Abstract
Cetuximab-induced interstitial lung disease (ILD), especially for tumors in the head and neck area, has rarely been reported. The present case may serve as an important reference to alert clinicians who use this chemotherapeutic agent. We report a case of acute ILD in a 66-year-old male with double cancers (oral floor cancer T4aN0M0 and supraglottic cancer T2N0M0), who received concurrent cetuximab and radiation therapy. He had severe dyspnea on day 51 along with worsened bilateral, progressive infiltration in chest X-ray, elevated C-reactive protein, Krebs von den Lungen 6 (KL-6), Surfactant Protein D (SP-D) and Surfactant Protein A (SP-A), and was diagnosed with ILD. He was treated with High-dose methylprednisone (1 mg/kg) soon after the diagnosis was made. He recovered remarkably and was discharged at day 100. For early detection of ILD, we suggest weekly chest X-rays for cetuximab-treated patients. In suspicious cases, High-resolution computed tomography scan and measurements of KL-6, SP-A, and SP-D levels should be considered.
Public Interest statement
The treatment with cis-diamminedichloroplatinum has been the first choice for the patients with head and neck squamous cell carcinoma (HNSCC). Although, the complication such as severe radiation induced dermatitis, mucositis or renal damage has lead us to seek for alternative anti-cancer agent. Cetuximab has been introduced as “safer” alternative to cis-diamminedichloroplatinum, although we have experienced several severe complications including interstitial lung disease. Interstitial lung disease is not a very common disease that otolaryngologist confront with in daily practice, but it could be lethal without a quick diagnose and prompt treatment, thus we believe it’s very important to acknowledge this complication and to try to detect the early signs during the treatment. We present a case we were successful in diagnosing and treating the patient with interstitial lung disease in the course of HNSCC treatment with cetuximab.
Competing Interests
The content is solely the responsibility of the authors and does not necessarily represent the official views of Jichi Medical University.
1. Introduction
Interstitial lung disease (ILD) is a severe and potentially fatal adverse event. ILD is an umbrella term for a group of disorders that have similar clinical or physiologic manifestations. Specific forms of ILD can be differentiated from one another by combination of clinical data, radiologic imaging, and if needed, pathologic findings by performing Bronchoalveolar Lavage (BAL) if possible. As ILD can give rise to a broad range of pathological patterns of lung disease, the corresponding imaging patterns vary and are nonspecific. Biomarkers play a supplementary role in the diagnosis of ILD. Applicable blood tests for suspected ILD include Krebs von der Lungen-6 (KL-6), pulmonary surfactant protein-A (SP-A) and pulmonary surfactant protein-D (SP-D). In drug induced ILD, treatment is avoidance of further exposure of the causing agent and systemic corticosteroids in patients with progressive or disabling disease. ILD has been reported in 3 of 774 (<0.5%) patients with advanced colorectal cancer receiving cetuximab (Erbitux, Citation2013). There have been only a few case reports of cetuximab-induced ILD in patients treated for head and neck squamous cell carcinoma (HNSCC). ILD is reported to be very rare in the past reports, but we have had 2 cases among 19 such patients in the past 18 months, which is a high incidence. Compared to the past reports, ILD may be a relative common adverse event among Japanese patients treated by irradiation and cetuximab. A case of a patient who developed ILD after receiving radiation and cetuximab in the course of HNSCC treatment is described. Fortunately, the patient survived. Thus, this case is described to propose methods of early detection and effective treatment of cetuximab induced ILD.
2. Case report
A 66-year-old male with double cancers (oral floor cancer T4aN0M0 and supraglottic cancer T2N0M0) received concurrent cetuximab and hyper fractionated radiation therapy. He was a former smoker (Brinkman Index 1600) who had stopped smoking when he underwent subtotal esophageal resection due to esophageal cancer (cT1N0M0, pT1N0M0) at age 60 years. He did not get adjuvant radiotherapy on the esophageal cancer. On the course of concurrent cetuximab and radiation therapy, no part of the lung field was involved. In addition, his past history did not include any known pre-existing lung disease or allergy. The Baseline chest X-ray is shown in Figure (A). Patient’s baseline swallowing function before the treatment was normal according to the endoscopic water drinking test. The patient’s course of treatment and the progression of laboratory data are shown in Figure .
Figure 1. Patient’s treatment course.
