Abstract
Vetiver (Vetiveria zizanioides) essential oil (VEO) has a long history of use. However, research on its biological activity in human skin cells is scarce. In this study, we investigated the biological activity of VEO in a pre-inflamed human dermal fibroblast model, which was designed to mimic the disease biology of chronic inflammation and fibrosis. We analyzed the impact of VEO on the levels of 17 important protein biomarkers pertinent to immune response and tissue remodeling. VEO exhibited strong antiproliferative activity in these cells and significantly inhibited the production of collagen III, an important molecule for skin and tissue remodeling processes. We also studied the effect of VEO on regulating genome-wide gene expression. VEO robustly impacted many genes and signaling pathways that are closely related to tissue remodeling and metabolism, among others. Specifically, VEO significantly impacted pathways for cholesterol synthesis and metabolism. This study provides the first evidence of the biological activity of VEO in human dermal fibroblasts. Though a definite conclusion remains elusive, the data suggest that VEO has therapeutic potential for both cosmetic and metabolic health care products. Further research into VEO’s biological and pharmacological mechanisms of action is recommended.
Public Interest Statement
Vetiver essential oil (VEO) has become increasingly popular for health purposes globally. This study looks at the impact of VEO in a human skin disease model of chronic inflammation. It was found that VEO has potential wound-healing activities. The impact of VEO on global gene expression was also analyzed. VEO diversely affects many genes involving metabolism and tissue remodeling processes; specifically, it influences cholesterol metabolism. These findings suggest that VEO may be a good therapeutic candidate for skincare and obesity. Therefore, this study provides an important stepping stone for further research on VEO and its health benefits for human beings. Additional research focusing on how VEO impacts these genes and processes is recommended.
Competing Interest
Xuesheng Han and Tory L. Parker are employees of dōTERRA, where the study agent VEO was manufactured.
1. Introduction
Vetiver essential oil (VEO) is produced by steam distillation of the aromatic roots of the tropical grass Vetiveria zizanioides, which is native to the Indian subcontinent. VEO has a long history of use primarily due to its insect-repellent property and persistent green-woody note. Scientific studies have evaluated its insect-repellent, anti-inflammatory, antioxidant, and metabolic activities in several settings (Chou, Lai, Lin, & Shih, Citation2012; Nararak et al., Citation2016). Anxiolytic and refreshing effects of VEO aromatherapy also have been demonstrated in rats (Cheaha et al., Citation2016; Saiyudthong, Pongmayteegul, Marsden, & Phansuwan-Pujito, Citation2015). Therefore, VEO has become increasingly popular in products for topical use as well as aromatherapy. However, research on the biological activity of VEO in a human skin model is scarce.
In this study, we investigated the effect of a commercially available VEO on human dermal fibroblasts. First, we evaluated the impact of VEO on 17 important protein biomarkers that are closely related to the processes of inflammation, immune response, and tissue remodeling. We also studied the effect of VEO on genome-wide gene expression. This study provides the first important evidence of the biological activity of VEO in human skin cells, suggesting its therapeutic potential.
2. Materials and methods
All experiments were conducted in a BioMAP HDF3CGF system, a cell culture of human dermal fibroblasts that is designed to model chronic inflammation and fibrosis in a robust and reproducible way. The system consists of three components: a cell type, stimuli to create the disease environment, and a set of biomarker (protein) readouts to examine how treatments affect that disease environment (Berg et al., Citation2010). The methodologies used in this study were essentially the same as those previously described (Han & Parker, Citationn.d.; Han, Rodriguez, & Parker, Citation2017).
2.1. Cell culture
Primary human neonatal fibroblasts were obtained as described previously (Bergamini et al., Citation2012) and were plated in a 96-well format and cultured under low-serum conditions for 24 h. Then they were stimulated with a mixture of interleukin-1β, tumor necrosis factor-α, interferon-ϒ, basic fibroblast growth factor, epidermal growth factor, and platelet-derived growth factor. Cell culture and stimulation conditions for the HDF3CGF assays were performed as described in detail previously (Bergamini et al., Citation2012; Development Core Team, Citation2011).
