Abstract
Bronchopulmonary dysplasia (BPD) is an emerging risk factor for chronic obstructive pulmonary disease. For BPD survivors, there are no guidelines for the management of lung disease that is often misdiagnosed as asthma. Pulmonary magnetic resonsance imaging (MRI) provides clinically-relevant lung biomarkers of ventilation abnormalities and emphysema. Here our objective was to quantify lung MRI biomarkers in adults with BPD to understand the underlying pathophysiologies responsible for their symptoms and abnormal pulmonary-function. We hypothesized that MRI measurements would be abnormal and reflect emphysema, not airways disease. Patients aged 20–29 year and born ≤32 weeks gestational age were included and those with MRI contraindications were excluded. A 25-year-old female never-smoker born <28 weeks gestation (S1) and a 27-year-old male ex-smoker born ~30 weeks gestation (S2) provided written-informed-consent and underwent pulmonary-function-tests and MRI. Lung abnormalities were quantified using ventilation defect percent (VDP), apparent diffusion coefficients (ADC) and mean linear intercept (Lm). Forced expiratory volume-in 1 sec (S1 = 46%pred/S2 = 33%pred), residual-volume (S1 = 192%pred/S2 = 267%pred) and diffusing-capacity-of-the-lung-for-carbon-monoxide (S1 = 73%pred/S2 = 72%pred) were abnormal. Chest–X-ray and computed tomography (CT) revealed mild structural abnormalities, while MRI VDP (S1 = 6%/S2 = 10%), ADC (S1 = 0.36 cm2/s/S2 = 0.37 cm2/s) and Lm (S1 = 400 μm/S2 = 430 μm) were markedly abnormal with ventilation defects spatially concordant with regions of low MRI signal-intensity and greater Lm, reflecting emphysema and/or gas-trapping. In BPD survivors, MRI biomarkers have the potential to serve as intermediate endpoints and help evaluate therapy.
Public Interest Statement
Bronchopulmonary dysplasia (BPD) is the most common chronic lung disease in infants born extremely premature and is an emerging risk factor for chronic obstructive pulmonary disease (COPD). Adult survivors of premature birth have abnormally low lung function and are more likely to be prescribed asthma medication but unfortunately, there are no guidelines for their long-term follow-up. Pulmonary imaging methods have the potential to measure underlying lung pathophysiologies in these patients with the potential to guide therapy decisions. In this article, we describe the use of radiation-free pulmonary magnetic resonance imaging (MRI) methods to measure lung structure-function biomarkers in young adult survivors of bronchopulmonary dysplasia. We observed that such MRI-derived lung structure-function abnormalities were consistent with emphysema and chronic obstructive pulmonary disease. The development of radiation-free lung imaging measurements of COPD in survivors of BPD is especially important in young adults in whom lifelong treatment and monitoring is required.
Competing Interest
The authors declare no competing interests.
1. Introduction
Prematurity of birth (<37 weeks gestation) currently represents ten percent of all births worldwide translating to approximately 15 million preterm births a year (Behrman & Butler, Citation2007; Blencowe, Cousens et al., Citation2013; Howson, Kinney, McDougall, & Lawn, Citation2013). Direct complications of preterm birth account for one million deaths each year and many of the remaining surviving babies face a lifetime of significant disability (Blencowe, Lee et al., Citation2013). It is estimated that 3% of these survivors have moderate to severe neurodevelopmental impairment and a further 4% have mild neurodevelopmental impairment (Blencowe, Lee et al., Citation2013). Another complication associated with extreme prematurity is bronchopulmonary dysplasia (BPD) which is the most common chronic lung disease in preterm-birth infants. It is commonly diagnosed in infants born 24–28 weeks gestation in whom alveolarization may have been incomplete and for whom oxygen therapy, mechanical-ventilation, and/or surfactant augmentation (Jobe & Bancalari, Citation2001) may have been required. It is estimated that infants born at 23–24 weeks have a 17–30% chance of survival and survivors have more than 75% chance of developing BPD, whereas infants born at 25 weeks have a 50% chance of survival with a 57% chance of developing BPD (Kim et al., Citation2016; Kong et al., Citation2016; Manuck et al., Citation2016). In addition to developing BPD, these infants are also at risk for developing cerebral palsy and poor neuro-functional outcomes (Halliday, Ehrenkranz, & Doyle, Citation2003; Jefferies, Citation2012). Taken together, infants with BPD are at greater risk of exhibiting abnormal neurodevelopment, however, it is unclear to what extent neurodevelopmental defects influence the severity and/or appearance of BPD. Recent advances in neonatal care have increased survival rates for preterm infants born at very early gestational ages (Isayama et al., Citation2012), and a number of these patients are now entering young adulthood with pulmonary function test results consistent with chronic obstructive pulmonary disease (COPD); in this regard, extreme prematurity of birth is now considered an emerging risk factor for the development of COPD (Boucherat, Morissette, Provencher, Bonnet, & Maltais, Citation2016; de Marco et al., Citation2004) in young adults.