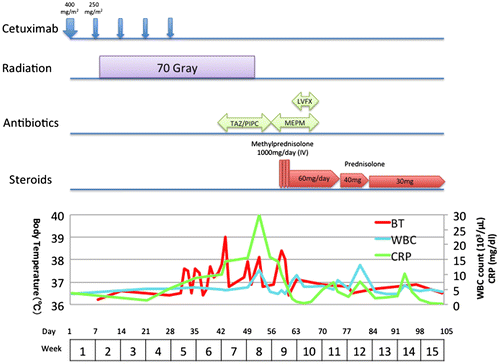
He received three courses of induction chemotherapy with DCF (Day 1: docetaxel 75 mg/m2, Day 1: cisplatin 75 mg/m2, Days 1–5: fluorouracil 750 mg/m2) every 3 weeks. Magnetic resonance imaging (MRI) was performed 1.5 months after the series of induction chemotherapy treatments for evaluation. The three courses of induction chemotherapy showed partial response (RECIST: Response Evaluation Criteria in Solid Tumors). Based on that evaluation, treatment with concurrent cetuximab and radiation therapy was continued.
The patient received cetuximab 400 mg/m2 followed by a total of five courses of weekly cetuximab 250 mg/m2 infusion (Day 9, 15, 28, 35, 44), along with a total dose of 70 Gy with 1.2 Gy fractional dose of hyperfractionated radiotherapy from Day 8 through Day 51. He had difficulty in swallowing because of the pain caused by severe mucosal injury of mouth, pharynx and larynx as an adverse effect of chemotherapy. However, he did not present any coughing on swallowing or stifling. At this point massive aspiration was not positively suspected.
On Day 42, a chest X-ray showed decreased radiolucency in both lower lung fields, apart far from the upper neck irradiation field (Figure (B)). The patient was assumed to have aspiration pneumonia and was treated with intravenous antibiotics. Sputum Gram stain and culture was negative.
On day 51, the patient’s temperature rose to 38°C, and a chest X-ray revealed worsened, bilateral, progressive infiltration (Figure (C)). On day 57, he had severe dyspnea, and his arterial oxygen saturation level decreased to 70% on room air. High-resolution computer tomography (HRCT) of the chest showed extensive consolidation, ground glass changes and traction bronchiectasis in both lungs that were not seen on the baseline CT (Figure ). Laboratory tests showed an elevated C-reactive protein (CRP) level of 30 mg/dL and a white blood cell (WBC) count of 11,500/μL in spite of intravenous antibiotics treatment. KL-6 was also as high as 1,671 U/mL, along with elevations of SP-A (359 ng/mL) and SP-D (162 ng/mL). He had further investigation according to ILD classification (Lauren & Tamera, Citation2015). He did not have any positive findings in connective tissue disease serology, heart function nor symptom for sleep apnea. We could not perform lung biopsy nor BAL due to his severe condition. Based on the clinical course and the laboratory and radiologic findings, he was finally diagnosed as having ILD secondary to cetuximab.
Figure 2. IP progression observed on chest X-rays. (A) Baseline Chest X-ray before the treatment. (B) On Day 42, a chest X-ray shows decreased radiolucency in both lower lung fields. (C) On day 55, a chest X-ray shows worsened bilateral, progressive infiltration. (D) Chest X-rays after treatment with prednisone show significant improvement.
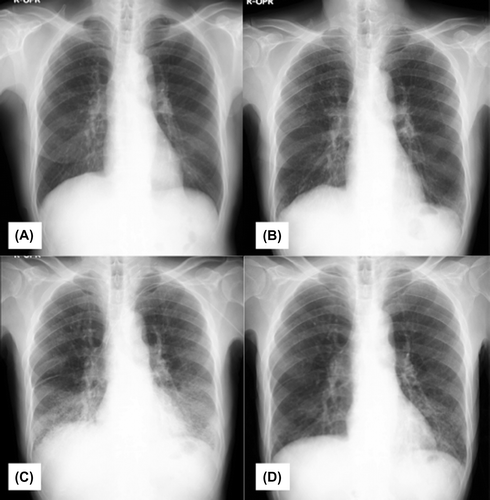
Figure 3. Chest HRCT on day 57 shows extensive consolidation and ground glass changes in both lungs that were not seen on the baseline HRCT.
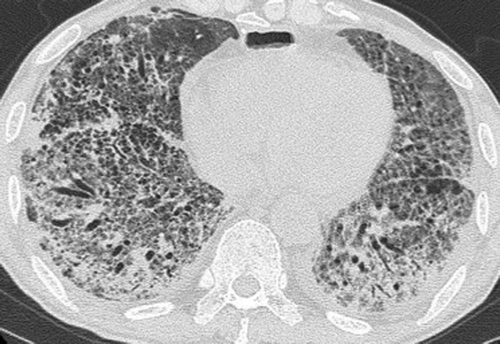
Figure 4. HRCT scans after treatment with prednisone show disappearance of broad ground glass opacity seen in the previous chest CT scan.
