed by classical phenotypic tests (Koneman & Winn, Citation2006) and by Vitek or Vitek 2 (bioMérieux, Inc., Durham, NC, USA) automated systems.
The in vitro susceptibilities to antimicrobial agents, chosen according to the bacterial agent and the Clinical and Laboratory Standards Institute (CLSI) recommendations, were evaluated based on minimal inhibitory concentrations (MIC) determined by the Etest (bioMérieux, Inc., Durham, NC, USA), according to the manufacturer’s instructions. The following antimicrobial agents were evaluated: penicillin G, oxacillin, cephalothin, levofloxacin, netilmicin, vancomycin, for gram-positive agents, and gentamicin, ceftazidime, cefepime, gatifloxacin, ofloxacin and imipenem for gram-negative ones. MIC was categorized in susceptible or resistant (strains with intermediate susceptibility were considered resistant) according to CLSI breakpoints (Clinical & Laboratory Standards Institute, Citation2015). Quality control strains (S. aureus ATCC 29213; E. coli ATCC 25922; P. aeruginosa ATCC 27853) were used to ensure test performance. MIC50 and MIC90 values were calculated based on the MIC value distribution: MIC50 is defined as the median value of MIC distribution; that is, the MIC value that can inhibit the growth of 50% of the bacterial population; MIC90 is the MIC value that can inhibit the growth of 90% of the bacterial population.
Resistance pattern classes were created to compare different bacterial species and were determined according to the number of resistance presented for each strain: susceptible (zero resistance), simple resistant (resistance to only one drug) or multiple resistant (resistance to two or more drugs).
Chi-squared or Fisher’s exact tests were used to compare frequencies (www.openepi.com); p values below 0.05 were considered to be significant.
The Research Ethics Committee of the Faculty of Medicine of Botucatu, Brazil (OF. 028/08-CEP) approved this study. We were granted an exemption from the requirement to obtain written informed-consent from the participants and/or their legal guardians because the strains included in the study had already been isolated and stored, on an ongoing basis, in the Culture Collection of the Department of Microbiology and Immunology, UNESP, Botucatu, São Paulo, Brazil.
3. Results
3.1. Etiological agents
From January 1993 to July 2013, 400 bacterial strains, corresponding to 400 episodes of peritonitis, were included in this study: 85 strains of Staphylococcus aureus; 178 of coagulase-negative Staphylococcus (CoNS) and 137 strains of gram-negative bacilli ().
Table 1. Causative agents of 400 episodes of peritoneal dialysis-associated peritonitis caused by bacterial agents in a Brazilian healthcare center (1993–2013)
3.2. Temporal evolution of microorganisms over time
Reduction in overall frequency of PD-related peritonitis was observed over time: the highest number of episodes was observed between 1996 and 1998 (111 episodes) and the lowest between 2011 and 2013 (15 episodes). Despite this reduction in frequency of peritonitis, an increase in proportion of episodes due to GNB was observed between 1996 and 1998 and between 2005 and 2007, with less episodes due to S. aureus. In more recent periods (between 2008 and 2013), the CoNS species represented the main causative agent of PD-associated peritonitis (). We observed the impact of the APD system introduction in 1998 and the adoption of a twin bag system in 1999 in gram-positive frequency, but an increase in the number of GNB episodes. After the mupirocin cream prophylaxis, introduced in 2003, and the gentamicin cream prophylaxis, in 2007, a decline in the overall rate of all agents was observed ().
3.3. Susceptibility testing
Antimicrobial susceptibility testing results are presented in . Oxacillin susceptibility rate was significantly higher for S. aureus (83.5%) compared with CoNS strains (36.0%) (p < 0.01). Regarding Staphylococcus epidermidis and other CoNS strains, the oxacillin susceptibility rate was found to be equally low in both groups (37.4 and 34.5%, respectively; p = 0.40). Vancomycin resistance was not detected against Staphylococcus strains.
Table 2. Susceptibility parameters of causative agents of PD-related peritonitis in a single healthcare center (Botucatu Medical School, 1993–2013)
Imipenem and ceftazidime presented the highest susceptibility rates for P. aeruginosa strains (77.8 and 74.1%, respectively) but significantly lower than those found for Enterobacteriaceae (Fisher’s p < 0.01, for imipenem [96.3%] and ceftazidime [95.1%]). The Acinetobacter strains presented the highest susceptibility rates to imipenem (85.7%) and gentamicin (57.1%).