Studies in infants with BPD have consistently revealed abnormalities in the peripheral lung (Husain, Siddiqui, & Stocker, Citation1998), including failed alveolarization and a decreased number of alveoli that are abnormally enlarged and with simplistic and highly abnormal architecture (Jobe & Bancalari, Citation2001). Several studies have shown that adult survivors of preterm birth present with persistent and abnormally diminished lung function (Gough et al., Citation2014; Saigal & Doyle, Citation2008), reduced exercise capacity (Vrijlandt, Gerritsen, Boezen, Grevink, & Duiverman, Citation2006) and they are more likely to be prescribed bronchodilators and inhaled corticosteroids compared to controls born full-term (Crump, Winkleby, Sundquist, & Sundquist, Citation2011; Saigal et al., Citation2007). These patients are often diagnosed with asthma, although the underlying mechanisms for their apparent airways disease is unlikely due to allergy, inflammation or airway remodeling or hyperresponsiveness (Baraldi, Bonetto, Zacchello, & Filippone, Citation2005; Fawke et al., Citation2010; Priante et al., Citation2016). Unfortunately, there are no guidelines for the long-term follow-up of adult survivors of BPD, but biomarkers derived from a number of methods including thoracic imaging have the potential to guide treatment with the goal of improving symptoms and outcomes.
In this regard, BPD was first identified and diagnosed based on chest X-ray (Northway, Rosan, & Porter, Citation1967). Although chest radiograph is routinely used for acute evaluation, the technique’s sensitivity is limited because patients with significant respiratory dysfunction may exhibit only minor radiographic abnormalities (Oppenheim et al., Citation1994). X-ray computed tomography (CT) has been previously used (Howling et al., Citation2000) to evaluate BPD in infants but the radiation burden stemming from CT (Brenner, Elliston, Hall, & Berdon, Citation2001) renders longitudinal or serial evaluations unrealistic, especially in such young patients who are especially vulnerable to damage from ionizing radiation. Using radiation-free pulmonary magnetic resonance imaging (MRI) methods, abnormal regions of low-signal intensity were previously reported in infants with BPD (Adams et al., Citation2002; Hahn et al., Citation2016; Walkup et al., Citation2015), providing similar information as that of CT (Hahn et al., Citation2016; Walkup et al., Citation2015). In children, inhaled 3He gas diffusion-weighted MRI also revealed focal areas of increased microstructure (Altes et al., Citation2006). However, the clinical and physiological meaning of these MRI abnormalities is not well-understood and to our knowledge, there have been no prospective MRI biomarker examinations in symptomatic adult BPD patients. Therefore, the objective of this proof-of-concept investigation was to acquire and quantify non-contrast enhanced and inhaled hyperpolarized noble gas MRI biomarkers (Table ) of chronic lung disease in young adult survivors of BPD. We hypothesized that such measurements would reveal novel and complementary information not available using pulmonary function tests, chest X-ray or CT and would help generate a better understanding of underlying pathophysiology related to symptoms which may help guide treatment options.
Table 1. List of pulmonary MRI biomarkers
2. Methods
2.1. Study logistics
Participants provided written informed consent to a study protocol (NCT02723513; https://clinicaltrials.gov/ct2/show/NCT02723513) approved by a local research ethics board and compliant with the Health Insurance Portability and Accountability Act (HIPAA, USA). The patient inclusion criteria were: (1) age between 20 and 29 years and, (2) pre-term birth (<32 weeks gestational age), with or without a physician diagnosis of BPD. Exclusion criteria included: (1) contraindications to MRI (i.e. metal/electronic/magnetic implants, claustrophobia, etc.), and (2) serious co-morbidities. While pulmonary function tests, hyperpolarized noble gas MRI, and UTE MRI were performed pre- and post-salbutamol, CT was performed once within 30 min of post-salbutamol MRI. Clinical history was first determined by interview and validated using the patient electronic health record (PowerChart, Cerner Canada Ltd., Markham, Canada).
Forced expiratory volume in one second (FEV1), forced vital capacity (FVC), residual volume (RV), functional residual capacity (FRC) and total lung capacity (TLC) were measured according to American Thoracic Society (ATS) guidelines (MedGraphics Elite Series Plethysmograph, St. Paul, MN) (Miller et al., Citation2005). The diffusing capacity of the lung for carbon monoxide (DLCO) was measured using the attached gas analyser and the St. George’s Respiratory Questionnaire (SGRQ) was used to measure quality of life on a scale of 0–100 (where 0 was the best possible score).
2.2. Image acquisition
MRI was performed at 3T (MR750 Discovery, GEHC, Milwaukee, WI) using a whole-body gradient set with maximum gradient amplitude of 4.8G/cm and single-channel, rigid elliptical transmit/receive chest coil (RAPID Biomedical GmbH, Wuerzburg, Germany). 1H and 3He static ventilation images were acquired as previously described (Kirby et al., Citation2012). In a single breath-hold, a multi-slice 2D gradient echo diffusion-weighted sequence was used to generate multi-b value morphometry data (Paulin et al., Citation2015). Ultra-short echo time (UTE) MRI was acquired using a 32-channel torso coil (GEHC) and three-dimensional cones UTE sequence (GEHC) at full expiration and FRC + 1L. Coaching was done prior to and during imaging to achieve full expiration. FRC + 1L was achieved by the inhalation of a 1L bag of N2 from the mouth after the end of passive expiration. Whole lung UTE images were acquired in the coronal plane with the following parameters in breath-hold: 15s acquisition time, echo time (TE)/repetition time (TR)/flip angle = 0.03 ms/3.5 ms/5°, field-of-view = 40 × 40 cm, bandwidth = 125 kHz, matrix = 200 × 200, and reconstructed to a 10 mm slice thickness.