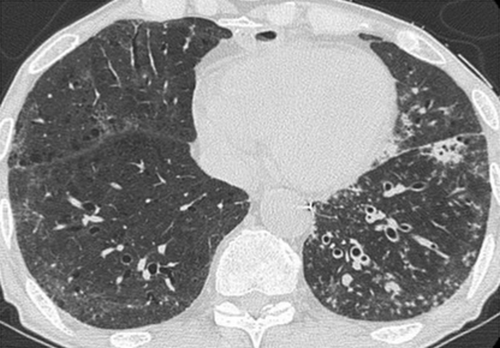
High-dose methylprednisone (1 mg/kg) was started immediately, and his condition improved rapidly. It was then switched to oral prednisone 60 mg daily, which was tapered to 25 mg/day. CRP decreased from 30 mg/dL to 0.8 mg/dL in 10 days, and both the chest X-ray and the CT scan showed significant improvements (Figure (D), 4). On day 76, the patient was able to walk on his own. He was admitted to the respiratory ward for further respiratory rehabilitation and was then discharged at day 100 on home oxygen therapy.
3. Discussion
Research emphasizes the importance of early diagnosis in cases of ILD due to the high mortality rate of advanced cases. Awareness and suspicion of ILD are important for early diagnosis, and we therefore advocate for greater monitoring of potential markers of ILD in patients on cetuximab treatment. Retrospective studies have suggested that pre-existing pulmonary fibrosis, pre-existing hypoxia, poor performance status, male sex, hypoalbuminemia, and current or former smoker status are risk factors for ILD. The most common symptoms for detecting ILD are fever, dyspnea, and cough. (Chua, Peters, Loneragan, & Clarke, Citation2009; Keishi et al., Citation2013)
Clinically, ILD has been defined as a syndrome consisting of dry cough, fever, and hypoxia accompanied by interstitial pulmonary infiltrates on chest X-ray or CT (Lenz, Citation2006). There is no single affirmative diagnostic tool for ILD. Normally the diagnosis of ILD is made by several factors including the clinical symptoms, the agents used for treatment, image studies and others. Establishing an accurate diagnosis of ILD can be challenging, as there are more than 200 different subtypes. The latest classification of ILD is shown in Table (Lauren & Tamera, Citation2015). We diagnosed our case as having ILD by the preceded use of cetuximab, poor response to antibiotics and typical chest CT finding of ground glass opacity. One may question about the possibility of aspiration pneumonitis, but in our case, sputum culture was negative and he had no symptom suggestive of aspiration during his clinical course. It is true that differential diagnosis with aspiration pneumonitis is important, because cetuximab and radiation cause severe oral/pharyngeal mucositis. Chest X-rays has wide variety in aspiration pneumonitis. It is difficult to differentiate ILD from aspiration pneumonia just by looking at the Chest X-rays. ILD can be differentiated from aspiration pneumonitis by combination of clinical data, HRCT imaging results and other findings. Evaluating swallowing disorder to see there is no aspiration or swallowing dysfunction is important. Antibiotics are usually ineffective in ILD. HRCT generally shows rapidly emerged extensive consolidation, ground glass changes in both lungs, although it varies by specific patterns. A complete lack of pulmonary parenchymal changes on HRCT imaging virtually excludes a diagnosis of ILD. The significance of serum markers such as KL-6, SP-A, and SP-D is yet controversial, but they are occasionally referred in diagnosis of the early stages of ILD in some countries including Japan, though they are not very common in the United States.
Table 1. Interstitial lung disease classification
KL-6 is a high molecular weight mucin-like glycoprotein that has been reported to serve as a sensitive marker for ILD (Ohtsuki, Nakanishi, & Fujita, Citation2007). The sensitivity of KL-6 for IP is 53–80% (Inomata & Azuma, Citation2012; Ohnishi, Yokoyama, & Yasuhara, Citation2003). It does not increase with bacterial pneumonia and is thus useful in differentiating ILD from aspiration pneumonitis. Elevated KL-6 levels are believed to be due to increased KL-6 production by regenerating alveolar type II pneumocytes and/or to enhanced permeability following destruction of the air-blood barrier in the affected lungs. Therefore, the serum KL-6 level may reflect the clinical activity and also the prognosis of ILD (Ohnishi et al., Citation2003; Hattori & Kohno, Citation2011).