Despite the intrinsic resistance to several antimicrobial classes, S. maltophilia and B. cepacia strains presented susceptibility rates of 75% (6/8 strains) and 100% (5/5 strains) for ceftazidime, respectively.
The P. fluorescens strain presented susceptibility to gentamicin (MIC 0.38 μg/mL), ceftazidime (1.5 μg/mL), cefepime (1.0 μg/mL), imipenem (0.5 μg/mL) but resistance to gatifloxacin (3 μg/mL) and ofloxacin (32 μg/mL). A. xylosoxidans strain was susceptible only to ceftazidime (4 μg/mL) and imipenem (1.0 μg/mL), and resistances to gentamicin (64 μg/mL) and cefepime (128 μg/mL) were detected.
3.4. Resistance patterns: Susceptible, simple and multiple resistances
Resistance patterns were defined according to the number of resistance presented for each strain: susceptible (zero resistance), simple resistant (resistance to only one drug) or multiple resistant (resistance to two or more drugs) (). Overall CoNS presented higher proportion of multiple resistant strains (61%) compared to S. aureus (17%) (p < 0.01), but similar rates of multiple resistant between S. epidermidis (62%) and the other CoNS staphylococci (60%) (p = 0.46). Regarding gram-negative bacilli, Enterobacteriaceae species presented a lower proportion of multiple resistant strains (10%) compared to P. aeruginosa (33%; Fisher’s p < 0.01) or Acinetobacter spp. (64%; Fisher’s p < 0.01).
Table 3. Distribution of resistance pattern classes [frequency (%)] of bacterial agents of peritoneal dialysis-associated peritonitis caused by bacterial agents in a Brazilian healthcare center (1993–2013)
3.5. Evolution of antimicrobial susceptibility over time
Staphylococcus spp. presented fluctuating values of penicillin G susceptibility rates, but constant rates of oxacillin (lower rates for CoNS), cephalothin, and vancomycin (always 100%) susceptibilities. Among Enterobacteriaceae strains, high susceptibility rates (about 90%) were verified over the periods for the drugs ceftazidime and imipenem; a statistically insignificant decrease in susceptibility rates of gentamicin (from 92.9% to 66.7%, Fisher’s p = 0.14) and cefepime (from 92.9% to 55.6%, Fisher’s p = 0.06) was observed in 2008–2010 compared with 2005–2007 period, respectively. For P. aeruginosa and Acinetobacter spp., imipenem showed the highest activity, but the remaining drug susceptibility rates oscillated over time ().
Table 4. Distribution of susceptibility rates for the main antimicrobial agents in prevalent causative agents of peritoneal-dialysis bacterial related peritonitis S. aureus; coagulase negative Staphylococcus (CoNS); Enterobacteriaceae; P. aeruginosa; Acinetobacter spp.
Over time, it was possible to observe a fluctuation in resistant pattern categories, as demonstrated in , with a slight (non-significant) trend of increasing strains susceptible to all evaluated drugs. The period of 1993–1995 presented the lowest value of susceptibility (19%) and the highest rate of multiple resistance strains (58%). From this period on, susceptibility ranged from 27% in 1996–1998 to the peak 53% in 2011–2013. Contrasting to emerging gentamicin resistance observed from 2007, increase in the susceptibility class was mainly influenced by Enterobacteriaceae strains.
4. Discussion
4.1. Etiological agents
In this largest series of PD-related peritonitis in a single Brazilian healthcare center, we evaluated the frequency of etiological agents and their antimicrobial susceptibility over 20 years. We verified the overall predominance of Staphylococcus species and a global reduction in the number of bacterial peritonitis over the years.