Thoracic CT was acquired post-salbutamol at inspiration under breath-hold conditions (FRC + 1L to allow for CT to MRI comparison) using a multi-detector, 64-slice Lightspeed VCT scanner (GEHC, Milwaukee, WI) (64 × 0.625 mm collimation, 120 kVp, 100 mA, tube rotation time = 500 ms, pitch = 0.98) (Kirby et al., Citation2013). A spiral acquisition was used and images were reconstructed using a Standard reconstruction algorithm and reconstructed slice thickness of 1.25 mm (Kirby et al., Citation2013).
2.3. Image analysis
Ventilation abnormalities were quantified using ventilation defect percent (VDP) (Kirby et al., Citation2012), while MRI apparent diffusion coefficients (ADC), mean linear intercept (Lm) and surface-to-volume ratio (S/V) were computed on a voxel-by-voxel basis (Paulin et al., Citation2015). UTE MR images were segmented and mean whole lung UTE signal-intensity was normalized to the mean liver signal-intensity (Ma et al., Citation2014). Pulmonary Workstation 2.0 (VIDA Diagnostics Inc., Coralville, Iowa, USA) was used to quantify the relative area of the CT density histogram with attenuation values <−950 HU (RA950). To identify potential spatial relationships between 3He MRI ventilation defects and UTE/CT measurements, 3He-UTE and 3He-CT co-registrations were performed using 3D Slicer registration software (http://www.slicer.org).
2.4. Statistics
Data were tested for normality using the Shapiro–Wilk normality test using SPSS 23.0 (IBM, Armonk, NY, USA). Non-parametric tests were performed because the data did not satisfy a normal distribution. A non-parametric repeated measures ANOVA (the Friedman test) was performed to evaluate the difference between baseline and post-salbutamol measurements. All statistical tests were performed using SPSS 23.0 (IBM, Armonk, NY, USA). Results were considered significant when the probability of making a Type I error was less than 5% (p < 0.05).
3. Results
We evaluated a 25 year old female (Subject S1) (height = 172 cm, weight = 58 kg) never-smoker who was referred to adult pulmonary specialist care based on a previous clinical diagnosis of BPD and respiratory symptoms; at the time of this study, medication included twice-daily combination inhaled budesonide and formoterol (100/6 mcg). She was born 27 weeks gestation with birth-weight of 1 lbs 14 oz and was mechanically-ventilated in a quaternary care neonatal intensive care unit for six weeks without surfactant therapy. She had been hospitalized on a single occasion at two years of age for acute pneumonia but otherwise had no history of exacerbations or evidence of pulmonary hypertension or clubbing. In addition, a 27 year old male (Subject S2) (height = 169 cm, weight = 80 kg) was evaluated after referral to adult pulmonary specialist care based on a clinical diagnosis of asthma and respiratory symptoms; at the time of this study, medication included twice-daily fluticasone (250 mcg) and once-daily of combination inhaled tiotropium and olodaterol (2.5/2.5 mcg). He reported a <1 pack-year smoking history and was a social smoker for 5 years having quit 2 years prior to the study visit. Acute dyspnea with wheeze and cough was reported to have initiated one year prior to the MRI evaluation. He was born 30 weeks gestation with birth-weight of 1 lbs 2 oz and administered oxygen (but not mechanically ventilated) in a quaternary care neonatal intensive care unit for 12 weeks before discharge.
As shown in Table , for Subject S1, SGRQ symptom-score and activity-score = 11, impact-score = 6, and total-score = 8 and indicative of mildly abnormal health status but close to normal ranges (Ferrer et al., Citation2002). Table also shows that there were no post-salbutamol improvements in FEV1 (46%pred/49%pred), FEV1/FVC (46%/48%), FRC (135%pred/129%pred), RV/TLC (47%/43%) or DLCO (73%pred/74%pred). For Subject S2, SGRQ symptom-score = 26, activity-score = 59, impact-score = 26, and total-score = 36 which were all indicative of abnormal health status and similar to scores observed in COPD and asthma patients (Ferrer et al., Citation2002). Similar to Subject S1, there were no post-salbutamol improvements in FEV1 (33%pred/36%pred), FEV1/FVC (36%/39%), FRC (163%pred/166%pred), RV/TLC (49%/43%) or DLCO (72%pred/76%pred) for Subject S2. For both subjects, the mean pre- and post-salbutamol pulmonary function test measurements (pre-/post-FEV1 = 40 ± 9%pred/43 ± 9%pred, p = 0.2; pre-/post-FEV1/FVC = 41 ± 7%/44 ± 6%, p = 0.2; pre-/post-FRC = 149 ± 20%pred/148 ± 26%pred, p = 0.7; pre-/post-RV/TLC = 48 ± 1%/43 ± 1%, p = 0.2; pre-/post-DLCO = 73 ± 1%pred/75 ± 1%pred, p = 0.2) were not significantly different (p > 0.05).
Table 2. Clinical, functional, and imaging measurements pre- and post-salbutamol
Figure shows the posterior-anterior (PA) and lateral chest X-ray for Subject S1 (who declined consent for CT imaging) and CT for Subject S2. For Subject S1, the chest X-ray revealed no obvious bullae or signs of emphysema but there was evidence of mild hyperinflation. For Subject S2, chest CT revealed mild centrilobular emphysema especially in the upper lobes with destructive changes in the apices but with no pleural or pericardial effusion or pneumothorax. Quantitative measurements of CT emphysema (RA950 = 16%) were consistent with moderate emphysematous destruction.