2.2. Protein-based readouts
A direct enzyme-linked immunosorbent assay (ELISA) was used to measure the biomarker levels of cell-associated and cell membrane targets. Soluble factors from supernatants were quantified using homogeneous time-resolved fluorescence detection, bead-based multiplex immunoassay, or capture ELISA. Overt adverse effects of the test agents on cell proliferation and viability (i.e. cytotoxicity) were measured using a sulforhodamine B assay. For proliferation assays, cells were cultured and then assayed after 72 h, which was optimized for the HDF3CGF system. Detailed information has been described elsewhere (Bergamini et al., Citation2012). Measurements were performed in triplicate wells, and a glossary of the biomarkers used in this study is provided in Supplementary Table S1.
Quantitative biomarker data are presented as the mean log10 relative expression level (compared to the respective mean vehicle control value) ± standard deviation of triplicate measurements. Differences in biomarker levels between VEO- and vehicle-treated cultures were tested for significance with the unpaired Student’s t test. A p-value < 0.05, with an effect size of at least 10% (more than 0.05 log10 ratio units), was regarded as statistically significant.
2.3. RNA isolation
Total RNA was isolated from cell lysates using the Zymo Quick-RNA MiniPrep kit (Zymo Research Corporation, Irvine, CA), according to the manufacturer’s instructions. The RNA concentration was determined using a NanoDrop ND-2000 instrument (Thermo Fisher Scientific). RNA quality was assessed with a Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA) and an Agilent RNA 6000 Nano Kit. All samples had an A260/A280 ratio between 1.9 and 2.1, and an RNA Integrity Number score greater than 8.0.
2.4. Microarray analysis for genome-wide gene expression
A 0.00033% (v/v) concentration of VEO was tested for its effect on the expression of 21,224 genes in the HDF3CGF system after a 24-h treatment. Samples for microarray analysis were processed by Asuragen, Inc. (Austin, TX), according to the company’s standard operating procedures. Biotin-labeled cRNA was prepared from 200 ng of total RNA with an Illumina TotalPrep RNA Amplification kit (Thermo Fisher Scientific) and one round of amplification. The cRNA yields were quantified via UV spectroscopy, and the distribution of transcript sizes was assessed using the Agilent Bioanalyzer 2100. Labeled cRNA (750 ng) was used to probe Illumina Human HT-12 v4 Expression BeadChips (Illumina, Inc., San Diego, CA). Hybridizing, washing, staining with streptavidin-conjugated Cyanine-3, and scanning of the Illumina arrays was performed according to the manufacturer’s instructions. Illumina BeadScan software was used to produce the data files for each array; raw data were extracted using Illumina BeadStudio software.
Raw data were uploaded into R (Development Core Team, Citation2011) and analyzed for quality-control metrics using the beadarray package (Dunning, Smith, Ritchie, & Tavare, Citation2007). Data were normalized using quantile normalization, then re-annotated and filtered to remove probes that were nonspecific or mapped to intronic or intragenic regions (Barbosa-Morais et al., Citation2010). The remaining probe sets comprised the data-set for the remainder of the analysis. Fold-change expression for each value was calculated as the log2 ratio of VEO to the vehicle control. These fold-change values were uploaded to Ingenuity Pathway Analysis (IPA, QIAGEN, Redwood City, CA, www.qiagen.com/ingenuity) to generate the network and pathway analyses.
2.5. Reagents
VEO (dōTERRA International LLC, Pleasant Grove, UT) was diluted in DMSO to 8 × the specified concentrations (the final DMSO concentration in the culture media was no more than 0.1% (v/v)); 25 μL of each 8 × solution was added to the cell culture to a final volume of 200 μL. DMSO (0.1% (v/v)) served as the vehicle control. Gas chromatography-mass spectrometry (GC-MS) analysis of VEO indicated that its major chemical constitutes (i.e. >5%) were trans-isovalencenol (14%), khusimol (13%), and vetiselinenol (5%).