Staphylococcus spp. are currently the main etiological agents of PD infections (Nieto-Ríos et al., Citation2014; Nishina et al., Citation2014; van Esch et al., Citation2014), and nasal colonization status seems to play an important role in the development of S. aureus peritonitis. Indeed, infection due to S. aureus were shown to increase the unfavorable outcomes compared to CoNS in our center (Camargo et al., Citation2014) with virulence factors such as beta-hemolysin and biofilm production (Barretti, Montelli, et al., Citation2009; Barretti et al., Citation2012) impacting negatively on outcomes. On the other hand, but in line with the largest series (Fahim et al., Citation2010; Szeto et al., Citation2008), infections due to CoNS were milder and with a favorable outcome. In our center, a favorable outcome was influenced by oxacillin susceptibility and initial treatment with vancomycin (Camargo et al., Citation2014), underscoring the importance of longitudinal analyses of antimicrobial susceptibility data towards optimizing empirical strategies and avoiding the emergence of antimicrobial resistance (Szeto, Citation2014). A high and constant frequency rate of oxacillin resistance CoNS required changes in the empirical treatment in our center, which currently is based on IP vancomycin and amikacin (Barretti et al., Citation2012). Contrasting to the reduction in the overall frequency of gram-positive pathogens, infection due to gram-negative pathogens increased in number, and consequently, in its proportion, in the early 2000s (Borràs, Citation2009). The same phenomenon was also reported earlier (Huang et al., Citation2011; Szeto et al., Citation2005), mainly attributed to improvements in connectology methodology that reduced the incidence of skin contaminating pathogens (Szeto, Citation2014). Importance of gram-negative pathogens have increased over the last years, particularly due to the emergence of different antimicrobial resistance determinants, such as extended spectrum beta-lactamase in Enterobacteriaceae species (Borràs, Citation2009; Feng et al., Citation2014) and carbapenemases in P. aeruginosa and Acinetobacter spp. (Prasad et al., Citation2014; Zhang et al., Citation2014).
Overall, a reduction in the number of infections in this series might be affected by changes in peritoneal practices over the years, initiated by the introduction of the Y-set system in the early 1990s; APD introduction in 1998 contributed to this decline, which was reinforced by the implementation of other measures (). Mupirocin cream prophylaxis was shown to be associated with the reduction in the S. aureus (Barretti et al., Citation2012) but not with CoNS peritonitis incidence in this center (Camargo et al., Citation2014). An absence of influence of mupirocin on PD-peritonitis pathogens other than S. aureus was verified by Xu, Tu, and Xu (Citation2010), analyzing three randomized controlled trials. Still CoNS have presented high oxacillin resistance rates in that casuistry; cross resistance to mupirocin was not investigated; the literature, however, does not confirm cross resistance between oxacillin and mupirocin (Cookson, Citation1998), likely ruling out this hypothesis and instigating the real role of mupirocin prophylaxis on S. aureus and coagulase-negative Staphylococcus peritonitis. Indeed, the role of mupirocin on peritonitis episodes seems to be less impacting in the daily practice than in the randomized trials, maybe due to the adherence to this practice (Szeto, Citation2014).
4.2. Antimicrobial susceptibility
Comprehensive evaluation of antimicrobial susceptibility was performed in all 400 bacterial strains isolated from PD-peritonitis. In line with a previous contemporary series (McGuire, Carson, Inglis, & Chakera, Citation2015; van Esch et al., Citation2014), vancomycin was the most potent antimicrobial agent against gram-positive bacteria (100% susceptible), even for CoNS strains more prone to present resistance to this drug (Srinivasan, Dick, & Perl, Citation2002). Oxacillin resistance was remarkably lower in S. aureus compared to CoNS. Oxacillin resistance is mainly mediated by mecA gene, which is allocated into the Staphylococcal chromosomal cassette mec (SCCmec), a transferable genetic element (Martins & Cunha, Citation2007). Higher frequency of oxacillin resistance in CoNS may be the result of the spread of those species and to the increased interaction among negative- and positive-mecA carrying strains, besides the higher diversity of SCCmec elements in CoNS, favoring the transmissibility of this resistance determinant (Zong, Peng, & Lü, Citation2011). In relation to gram-negative antimicrobial susceptibility, imipenem presented the best results but beta-lactams also achieved more than 90% activity. Imipenem was the most efficient drug against P. aeruginosa and Acinetobacter species, but presented consistently lower susceptibility rates than Enterobacteriaceae. For B. cepacia and S. maltophilia, ceftazidime presented good results, inhibiting all but two evaluated strains ().
Susceptibility categories defined by the number of resistances demonstrated the high frequency of multiple resistant CoNS and the predominance of susceptible Enterobacteriaceae strains. CoNS are recognized as a reservoir of resistance determinants in Staphylococcus (Martins & Cunha, Citation2007; Zhang, Agidi, & LeJeune, Citation2009; Zong et al., Citation2011) and yet this pathogen is associated with milder infections, virulence determinants, such as toxins and biofilm, may be present and to contribute to its pathogenicity, and, consequently, to unfavorable outcomes (Barretti, Montelli, et al., Citation2009; Cunha et al., Citation2005), underscoring the need for monitoring the antimicrobial resistance of these emerging causative organisms of PD infections. Kim et al. (Citation2004) reported an increase in MRCoNS among patients suffering from PD-peritonitis in a Korean healthcare center over 10 years. A recent German report also described the increase in Methicillin-resistant S. epidermidis in a single-center longitudinal study (Kitterer et al., Citation2015).