Figure 1. Chest X-ray and CT in adult survivors of bronchopulmonary dysplasia.
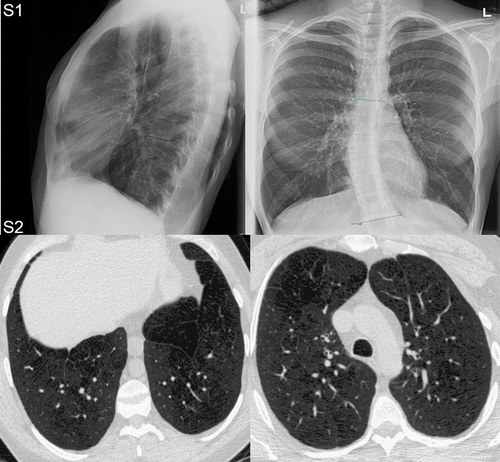
Figure shows post-salbutamol UTE (at full expiration and FRC + 1L) and 3He MRI for both patients in whom there were no obvious post-salbutamol improvements in ventilation, morphometry or UTE signal-intensity. For both subjects, the mean pre- and post-salbutamol imaging measurements (pre-/post-VDP = 8 ± 3%/9 ± 5%, p = 0.7; pre-/post-ADC = 0.37 ± 0.01 cm2/s/0.37 ± 0.07%, p ~ 1; pre-/post-Lm = 420 ± 20 μm/410 ± 10 μm, p = 0.3; pre-/post-R = 470 ± 10 μm/470 ± 10 μm, p ~ 1; pre-/post-S/V = 100 ± 0 cm−1/100 ± 0 cm−1, p ~ 1; pre-/post-signal-intensity at full expiration = 31 ± 8%/29 ± 9%, p = 0.2; pre-/post-signal-intensity at FRC + 1L = 27 ± 5%/24 ± 6%, p = 0.2) were not significantly different (p > 0.05). For Subject S1, UTE MRI at full expiration revealed regional cyst-like structures of low signal-intensity (signal-intensity = 35%) in the upper left lobe and middle and lower right lobes. There were focal ventilation defects (VDP = 5%) in the upper and lower lobes of both lungs. Abnormal ADC (ADC = 0.36 cm2/s) and Lm (Lm = 400 μm) were heterogeneously distributed throughout the lung. Figure also shows that the regions of low UTE signal-intensity and elevated ADC and Lm that were co-localized with focal ventilation defects. For Subject S2, UTE MRI at full expiration (signal-intensity = 22%) and FRC + 1L (signal-intensity = 20%) revealed no obvious cyst-like regions of low signal-intensity. Ventilation defects were heterogeneously distributed throughout the lung (VDP = 12%). Abnormal ADC (0.37 cm2/s) and Lm (420 μm) were heterogeneously distributed throughout the lung.
Figure 2. Multi-nuclear MRI in adult survivors of bronchopulmonary dysplasia.

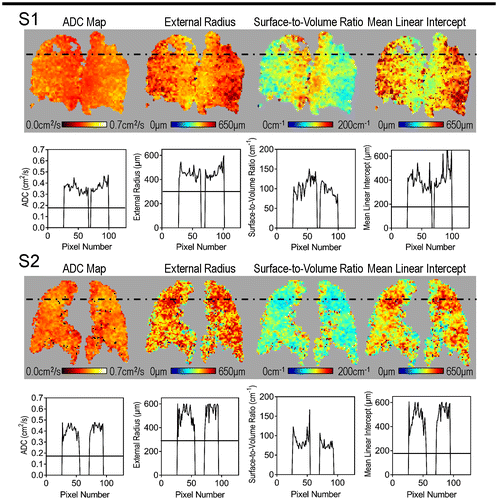
Figure shows diffusion-weighted multiple b-value 3He MRI maps for both subjects. The centre coronal ADC and Lm maps reflected abnormally large values (acinar duct enlargement) and the S/V map reflected abnormally small values, especially near focal ventilation defects for Subject S1 (as identified by the dotted black line). Mean 3He ADC = 0.36 cm2/s, Lm = 400 μm, R = 460 μm, and S/V = 100 cm−1 were abnormal and reflective of acinar duct or airspace enlargement. For Subject S2, the centre coronal ADC and Lm maps reflected abnormally large values as compared to those observed in full-term-born mid-20 year olds (Quirk et al., Citation2016). The S/V map also revealed abnormally small values distributed throughout the lung. Mean 3He ADC = 0.37 cm2/s, Lm = 420 μm, R = 480 μm, and S/V = 100 cm−1 were also highly abnormal. The solid black lines show normal values expected for an age-matched never-smoker born at full-term, as previously described (Quirk et al., Citation2016).
Figure 3. Quantitative analysis of 3He MRI cyst-like ventilation defects.