3. Results and discussion
3.1. Bioactivity profile of VEO in pre-inflamed human dermal fibroblasts
We analyzed the activity of VEO in a dermal fibroblast system, HDF3CGF, which features the microenvironment of inflamed human skin cells with boosted inflammatory and immune responses. Four concentrations of VEO (0.001, 0.00033, 0.00011, and 0.000037%, v/v in DMSO) were tested for cell viability. The highest VEO concentration (0.001%) was shown to be overtly cytotoxic to these cells; thus, it was excluded from further analyses. Biomarkers were designated as having key activity if their values were significantly different (p < 0.05) after cell treatment with 0.00033% VEO (the highest concentration that was not cytotoxic to these cells), compared to those of vehicle controls, with an effect size of at least 10% (more than 0.05 log ratio units) (Figure ).
Figure 1. The bioactivity profile of vetiver essential oil (VEO, 0.00033%, v/v in DMSO) in the BioMAP system HDF3CGF.
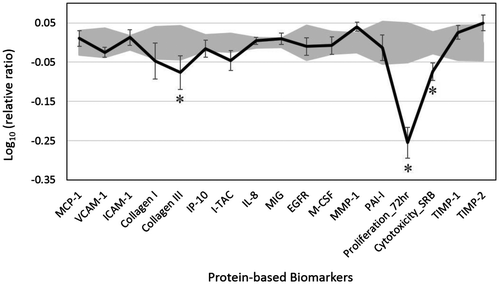
VEO showed significant antiproliferative activity in dermal fibroblasts (Figure ). In addition, VEO significantly inhibited the production of collagen III, an extracellular matrix protein and fibrillar collagen found extensively in connective tissues that is critically involved in the tissue remodeling process. This finding suggests that VEO might play a role in modulating the tissue remodeling process. VEO did not significantly affect the other analyzed biomarkers in the system.
VEO has shown strong anti-inflammatory activities in lipopolysaccharide (LPS)-induced RAW 264.7 macrophages by regulating the expression of the inflammation-related enzymes heme oxygenase-1, inducible nitric oxide synthase, and cyclooxygenase-2 (inducible cyclooxygenase) as well as the inflammatory cytokines tumor necrosis factor-α, interleukin-1β, and interferon-β (Chou et al., Citation2012). In contrast, our data did not show VEO to have an inhibitory effect on inflammatory responses in human dermal fibroblasts, suggesting different mechanisms of action. Further investigation is needed to clarify these findings.
The reported anti-inflammatory activity of VEO has been demonstrated to be associated with its antioxidant activity of decreasing LPS-induced superoxide anion production and malondialdehyde levels (Chou et al., Citation2012). Similarly, it has been reported that the protective role of VEO in the abatement of cisplatin-induced toxicity in mice may be attributed to its antioxidant activity (Sinha et al., Citation2015). Therefore, the inhibitory effect of VEO on collagen III production observed in our study, along with its antioxidant activity, suggest potential benefits of using VEO on the human skin.
3.2. Effects of VEO on genome-wide gene expression
Next, we analyzed the effect of 0.00033% VEO (the highest tested concentration that was noncytotoxic to these cells) on the RNA expression of 21,224 genes in the HDF3CGF system. The results showed that VEO had diverse effects on regulating human genes. Among the 163 most-regulated genes (with a fold-change ratio of expression over vehicle control ≥ |1.5|) by VEO, the majority of them (115 out of 163 genes) were significantly upregulated, and the remaining were downregulated (Table S2).
