Abstract
Despite improvements in the prognosis of acute myelogenous leukemia in patients with Down syndrome, the prognosis of acute lymphocytic leukemia (DS-ALL) in these patients is still poorer than that of ALL in the general population. We compared treatment results between 4 patients with DS-ALL and 16 non-Down syndrome patients with ALL (NDS-ALL). Four patients with DS-ALL treated with TCCSG protocols achieved complete remission by the end of the induction phase. During the high-dose methotrexate (HD-MTX) phase, the dosage of MTX was reduced from 3 to 2 g/m2 after the syndrome of inappropriate ADH secretion developed in the first patient. All DS-ALL patients had severe mucositis and a longer period of anorexia than NDS-ALL patients, even after dose reductions, resulting in a longer HD-MTX phase in DS-ALL patients than in NDS-ALL patients. The mean dosages of 6-MP and MTX during the maintenance phase were significantly lower in DS-ALL patients than in NDS-ALL patients (6-MP 22.1 ± 9.9 mg/m2 and MTX 16.1 ± 4.5 mg/m2 vs. 6-MP 43.5 ± 23.1 mg/m2 and MTX 25.2 ± 8.8 mg/m2, respectively). All DS-ALL patients have been in complete remission for a median of 10 years 0 month (range: 8 years 10 months-14 years 1 month) as of January 1, 2017. DS-ALL patients may receive the same treatment protocol as that for NDS-ALL patients with adequate care and few modifications, and this strategy may result in the expansion of treatment protocols suitable for DS-ALL patients in the future.
Public Interest Statement
Persons with Down syndrome are identified as vulnerable individuals. They develop leukemia 20 times more than people at large. Acute lymphocytic leukemia is one type of leukemia (ALL). Although prognosis of ALL is excellent in general population, it is still unfavorable in Down syndrome. There are many reasons for this. Persons with Down syndrome often have various congenital anomalies such as heart defects, obstruction of digestive tract, immunological weakness, etc. In addition to the fact that these conditions are likely to hinder adequate treatment, persons with Down syndrome are sensitive to anti-leukemic drugs and are often unable to tolerate modern treatment. On the assumption that main reason of this poor prognosis is inadequate treatment, we treated them with meticulous supportive care as intensively as they can tolerate. The result of our strategy seems satisfactory and all Down syndrome children with ALL are doing well for more than 8 years.
Competing Interests
The authors declare no competing interest.
1. Introduction
Patients with Down syndrome (DS) are at a high risk of developing leukemia (Ross, Citation2005). In contrast to the improvements achieved in the prognosis of acute myelogenous leukemia in patients with DS (Creutzig, Citation2005; Kojima, Citation2000), the prognosis of acute lymphocytic leukemia (DS-ALL) in these patients is still poorer than that of ALL in the general population (NDS-ALL) (Arico et al., Citation2008; Buitenkamp et al., Citation2014; Lundin et al., Citation2014; Patrick et al., Citation2014; Xavier, Ge, & Taub, Citation2009). Although the reason for this poorer prognosis is multifactorial, the main reasons appear to be the poor tolerance of DS-ALL patients to anti-leukemic drugs and high relapse rate. However, the high relapse rate may be not an independent factor, but secondary to poor tolerance to anti-leukemic drugs rather than decreases in the sensitivity of leukemic cells to these drugs (Izraeli, Citation2014; Whitlock, Citation2006). In addition to inherited immune incompetence (Burgio et al., Citation1978; Carsetti et al., Citation2015; Ram & Chinen, Citation2011), patients with DS often have severe mucosal damage and increased myelosuppression due to the pharmacokinetic and/or pharmacodynamic characteristics of these drugs, and frequently develop severe infectious complications. If the main factor of the poor tolerance of DS-ALL patients to anti-leukemic drugs is the sustained high blood levels of these drugs or the high sensitivity of DS-ALL cells to these drugs, titrations of the dosages of these drugs based on the degree of myelosuppression represent the best and easiest approach to avoid or minimize adverse effects, as reported previously for the maintenance of ALL remission in general (Bohnstedt et al., Citation2013; Schmiegelow, Citation2006). We herein present our results on the treatment of DS-ALL using this strategy in a single institute.