On the other hand, peritonitis due to Enterobacteriaceae presented better outcomes (Oliveira et al., Citation2012) and lower resistance rates (Barretti, Pereira, et al., Citation2009) than NFGNB. It is reasonable to attribute the worst outcomes in NFGNB peritonitis to antimicrobial resistance compared to Enterobacteriaceae infections. Pathogens enrolled into the NFGNB group are naturally more resistant than other GNB due to intrinsic and multiple acquired resistance mechanisms (Livermore, Winstanley, & Shannon, Citation2001). Differences in the membrane composition and permeability, and production of chromosomally encoded antibiotic degrading enzymes, most of the time plasmid-mediated, figure as the main resistance mechanisms in NFGNB. In more recent years, however, virulence (despite the antimicrobial susceptibility) was appointed as an important factor associated with worst outcome of E. coli peritonitis (Valdes-Sotomayor et al., Citation2003). These findings indicate that comprehensive evaluation of etiological agents and antimicrobial susceptibility testing must be performed to support adequate management of peritonitis after laboratory results are available.
As a post-hoc result, we observed a decrease in gentamicin susceptibility rates in Enterobacteriaceae, which led us to investigate the impact of introduction of gentamicin prophylaxis practice (initiated in 2007). Gentamicin susceptibility was determined in 165 strains (93 up to 2006 and 72 from 2007 on). Overall, changes in the percentage of gentamicin resistant strains was observed after gentamicin prophylaxis implementation, increasing from 12.9% in the strains isolated up to 2006, to 27.8% in the strains recovered from 2007 on (p = 0.01). This change is likely due to increasing in the resistance rate of Enterobacteriaceae strains (which rises from 1.6% to 21.1%, p < 0.01, in the periods before and after gentamicin prophylaxis implementation, respectively) whilst other pathogens did not show increases in the gentamicin resistance rates (p > 0.05). Although this finding deserves further studies, fortunately, the gentamicin prophylaxis is not widely employed (Pierce, Williamson, Mauck, Russell, & Palavecino, Citation2012). Differently, McGuire and colleagues (Citation2015) reported no increase in gentamicin resistance in their center, over a 5-year period in recent years but the gentamicin prophylaxis is not reported. On a practical point of view, the present utilization of vancomycin plus the aminoglycoside amikacin as empiric treatment of PD-peritonitis in our center maybe should be revised.
Finally, this study has several strengths but also potential limitations. The extrapolation of our results is limited because we used data from a single center; a large temporal series would be able to evaluate the impact of agents on mortality and morbidity, as we have already explored in earlier studies (Barretti, Montelli, et al., Citation2009; Barretti et al., Citation2012; Barretti, Pereira, et al., Citation2009; Camargo et al., Citation2014; Cunha et al., Citation2004, Citation2005; Oliveira et al., Citation2012). The strengths of this study, on the other hand, include the consistency in the presence of the same clinical staff in peritonitis diagnosis and treatments; highly meticulous laboratory work in strain maintenance; antimicrobial susceptibility testing done by the same laboratorial staff, which, for 20 years, provided quantitative results related to etiologic agents involved in PD-related peritonitis.
5. Conclusion
In summary, our results show that PD-peritonitis frequency declined over years; gram-positive infection proportion has dropped off and gram-negative pathogens proportion arise. Changes in the etiological agents are likely related to changes in PD-practices. Longitudinal antimicrobial susceptibility testing ensures that current empirical therapy covers the majority of prevalent agents of PD-related peritonitis in this center, although detection of gentamicin-resistant Enterobacteriaceae strains mainly after introduction of topic gentamicin prophylaxis alerts for the occurrence of aminoglycoside resistance. This study also supports the current recommendations regarding retrospective evaluation of antimicrobial susceptibility in order to define the optimal empiric therapy in each center (Barretti, Doles, Pinotti, & El Dib, Citation2014; Li et al., Citation2010; Piraino et al., Citation2005).