SP-A and SP-D are pulmonary surfactants produced in the cytoplasm of type II alveolar epithelial cells. They are important for microbial defense and control of pulmonary infection. They have been reported to be useful serum biomarkers for prognosis and disease activity in ILD (Ohtsuki et al., Citation2007). Both SP-A and SP-D levels are reported to increase with active inflammation. Thus, they correlate with acute phase findings on HRCT, such as ground glass opacities (Inomata & Azuma, Citation2012).
In order to identify and treat ILD early, we suggest weekly chest X-rays for every patient who is treated with cetuximab. When the chest X-ray shows a typical pattern, such as infiltration on both sides of lower lung fields, an HRCT scan should be considered. The drug induced ILD typically occur from 2–3 weeks to 2–3 months after starting to use the drug. So we should keep a watchful eye on it for this period (Lauren & Tamera, Citation2015). Specific serum markers such as SP-A, SP-D, and KL-6 may be useful to detect the early stages of ILD. So far, we have had one additional case that was very similar in its symptom pattern to this reported case, and following the diagnostic strategy suggested above, we were able to make the diagnosis rapidly. In a subsequent case, a male patient experienced a sudden onset of dyspnea with high fever. The patient’s chest X-ray was not typical. HRCT was performed and showed widespread ground glass opacities affecting all lung fields. KL-6, SP-A, and SP-D levels were within the normal limits, and he was diagnosed with heart failure.
Drug-induced ILD must be treated immediately with high-dose methylprednisone (1 mg/kg) for 3 days. Sivelestat sodium tetrahydrate 240 mg/body is often administered along with steroid therapy. When intravenous methylprednisone injection is effective, it may be tapered and switched to oral administration. HRCT and measurement of KL-6 levels should be repeated to monitor the clinical activity of ILD.
4. Conclusion
Cetuximab-induced interstitial pneumonia, especially in patients with head and neck cancer, has rarely been reported, and the present case may serve as an important reference to alert clinicians who use these chemotherapeutic agents. In order to identify ILD early, we suggest weekly chest X-rays for cetuximab-treated patients. In suspicious cases, HRCT scan and measurements of KL-6, SP-A, and SP-D levels should be considered.
Additional information
Funding
Notes on contributors
Mari Shimada
We treat various patients with head and neck squamous cell carcinoma. In the last few years, concurrent cetuximab and radiation therapy has become one of the major choices of treatment for advanced head and neck cancer patients. Together with the oncologists, we have been actively gaining the cetuximab treated cases. Among those cases, we experienced some severe complications including interstitial lung disease. Cetuximab has started to be used as an alternative for cis-diamminedichloroplatinum for its expectation for less complication and safety, although our experience might give an important alert to the use of cetuximab.
References
- Chua, W., Peters, M., Loneragan, R., & Clarke, S. (2009). Cetuximab-associated pulmonary toxicity. Clinical Colorectal Cancer, 8, 118–120.10.3816/CCC.2009.n.019
- Erbitux (Cetuximab). (2013). [Package insert]. Princeton, NJ: Bristol-Myers Squibb.
- Hattori, N., & Kohno, N. (2011). KL-6. Mebio, 28, 38–46.
- Inomata, M., & Azuma, A. (2012). Interstitial Pneumonia. Kokyu (Respiratory), 31, 1028–1033.
- Keishi, K., Arata, A., Minoru, K., Hideto, K., Masahiko, K., & Akihiko, G. (2013). Consensus statement for the diagnosis and treatment of drug-induced lung injuries. Respiratory Investigation, 51, 260–277.
- Lauren, T., & Tamera, C. (2015). Interstitial lung disease in 2015: where are we now? Thorax, 44, 546–552.
- Lenz, H. J. (2006). Anti-EGFR mechanism of action: Antitumor effect and underlying cause of adverse events. Oncology, 20, 5–10.
- Ohnishi, H., Yokoyama, A., & Yasuhara, Y. (2003). Circulating KL-6 levels in patients with drug induced pneumonitis. Thorax, 58, 872–875.10.1136/thorax.58.10.872
- Ohtsuki, Y., Nakanishi, N., & Fujita, J. (2007). Immunohistochemical distribution of SP-D, compared with that of SP-A and KL-6, in interstitial pneumonias. Medical Molecular Morphology, 40, 163–167.10.1007/s00795-007-0360-0