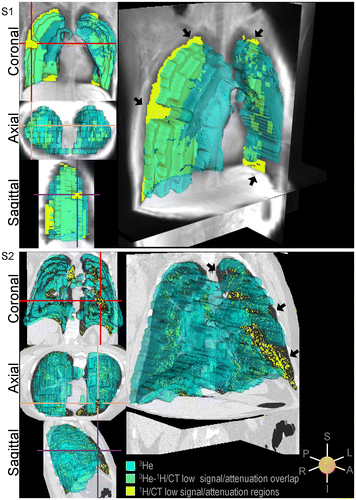
To better understand the spatial relationships between lung structural and functional measurements, Figure shows 3He ventilation images co-registered with anatomical MRI or CT images. For Subject S1, the yellow mask represents lung regions <15th percentile of the UTE signal-intensity distribution that are co-spatially localized with ventilation defects while green reflects co-spatially localized UTE low signal intensity and well-ventilated lung. For Subject S2, the yellow mask represents lung regions <−950 HU co-spatially located with ventilation defects while green reflects low CT density spatially concordant with well-ventilated lung.
Figure 4. Imaging structure-function relationships.
4. Discussion
Pulmonary MRI provides lung structural and functional biomarkers that may be acquired rapidly without radiation burden and have the potential to provide a better understanding of the underlying pathophysiologies responsible for lung symptoms and disease worsening in BPD patients. In this proof-of-concept investigation, we explored conventional and inhaled gas pulmonary MRI biomarkers of lung disease in young adult survivors of BPD and observed: (1) structural and functional pulmonary abnormalities common in COPD patients that were not reversible with salbutamol, (2) abnormally-elevated lung microstructure measurements compared to previously reported values age-matched subjects born at full-term (Quirk et al., Citation2016), and, (3) spatially correlated structural and functional abnormalities including enlarged lung microstructure, regions of low CT radiodensity/UTE signal-intensity and ventilation defects.
In both BPD patients, MRI ventilation was clearly abnormal and ventilation defects were irreversible which is consistent with COPD and not asthma. Subject S1 displayed ventilation defects that were spatially concordant with cyst-like regions of low UTE signal-intensity. These cyst-like structures have also been previously observed in infants using MRI (Adams et al., Citation2002; Hahn et al., Citation2016; Walkup et al., Citation2015) and adults using CT (Howling et al., Citation2000) and may reflect emphysema and/or gas-trapping. These abnormalities were hypothesized to be related to prolonged mechanical-ventilation (Adams et al., Citation2002) which is often injurious to immature, surfactant-deficient lungs (Dreyfuss & Saumon, Citation1998). For this patient, there was no history of surfactant treatment, nor was there documentation of the timeframe for mechanical ventilation, so we cannot be certain about the etiology of these findings. It should be noted that in addition to regions of low signal-intensity, there were cyst-like ventilation defects and elevated lung microstructure measurements spatially related to these, both of which are suggestive of emphysematous “holes” with long time constants for air filling. For Subject S2, MRI ventilation was abnormal with relatively small and numerous ventilation defects (or patchy ventilation) heterogeneously distributed throughout the lung. Similar to Subject S1, ventilation abnormalities were not reversible after the administration of salbutamol. Abnormally elevated MRI ADC and Lm along with visually obvious CT low attenuating irregular-shaped voids were also observed in Subject S2, although there was no evidence of the larger spherical, cyst-like structures observed in Subject S1. Taken together, all of these results are consistent with previous imaging findings in patients with COPD and reflective of emphysema and/or gas-trapping (van Mastrigt et al., Citation2016).
In both patients, heterogeneously distributed ADC were observed with abnormal mean ADC of 0.36 and 0.37 cm2/s for Subjects S1 and S2, respectively. The relationship between lung age and ADC in healthy participants was previously reported (Fain et al., Citation2005) and based on this previous work, ADC values for a healthy normal 25 year old are estimated at 0.18 cm2/s. Based on the previously described age-ADC curve, the lung age for both of these BPD patients was estimated at ~130 years and this significant finding may alter their medical management. A lung age of 130 years was extrapolated from the age-ADC curve and it was assumed that ADC increased with age beyond the age of 70 (Fain et al., Citation2005). Mean Lm and S/V were also abnormal compared to previous estimates for 25 year old born full-term (Quirk et al., Citation2016). These findings suggest enlarged alveoli, which may reflect ventilator-induced lung injury (Dreyfuss & Saumon, Citation1998) or abnormal alveolar development including fewer and larger alveoli (Husain et al., Citation1998).
Finally, there was visual evidence of structure-function relationships. Hyperpolarized noble MRI ventilation defects were spatially related to regions of low signal-intensity/CT radiodensity and elevated ADC and morphometry measurements. The decreased signal-intensity/CT radiodensity and elevated ADC and Lm measurements near ventilation defects may reflect alveolar enlargement. This was consistent with the pulmonary function test measurements that provided evidence of both gas-trapping and emphysema. Taken together, these results may provide a deeper understanding of the source of symptoms in young adult survivors of premature birth. Future work should aim to quantify these spatial relationships as this may provide a better understanding of the etiology of these structure-function abnormalities.
Several questions arise from our results such as: is there a relationship between ventilation defects, lung injury and arrested lung development due to premature birth? Is there a clinical role for MRI in BPD patients? There is evidence to suggest that early lung injury in preterm patients has lifetime consequences (Kotecha et al., Citation2013; Narang, Citation2010). Survivors of premature birth, who are increasing in prevalence because of modern neonatal intensive care strategies, are now entering adulthood, sometimes with previously unrecognized chronic lung disease (Priante et al., Citation2016). Understanding the mechanisms leading to altered pulmonary structure and function following extreme prematurity may help us intervene early and optimize therapy.