Acknowledgments
The authors are thankful to staff of the Clinical Laboratory Division and of Dialysis Unit of the Botucatu Medical School for their routine procedures. Authors thank Dra. Erica Chimara for the manuscript revision and suggestions.
Additional information
Funding
Notes on contributors
Carlos H. Camargo
After the introduction of peritoneal dialysis in Botucatu Medical School (BMS) on 1990s, a great number of patients presented peritonitis. In 1993, a multidisciplinary team of medicine doctors from BMS and biologists from Botucatu Biosciences Institute, UNESP, was structured to investigate the clinical, epidemiological and microbiological aspects of peritonitis and to reduce the infection rates in our center, composed by clinical nephrologists (Pasqual Barretti, Jacqueline Caramori), clinical pathologists (Augusto Montelli, Alessandro Mondelli), microbiologists (Terue Sadatsune, Maria Cunha) and molecular microbiologist (Carlos Camargo). The ongoing work of the group over the last 20 years generated numerous national and international publications and academic thesis about peritonitis, with microbiological investigations in all of them. In this paper we present a synthesis of all microbiological information obtained from 400 bacterial strains studied from 1993 to 2013, including identification and antimicrobial susceptibility. The use of our results for improving patient care is suggested.
References
- Barretti, P., Doles, J. V. P., Pinotti, D. G., & El Dib, R. (2014). Efficacy of antibiotic therapy for peritoneal dialysis-associated peritonitis: A proportional meta-analysis. BMC Infectious Diseases, 14, 445. doi:10.1186/1471-2334-14-445
- Barretti, P., Montelli, A. C., Batalha, J. E., Caramori, J. C., & Maria de Lourdes, R. S. (2009). The role of virulence factors in the outcome of staphylococcal peritonitis in CAPD patients. BMC Infectious Diseases, 9, 297. doi:10.1186/1471-2334-9-212
- Barretti, P., Moraes, T. M., Camargo, C. H., Caramori, J. C., Mondelli, A. L., Montelli, A. C., & Maria de Lourdes, R. S. (2012). Peritoneal dialysis-related peritonitis due to staphylococcus aureus: A single-center experience over 15 years. PLoS ONE, 7, e31780. doi:10.1371/journal.pone.0031780
- Barretti, P., Pereira, D., Brasil, M. A., de Lourdes Cunha, M., Caramori, J., & Montelli, A. C. (2009). Evolution of gram-negative bacilli susceptibility in peritoneal dialysis-related peritonitis in Brazil: A single center’s experience over nine years. Peritoneal Dialysis International, 29, 230–233.
- Borràs, M. (2009). Antibiotic resistance in gram-negative peritonitis. Peritoneal Dialysis International, 29, 274–276.
- Camargo, C. H., da Cunha, M. D. L. R. D. S., Caramori, J. C., Mondelli, A. L., Montelli, A. C., & Barretti, P. (2014). Peritoneal dialysis-related peritonitis due to coagulase-negative Staphylococcus: A review of 115 cases in a Brazilian center. Clinical Journal of the American Society of Nephrology, 9, 1074–1081. doi:10.2215/CJN.09280913
- Clinical and Laboratory Standards Institute. (2015). Performance standards for antimicrobial susceptibility testing (M100–S25). Wayne, PA: Author.
- Cookson, B. D. (1998). The emergence of mupirocin resistance: A challenge to infection control and antibiotic prescribing practice. Journal of Antimicrobial Chemotherapy, 41, 11–18.10.1093/jac/41.1.11
- Cunha, M. L. R. S., Caramori, J. C., Fioravante, A. M., Batalha, J. E., Montelli, A. C., & Barretti, P. (2004). Significance of slime as virulence factor in coagulase-negative staphylococcus peritonitis in CAPD. Peritoneal Dialysis International, 24, 191–193.