Furthermore, IPA showed that the bioactivity of VEO matched significantly with many canonical signaling pathways from the literature-validated database (Figure ). Many of these pathways are critical for metabolism and tissue remodeling processes. For example, the top four pathways are inositol pyrophosphate biosynthesis, superpathway of cholesterol biosynthesis, pyridoxal 5’-phosphate salvage, and the cholesterol biosynthesis I pathway. These findings suggest that VEO might play potential roles in regulating metabolism; specifically, VEO might play roles in maintaining cholesterol homeostasis.
Figure 2. The top 20 canonical pathways matching the gene expression bioactivity profile of vetiver essential oil (VEO) in the HDF3CGF system, produced via Ingenuity pathway analysis (QIAGEN, www.qiagen.com/ingenuity).
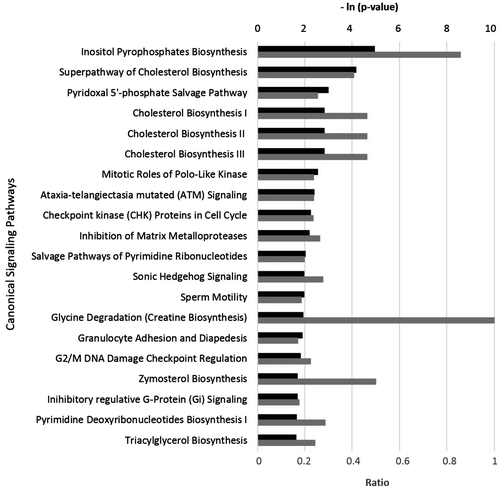
Karan, Pal, Mishra, and Mondal (Citation2013) have reported that an ethanol extract of vetiver roots (similar chemical composition to VEO) has antihyperglycemic activity in diabetic rats, suggesting the therapeutic and antidiabetic potential of VEO. The data from our genome-wide gene expression study seem to support this idea because VEO showed a robust impact on signaling pathways related to metabolism (Figure ).
The current study has several limitations. Though the disease model was designed to simulate the disease biology of chronic inflammation and fibrosis, the in vitro study results cannot be directly translated to the more complex human system. The impact of VEO on gene expression was evaluated after short-term intervention. How VEO impacts global gene expression over a longer term is unclear. Nevertheless, based on the protein and gene expression data, this study provides evidence of the biological effect of VEO on human skin cells and will likely stimulate further research into VEO’s mechanisms of action.
4. Conclusions
To the best of our knowledge, this study provides the first evidence of the biological activity of VEO in a human skin disease model. VEO showed significant antiproliferative activity and also inhibited collagen III production. In addition, the genome-wide gene expression study demonstrated that VEO robustly modulated global gene expression and impacted signaling pathways that are closely related to several critical physiological processes, including tissue remodeling and metabolism. Specifically, VEO robustly impacted pathways critical for cholesterol metabolism. Thus, the data suggest that VEO has therapeutic potential for both cosmetic and metabolic health care products.
Funding
This study was funded by dōTERRA (Pleasant Grove, UT, USA) and conducted at DiscoverX (Freemont, CA, USA).
VEO_Supplementary_Material.docx
Download MS Word (482.9 KB)Additional information
Notes on contributors
Xuesheng Han
At dōTERRA, our group primarily studies the health benefits of essential oils. We are specifically interested in the efficacy and safety of essential oils and their active components. Our studies of essential oils in both in vitro and clinical settings utilize a variety of experimental approaches, including analytical, biological, biochemical, and biomedical methodologies. We work closely with hospitals, clinics, and research institutes towards developing quality essential oils with therapeutic benefits. The research work discussed in this paper represents one part of a large research project, which was designed to extensively examine the impact of essential oils on human cells. This study, along with others, will further the understanding of the health benefits of essential oils for a wide research audience. We believe that a full understanding of these health benefits will ultimately lead to the evaluation and use of essential oils as an adjunctive therapy for a variety of diseases.