Although this exploratory proof-of-concept study provides promising preliminary results, we must acknowledge that this study was limited by the small sample size and therefore, caution must be taken when extrapolating these results to a larger sample size. We must also acknowledge that CT was not acquired for Subject S1 for this study, nor at any time during the patient’s childhood or young adult life. This makes comparisons and an understanding of the cyst-like structures difficult to determine. We also recognize that ADC values and morphometric parameters may be overestimated at higher magnetic fields (Parra-Robles et al., Citation2012), however, in healthy young never-smokers, ADC estimates at 3 and 1.5 T were in good agreement (Paulin et al., Citation2015). The lung age of 130 years was extrapolated from an age-ADC curve, however, it should be noted that several other factors could affect the trajectory of ADC values beyond 70 years of age. Regardless, based on the ADC data, the physiological lung age for these patients is older than their calendar age and markedly abnormal. With limited 3He access and the high cost of 3He gas there has been transition towards 129Xe gas, which is a less expensive and more readily available contrast agent. It should be noted that BPD is a relatively new disease for which there are no consensus treatment guidelines, the exploratory inclusion of MRI biomarkers into patient-based research and care is helping to build our understanding of how the disease progresses and responds to therapy. Currently, patient diagnosis, monitoring, and response to therapy are evaluated using airflow measurements that may conceal the independent contributions of underling pathologies. While relatively expensive and complex to acquire, thoracic MR imaging provides a way to regionally identify the underlying pathologies associated with BPD and offer quantitative biomarkers. As the first step to identify BPD patients with specific underlying pathologies, we think it is important to understand the physiological and clinical consequences of these MR imaging derived biomarkers. These biomarkers have the potential to serve and intermediate endpoints of outcomes (e.g. symptoms and quality of life) or serve as endpoints that might be helpful in guiding treatment decisions aimed at improving symptoms.
5. Conclusions
In this first exploration of conventional and inhaled gas MRI biomarkers in young adult survivors of BPD, we demonstrated feasibility and measured novel lung structure-function abnormalities, consistent with COPD and otherwise not readily available using conventional clinical measurements. For young adults in whom lifelong treatment and monitoring of response to COPD therapy is required, such biomarkers may play an important clinical role.
Funding
Dr Ouriadov gratefully acknowledges salary support from the Alpha-one Foundation (USA) and Dr Parraga gratefully acknowledges support from the Canadian Institutes of Health Research (CIHR) New Investigator Award program and operational funding from the CIHR-funded Canadian Respiratory Research Network.
Cover Image
Source: Courtesy Parraga laboratory, Robarts Research Institute.
Acknowledgements
We thank L. Reid-Jones for clinical coordination and clinical database management. We thank D. Reese for MRI of research volunteers and are grateful Dr Rob Peters and Dr Michael Carl for their continued support of the development of pulmonary MRI pulse sequences.
Additional information
Notes on contributors
Khadija Sheikh
Our research is focused on developing a better understanding of lung structure and function in patients with COPD and asthma using imaging tools our lab discovered and developed including pulmonary functional magnetic resonance imaging. The research in this paper describes methods we have developed to better understand lung structure and functional and chronic obstructive pulmonary disease in young adult survivors of bronchopulmonary dysplasia. Such patients have abnormally low lung function in young adulthood and symptoms of airflow obstruction. This is important because it is not clear whether such respiratory abnormalities in preterm born adults reflect non-progressive lung disease stemming from lung structural abnormalities that occurred early in life or if there is ongoing and progressive lung disease that increase the risk of COPD.
References
- Adams, E. W., Counsell, S. J., Hajnal, J. V., Cox, P. N., Kennea, N. L., Thornton, A. S., … Edwards, A. D. (2002). Magnetic resonance imaging of lung water content and distribution in term and preterm infants. American Journal of Respiratory and Critical Care Medicine, 166, 397–402.10.1164/rccm.2104116
- Altes, T., Mata, J., Froh, D., Paget-Brown, A., de Lange, E., & Mugler, J. (2006). Abnormalities of lung structure in children with bronchopulmonary dysplasia as assessed by diffusion hyperpolarized helium-3 MRI. Proceedings of the International Society for Magnetic Resonance in Medicine, 14, 86.
- Baraldi, E., Bonetto, G., Zacchello, F., & Filippone, M. (2005). Low exhaled nitric oxide in school-age children with bronchopulmonary dysplasia and airflow limitation. American Journal of Respiratory and Critical Care Medicine, 171, 68–72.10.1164/rccm.200403-298OC
- Behrman, R. E., & Butler, A. S. (Eds.). (2007). Preterm birth: Causes, consequences, and prevention. Washington, DC: National Academy of Sciences.