- Cunha, M. L. R. S., Montelli, A. C., Fioravante, A. M., Batalha, J. E. N., Caramori, J. C. T., & Barretti, P. (2005). Predictive factors of outcome following staphylococcal peritonitis in continuous ambulatory peritoneal dialysis. Clinical Nephrology, 64, 378–382.10.5414/CNP64378
- Fahim, M., Hawley, C. M., McDonald, S. P., Brown, F. G., Rosman, J. B., Wiggins, K. J., ... Johnson, W. D. (2010). Coagulase-negative staphylococcal peritonitis in Australian peritoneal dialysis patients: Predictors, treatment and outcomes in 936 cases. Nephrology Dialysis Transplantation, 25, 3386–3392. doi:10.1093/ndt/gfq222
- Feng, X., Yang, X., Yi, C., Guo, Q., Mao, H., Jiang, Z., ... Yu, X. (2014). Escherichia coli peritonitis in peritoneal dialysis: The prevalence, antibiotic resistance and clinical outcomes in a South China dialysis center. Peritoneal Dialysis International, 34, 308–316. doi:10.3747/pdi.2013.00012
- Huang, S. T., Chuang, Y. W., Cheng, C. H., Wu, M. J., Chen, C. H, Wu, T. M., & Shu, K. H. (2011). Evolution of microbiological trends and treatment outcomes in peritoneal dialysis-related peritonitis. Clinical Nephrology, 75, 416–425.
- Kim, D. K., Yoo, T. H., Ryu, D. R., Xu, Z. G., Kim, H. J., Choi, K. H., … Kang, S. W. (2004). Changes in causative organisms and their antimicrobial susceptibilities in CAPD peritonitis: A single center’s experience over one decade. Peritoneal Dialysis International, 24, 424–432.
- Kitterer, D., Latus, J., Pöhlmann, C., Alscher, M. D., Kimmel, M., & Al-Ahmad, A. (2015). Microbiological surveillance of peritoneal dialysis associated peritonitis: Antimicrobial susceptibility profiles of a referral center in Germany over 32 years. PLOS ONE, 10, e0135969. doi:10.1371/journal.pone.0135969
- Koneman, E. W., & Winn, W. C. (2006). Koneman’s color atlas and textbook of diagnostic microbiology (6th ed.). Philadelphia, PA: Lippincott Williams & Wilkins.
- Li, P. K., Szeto, C. C., Piraino, B., Bernardini, J., Figueiredo, A. E., Gupta, A., ... Struijk, D. G. (2010). Peritoneal dialysis-related infections recommendations: 2010 update. Peritoneal Dialysis International, 30, 393–423. doi:10.3747/pdi.2010.00049
- Livermore, D. M., Winstanley, T. G., & Shannon, K. P. (2001). Interpretative reading: Recognizing the unusual and inferring resistance mechanisms from resistance phenotypes. Journal of Antimicrobial Chemotherapy, 48, 87–102.10.1093/jac/48.suppl_1.87
- Martins, A., & Cunha, M. (2007). Methicillin resistance in Staphylococcus aureus and coagulase-negative staphylococci: Epidemiological and molecular aspects. Microbiology and Immunology, 51, 787–795. doi: JST.JSTAGE/mandi/51.787
- McGuire, A. L., Carson, C. F., Inglis, T. J. J., & Chakera, A. (2015). Effects of a Statewide Protocol for the Management of Peritoneal Dialysis-Related Peritonitis on Microbial Profiles and Antimicrobial Susceptibilities: A Retrospective Five-Year Review. Peritoneal Dialysis International, 35, 722–728. doi:10.3747/pdi.2014.00117
- Nieto-Ríos, J. F., Díaz-Betancur, J. S., Arbeláez-Gómez, M., García-García, A., Rodelo-Ceballos, J., Reino-Buelvas, A., ... Henao-Sierra, J. E. (2014). Peritoneal dialysis-related peritonitis: Twenty-seven years of experience in a Colombian medical center. Nefrologia, 34, 88–95. doi:10.3265/Nefrologia.pre2013.Nov.12002
- Nishina, M., Yanagi, H., Kakuta, T., Endoh, M., Fukagawa, M., & Takagi, A. (2014). A 10-year retrospective cohort study on the risk factors for peritoneal dialysis-related peritonitis: A single-center study at Tokai University hospital. Clinical and Experimental Nephrology, 18, 649–654. doi:10.1007/s10157-013-0872-y
- Oliveira, L. G., Luengo, J., Caramori, J. C., Montelli, A. C., Maria de Lourdes, R. S., & Barretti, P. (2012). Peritonitis in recent years: Clinical findings and predictors of treatment response of 170 episodes at a single Brazilian center. International Urology and Nephrology, 44, 1529–1537. doi:10.1007/s11255-011-0107-7
- Pérez Fontan, M., Rodríguez-Carmona, A., García-Naveiro, R., Rosales, M., Villaverde, P., & Valdés, F. (2005). Peritonitis-related mortality in patients undergoing chronic peritoneal dialysis. Peritoneal Dialysis International, 25, 274–284.