References
- Barbosa-Morais, N. L., Dunning, M. J., Samarajiwa, S. A., Darot, J. F. J., Ritchie, M. E., Lynch, A. G., & Tavare, S. (2010). A re-annotation pipeline for illumina beadarrays: Improving the interpretation of gene expression data. Nucleic Acids Research, 38, e17. doi:10.1093/nar/gkp942
- Berg, E. L., Yang, J., Melrose, J., Nguyen, D., Privat, S., Rosler, E., ... Ekins, S. (2010). Chemical target and pathway toxicity mechanisms defined in primary human cell systems. Journal of Pharmacological and Toxicological Methods, 61, 3–15. doi:10.1016/j.vascn.2009.10.001
- Bergamini, G., Bell, K., Shimamura, S., Werner, T., Cansfield, A., Müller, K., ... Neubauer, G. (2012). A selective inhibitor reveals PI3Kγ dependence of TH17 cell differentiation. Nature Chemical Biology, 8, 576–582. doi:10.1038/nchembio.957
- Cheaha, D., Issuriya, A., Manor, R., Kwangjai, J., Rujiralai, T., & Kumarnsit, E. (2016). Modification of sleep-waking and electroencephalogram induced by vetiver essential oil inhalation. Journal of Intercultural Ethnopharmacology, 5, 72–78. doi:10.5455/jice.20160208050736
- Chou, S. T., Lai, C. P., Lin, C. C., & Shih, Y. (2012). Study of the chemical composition, antioxidant activity and anti-inflammatory activity of essential oil from Vetiveria zizanioides. Food Chemistry, 134, 262–268. doi:10.1016/j.foodchem.2012.02.131
- Development Core Team. (2011). R: A language and environment for statistical computing. Vienna: The R Foundation for Statistical Computing. Retrieved from http://www.R-project.org/
- Dunning, M. J., Smith, M. L., Ritchie, M. E., & Tavare, S. (2007). Beadarray: R classes and methods for illumina bead-based data. Bioinformatics, 23, 2183–2184. doi:10.1093/bioinformatics/btm311
- Han, X. & Parker, T. L. (n.d.). Arborvitae (Thuja plicata) essential oil significantly inhibited critical inflammation- and tissue remodeling-related proteins and genes in human dermal fibroblasts. Biochimie Open. doi:10.1016/j.biopen.2017.02.003
- Han, X., Rodriguez, D., & Parker, T. L. (2017). Biological activities of frankincense essential oil in human dermal fibroblasts. Biochimie Open, 4, 31–35. doi:10.1016/j.biopen.2017.01.003
- Karan, S. K., Pal, D., Mishra, S. K., & Mondal, A. (2013). Antihyperglycaemic effect of Vetiveria zizanioides (L.) nash root extract in alloxan induced diabetic rats. Asian Journal of Chemistry, 25, 1555–1557. doi:10.14233/ajchem.2013.13137
- Nararak, J., Sathantriphop, S., Chauhan, K., Tantakom, S., Eiden, A. L., & Chareonviriyaphap, T. (2016). Avoidance behavior to essential oils by anopheles minimus, a malaria vector in Thailand. Journal of the American Mosquito Control Association, 32, 34–43. doi:10.2987/moco-32-01-34-43.1
- Saiyudthong, S., Pongmayteegul, S., Marsden, C. A., & Phansuwan-Pujito, P. (2015). Anxiety-like behaviour and c-fos expression in rats that inhaled vetiver essential oil. Natural Product Research, 29, 2141–2144. doi:10.1080/14786419.2014.992342
- Sinha, S., Jothiramajayam, M., Ghosh, M., Jana, A., Chatterji, U., & Mukherjee, A. (2015). Vetiver oil (Java) attenuates cisplatin-induced oxidative stress, nephrotoxicity and myelosuppression in Swiss albino mice. Food and Chemical Toxicology, 81, 120–128. doi:10.1016/j.fct.2015.04.018