- Blencowe, H., Cousens, S., Chou, D., Oestergaard, M., Say, L., Moller, A. B., … Lawn, J. (2013). Born too soon: The global epidemiology of 15 million preterm births. Reproductive Health, 10(Suppl. 1), S2.10.1186/1742-4755-10-S1-S2
- Blencowe, H., Lee, A. C., Cousens, S., Bahalim, A., Narwal, R., Zhong, N., … Vos, T. (2013). Preterm birth-associated neurodevelopmental impairment estimates at regional and global levels for 2010. Pediatric Research, 74, 17–34.10.1038/pr.2013.204
- Boucherat, O., Morissette, M. C., Provencher, S., Bonnet, S., & Maltais, F. (2016). Bridging lung development with chronic obstructive pulmonary disease. Relevance of developmental pathways in chronic obstructive pulmonary disease pathogenesis. American Journal of Respiratory and Critical Care Medicine, 193, 362–375.10.1164/rccm.201508-1518PP
- Brenner, D., Elliston, C., Hall, E., & Berdon, W. (2001). Estimated risks of radiation-induced fatal cancer from pediatric CT. American Journal of Roentgenology, 176, 289–296.10.2214/ajr.176.2.1760289
- Crump, C., Winkleby, M. A., Sundquist, J., & Sundquist, K. (2011). Risk of asthma in young adults who were born preterm: A Swedish national Cohort study. Pediatrics, 127, e913–e920.10.1542/peds.2010-2603
- de Marco, R., Accordini, S., Cerveri, I., Corsico, A., Sunyer, J., Neukirch, F., … Vermeire, P. (2004). An international survey of chronic obstructive pulmonary disease in young adults according to GOLD stages. Thorax, 59, 120–125.10.1136/thorax.2003.011163
- Dreyfuss, D., & Saumon, G. (1998). Ventilator-induced Lung Injury. American Journal of Respiratory and Critical Care Medicine, 157, 294–323.10.1164/ajrccm.157.1.9604014
- Fain, S. B., Altes, T. A., Panth, S. R., Evans, M. D., Waters, B., Mugler, J. P., … Owers-Bradley, J. (2005). Detection of age-dependent changes in healthy adult lungs with diffusion-weighted 3He MRI. Academic Radiology, 12, 1385–1393.10.1016/j.acra.2005.08.005
- Fawke, J., Lum, S., Kirkby, J., Hennessy, E., Marlow, N., Rowell, V., … Stocks, J. (2010). Lung function and respiratory symptoms at 11 years in children born extremely preterm: The EPICure study. American Journal of Respiratory and Critical Care Medicine, 182, 237–245.10.1164/rccm.200912-1806OC
- Ferrer, M., Villasante, C., Alonso, J., Sobradillo, V., Gabriel, R., Vilagut, G., … Miravitlles, M. (2002). Interpretation of quality of life scores from the St George’s Respiratory Questionnaire. European Respiratory Journal, 19, 405–413.10.1183/09031936.02.00213202
- Gough, A., Linden, M., Spence, D., Patterson, C. C., Halliday, H. L., & McGarvey, L. P. (2014). Impaired lung function and health status in adult survivors of bronchopulmonary dysplasia. European Respiratory Journal, 43, 808–816.10.1183/09031936.00039513
- Hahn, A. D., Higano, N. S., Walkup, L. L., Thomen, R. P., Cao, X., Merhar, S. L., … Fain, S. B. (2016). Pulmonary MRI of neonates in the intensive care unit using 3D ultrashort echo time and a small footprint MRI system. Journal of Magnetic Resonance Imaging.
- Halliday, H., Ehrenkranz, R., & Doyle, L. (2003). Early postnatal (<96 hours) corticosteroids for preventing chronic lung disease in preterm infants. The Cochrane Library.
- Howling, S. J., Northway, W. H., Jr, Hansell, D. M., Moss, R. B., Ward, S., & Müller, N. L. (2000). Pulmonary sequelae of bronchopulmonary dysplasia survivors: High-resolution CT findings. American Journal of Roentgenology, 174, 1323–1326.10.2214/ajr.174.5.1741323
- Howson, C. P., Kinney, M. V., McDougall, L., & Lawn, J. E. (2013). Born toon soon: Preterm birth matters. Reproductive Health, 10(Suppl. 1), S1.10.1186/1742-4755-10-S1-S1
- Husain, A. N., Siddiqui, N. H., & Stocker, J. T. (1998). Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Human Pathology, 29, 710–717.10.1016/S0046-8177(98)90280-5
- Isayama, T., Lee, S. K., Mori, R., Kusuda, S., Fujimura, M., Xiang, Y. Y., & Shah, P. S. (2012). Comparison of mortality and morbidity of very low birth weight infants between Canada and Japan. Pediatrics, 130, e957–e965.10.1542/peds.2012-0336
- Jefferies, A. L. (2012). Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Paediatrics & Child Health, 17, 573.