- Pierce, D. A., Williamson, J. C., Mauck, V. S., Russell, G. B., Palavecino, E., & Burkart, J. M. (2012). The effect on peritoneal dialysis pathogens of changing topical antibiotic prophylaxis. Peritoneal Dialysis International, 32, 525–530. doi:10.3747/pdi.2011.00183
- Piraino, B., Bailie, G. R., Bernardini, J., Boeschoten, E., Gupta, A., Holmes, C., … ISPD Ad Hoc Advisory Committee. (2005). Peritoneal dialysis-related infections recommendations: 2005 update. Peritoneal Dialysis International, 25, 107–131.
- Prasad, K. N., Singh, K., Rizwan, A., Mishra, P., Tiwari, D., Prasad, N., & Gupta, A. (2014). Microbiology and outcomes of peritonitis in Northern India. Peritoneal Dialysis International, 34, 188–194. doi:10.3747/pdi.2012.00233
- Siva, B., Hawley, C. M., McDonald, S. P., Brown, F. G., Rosman, J. B., Wiggins, K. J., ... Johnson, D. W. (2009). Pseudomonas peritonitis in Australia: Predictors, treatment, and outcomes in 191 cases. Clinical Journal of the American Society of Nephrology, 4, 957–964. doi:10.2215/CJN.00010109
- Srinivasan, A., Dick, J. D., & Perl, T. M. (2002). Vancomycin resistance in staphylococci. Clinical Microbiology Reviews, 15, 430–438.10.1128/CMR.15.3.430-438.2002
- Szeto, C. C. (2014). Peritonitis rates of the past thirty years: From improvement to stagnation. Peritoneal Dialysis International, 34, 151–153. doi:10.3747/pdi.2014.00007
- Szeto, C. C., Kwan, B. C., Chow, K. M., Lau, M. F., Law, C. M., Chung, K. Y., ... Li, P. K. T. (2008). Coagulase negative staphylococcal peritonitis in peritoneal dialysis patients: Review of 232 consecutive cases. Clinical Journal of the American Society of Nephrology, 3, 91–97. doi:10.2215/CJN.03070707
- Szeto, C. C., Leung, C. B., Chow, K. M., Kwan, B. S.-H., Law, C. M., Wang, A. Y.-M., ... Li, P. K.-T. (2005). Change in bacterial aetiology of peritoneal dialysis-related peritonitis over 10 years: Experience from a centre in South-East Asia. Clinical Microbiology and Infection, 11, 837–839. doi:10.1111/j.1469-0691.2005.01222.x
- Tokgoz, B. (2009). Clinical advantages of peritoneal dialysis. Peritoneal Dialysis International, 29, S59–S61.
- Valdes-Sotomayor, J., Cirugeda, A., Bajo, M. A., del Peso, G., Escudero, E., Bajo, M. A., … Grupo de Estudios Peritoneales de Madrid. (2003). Increased severity of Escherichia coli peritonitis in peritoneal dialysis patients independent of changes in in vitro antimicrobial susceptibility testing. Peritoneal Dialysis International, 23, 450–455.
- van Esch, S., Krediet, R. T., & Struijk, D. G. (2014). 32 years’ experience of peritoneal dialysis-related peritonitis in a university hospital. Peritoneal Dialysis International, 34, 162–170. doi:10.3747/pdi.2013.00275
- Xu, G., Tu, W., & Xu, C. (2010). Mupirocin for preventing exit-site infection and peritonitis in patients undergoing peritoneal dialysis. Nephrology Dialysis Transplantation, 25), 587–592. doi:10.1093/ndt/gfp411
- Zhang, Y., Agidi, S., & LeJeune, J. T. (2009). Diversity of staphylococcal cassette chromosome in coagulase-negative staphylococci from animal sources. Journal of Applied Microbiology, 107, 1375–1383. doi:10.1111/j.1365-2672.2009.04322.x
- Zhang, W., Wu, Y. G., Qi, X. M., Dai, H., Lu, W., & Zhao, M. (2014). Peritoneal dialysis-related peritonitis with acinetobacter baumannii: A review of seven cases. Peritoneal Dialysis International, 34, 317–321. doi:10.3747/pdi.2012.00198
- Zong, Z., Peng, C., & Lü, X. (2011). Diversity of SCCmec elements in methicillin-resistant coagulase-negative staphylococci clinical isolates. PLoS ONE, 6, e20191. doi:10.1371/journal.pone.0020191