- Jobe, A. H., & Bancalari, E. (2001). Bronchopulmonary dysplasia. American Journal of Respiratory and Critical Care Medicine, 163, 1723–1729.10.1164/ajrccm.163.7.2011060
- Kim, J. K., Chang, Y. S., Sung, S., Ahn, S. Y., Yoo, H. S., & Park, W. S. (2016). Trends in survival and incidence of bronchopulmonary dysplasia in extremely preterm infants at 23–26 weeks gestation. Journal of Korean Medical Science, 31, 423–429.10.3346/jkms.2016.31.3.423
- Kirby, M., Heydarian, M., Svenningsen, S., Wheatley, A., McCormack, D. G., Etemad-Rezai, R., & Parraga, G. (2012). Hyperpolarized 3He magnetic resonance functional imaging semiautomated segmentation. Academic Radiology, 19, 141–152.10.1016/j.acra.2011.10.007
- Kirby, M., Owrangi, A., Svenningsen, S., Wheatley, A., Coxson, H. O., Paterson, N. A., … Parraga, G. (2013). On the role of abnormal DLCO in ex-smokers without airflow limitation: Symptoms, exercise capacity and hyperpolarised helium-3 MRI. Thorax, 68, 752–759.10.1136/thoraxjnl-2012-203108
- Kong, X., Xu, F., Wu, R., Wu, H., Ju, R., Zhao, X., … Xu, P. (2016). Neonatal mortality and morbidity among infants between 24 to 31 complete weeks: A multicenter survey in China from 2013 to 2014. BMC Pediatrics, 16, 2162.10.1186/s12887-016-0716-5
- Kotecha, S. J., Edwards, M. O., Watkins, W. J., Henderson, A. J., Paranjothy, S., Dunstan, F. D., & Kotecha, S. (2013). Effect of preterm birth on later FEV1: A systematic review and meta-analysis. Thorax, 68, 760–766.10.1136/thoraxjnl-2012-203079
- Ma, W., Sheikh, K., Svenningsen, S., Pike, D., Guo, F., Etemad‐Rezai, R., … Parraga, G. (2014). Ultra-short echo-time pulmonary MRI: Evaluation and reproducibility in COPD subjects with and without bronchiectasis. Journal of magnetic resonance imaging: JMRI.
- Manuck, T. A., Rice, M. M., Bailit, J. L., Grobman, W. A., Reddy, U. M., Wapner, R. J., … Saade, G. R. (2016). Preterm neonatal morbidity and mortality by gestational age: A contemporary cohort. American journal of obstetrics and gynecology.
- Miller, M. R., Hankinson, J., Brusasco, V., Burgos, F., Casaburi, R., Coates, A., & Wanger, J. (2005). Standardisation of spirometry. European Respiratory Journal, 26, 319–338.10.1183/09031936.05.00034805
- Narang, I. (2010). Review series: What goes around, comes around: childhood influences on later lung health?: Long-term follow-up of infants with lung disease of prematurity. Chronic Respiratory Disease, 7, 259–269.10.1177/1479972310375454
- Northway Jr., W. H., Rosan, R. C., & Porter, D. Y. (1967). Pulmonary disease following respirator therapy of hyaline-membrane disease: Bronchopulmonary dysplasia. New England Journal of Medicine, 276, 357–368.10.1056/NEJM196702162760701
- Oppenheim, C., Mamou-Mani, T., Sayegh, N., de Blic, J., Scheinmann, P., & Lallemand, D. (1994). Bronchopulmonary dysplasia: Value of CT in identifying pulmonary sequelae. American Journal of Roentgenology, 163, 169–172.10.2214/ajr.163.1.8010206
- Parra-Robles, J., Ajraoui, S., Marshall, H., Deppe, M. H., Xu, X., & Wild, J. M. (2012). The influence of field strength on the apparent diffusion coefficient of 3He gas in human lungs. Magnetic Resonance in Medicine, 67, 322–325.10.1002/mrm.23187
- Paulin, G. A., Ouriadov, A., Lessard, E., Sheikh, K., McCormack, D. G., & Parraga, G. (2015). Noninvasive quantification of alveolar morphometry in elderly never- and ex-smokers. Physiological reports, 3(10).
- Priante, E., Moschino, L., Mardegan, V., Manzoni, P., Salvadori, S., & Baraldi, E. (2016). Respiratory outcome after preterm birth: A long and difficult journey. American Journal of Perinatology, 33, 1040–1042.10.1055/s-0036-1586172
- Quirk, J. D., Sukstanskii, A. L., Woods, J. C., Lutey, B. A., Conradi, M. S., Gierada, D. S., … Yablonskiy, D. A. (2016). Experimental evidence of age-related adaptive changes in human acinar airways. Journal of Applied Physiology, 120, 159–165.10.1152/japplphysiol.00541.2015
- Saigal, S., & Doyle, L. W. (2008). An overview of mortality and sequelae of preterm birth from infancy to adulthood. The Lancet, 371, 261–269.10.1016/S0140-6736(08)60136-1
- Saigal, S., Stoskopf, B., Boyle, M., Paneth, N., Pinelli, J., Streiner, D., & Goddeeris, J. (2007). Comparison of current health, functional limitations, and health care use of young adults who were born with extremely low birth weight and normal birth weight. Pediatrics, 119, e562–e573.10.1542/peds.2006-2328
- van Mastrigt, E., Logie, K., Ciet, P., Reiss, I. K., Duijts, L., Pijnenburg, M. W., & Tiddens, H. A. (2016). Lung CT imaging in patients with bronchopulmonary dysplasia: A systematic review. Pediatric Pulmonology, 51, 975–986.10.1002/ppul.23446
- Vrijlandt, E. J., Gerritsen, J., Boezen, H. M., Grevink, R. G., & Duiverman, E. J. (2006). Lung function and exercise capacity in young adults born prematurely. American Journal of Respiratory and Critical Care Medicine, 173, 890–896.10.1164/rccm.200507-1140OC
- Walkup, L. L., Tkach, J. A., Higano, N. S., Thomen, R. P., Fain, S. B., Merhar, S. L., … Woods, J. C. (2015). Quantitative magnetic resonance imaging of bronchopulmonary dysplasia in the neonatal intensive care unit environment. American Journal of Respiratory and Critical Care Medicine, 192, 1215–1222.10.1164/rccm.201503-0552OC