2. Materials and methods
Three patients with DS-ALL and 16 patients with non-Down syndrome ALL (NDS-ALL) were newly diagnosed and treated at Kyorin University Hospital between September 2001 and February 2008. One patient with DS-ALL that recurred in the testis 4 years after initial treatment completion was also treated during this period and added to the analysis. A summary of the patients examined was shown in Table . The median age of DS patients was 7 years 5 months (range: 4 years 0 months-8 years 5 months) and the median WBC was 6,950/μl (range: 5,800–53,500/μl) with a median blast number of 1,739/μl (range: 0–48,685/μl), while the median hemoglobin (Hgb) concentration was 9.8 g/dl (range: 5.7–15.4 g/dl) and median platelet count was 51.5x103/μl (range; 29.2–108 × 103/μl). The karyotypes of DS patients were 47, XY,+21 or 47, XX,+21 and an immunological surface marker study revealed the B-cell precursor type (BCP-ALL) for all patients. BCP-ALL was defined as the immunophenotype of CD 19-positive, CD 10-positive or -negative early pre-B lymphoblasts. None of the DS-ALL patients had central nervous system (CNS) infiltration. The median age of 16 NDS-ALL patients was 5 years 1 month (range: 0 years 11 months-14 years 6 months) and the median WBC was 7,900/μl (range: 1,700–65,600/μl) with a median blast number of 4,101/μl (range: 0–62,976/μl), while the median Hgb concentration was 8.2 g/dl (range: 4.9–12.7 g/dl) and median platelet count was 7.05x103/μl (range; 1.1–28.6x103/μl). All 16 NDS-ALL were BCP-ALL and none had CNS involvement. All of these patients were registered into the TCCSG L99–15 (Manabe et al., Citation2008) or L04–16 protocol for statistical analyses, whereas DS-ALL patients were not enrolled for treatment. All but one patient with DS-ALL were classified as standard-risk (SR) according to the definition of the protocol and all patients including one at high-risk (HR) were treated with the regimen of the SR arm of these protocols. Nine out of 16 NDS-ALL were classified as SR and treated with the same treatment regimen as DS-ALL. Seven NDS-ALL were classified as HR and treated with the regimens of the HR arm. These two protocols differed in the selection of the methotrexate (MTX) infusion time, as described below in the CNS leukemia prophylaxis phase. MTX blood concentrations were measured using an EmitⓇ Methotrexate Assay (Siemens Healthcare Diagnostics, Ltd.). Adverse effects were evaluated according to National Cancer Institute-Common Toxicity Criteria Version 2.0 (NCI-CTC). Statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan) (Kanda, Citation2013).
Table 1. Summary of characteristics of patients with Down syndrome
2.1. Outlines of treatment protocols: treatment phases in the present study
2.1.1. Remission induction phase
The regimen of the SR arm of TCCSG L99–15 included prednisolone at 60 mg/m2 for 4 weeks, vincristine at 1.5 mg/m2, 5 weekly IV doses, and THP-Adriamycin at 20 mg/m2, 2 weekly IV doses. This phase also included L-asparaginase 6,000 U/m2, 9 triweekly IV or IM doses, and 5 intrathecal (IT) doses of MTX, cytosine arabinoside, and hydrocortisone (Triple IT). IT dosages were decided based on age rather than on body size. In the regimen of the HR arm of TCCSG L99-15, THP-Adriamycin was replaced with daunorubicin 25 mg/m2, 4 IV doses and cyclophosphamide 1,000 mg/m2, 2 IV doses. Both arms were the same in the CNS phase with high-dose MTX (HD-MTX) IV infusions and in the maintenance phase with weekly oral MTX and daily oral 6-mercaputopurine.
2.1.2. CNS leukemia prophylaxis phase
MTX at 3 g/m2 weekly IV over 24 h for 3 doses was followed with leucovorin rescue and concomitant triple IT. In the TCCSG L04-16 protocol, the infusion time was reduced to 12 h for the 2nd and 3rd doses if mucositis and/or myelosuppression due to NCI-CTC toxicity more than Grade 4 occurred in the 1st dose. Blood MTX concentrations were measured 48 and 72 h after starting the infusion and leucovorin rescue was given every 6 h, 6 doses starting 36 h after the initiation of the MTX infusion. In cases in which the MTX concentration at 48 h was greater than 1 μmol, the 7th dose of leucovorin was given and leucovorin rescue was continued every 6 h until the MTX concentration reached safer levels.
During the HD-MTX phase, other drugs, including trimethoprim-sulfamethoxazole, were withheld.
2.1.3. Intermediate-dose MTX phase
Three doses of MTX 500 mg/m2 were given every 6 weeks. The 1st dose was infused over 6 h without leucovorin rescue and the 2nd and 3rd doses were infused over 24 h with a single dose of leucovorin rescue. Each dose was associated with IT-MTX and IT-hydrocortisone.
2.1.4. Maintenance phase
Weekly doses of MTX and daily 6-MP were administered for 2 years. The initial doses of MTX and 6-MP were 25 mg/m2 and 40 mg/m2, respectively. These doses were adjusted to maintain the white blood cell count (WBC) between 2,000 and 3,000/μl; the doses of both drugs were simultaneously increased by 25% if 2 consecutive WBC were more than 3,000/μl in WBC biweekly measurements, were simultaneously reduced by 50% if WBC were less than 2,000/μl, and were both withdrawn if WBC were 1,500/μl until they became 3,000/μl.
3. Results
3.1. Responses to the treatment
We treated all DS-ALL patients as those belonging to the SR group. Since the 1st patient developed severe adverse effects including septic shock and the syndrome of inappropriate antidiuretic hormone secretion (SIADH) after the 3rd infusion of HD-MTX 3 g/m2, the dosage of HD-MTX was reduced to 2 g/m2 for the remaining 3 DS-ALL patients. All DS-ALL patients completed 3-year maintenance therapy including the first year of intermittent courses of intensification and have maintained complete remission for a median of 10 years 0 months (range: 8 years 10 months-14 years 1 month) as of January 1, 2017. All DS-ALL patients, including those with congenital cardiac defects, are currently doing well and the deterioration of cardiac function has not yet been observed.
3.2. Consideration of each phase
3.2.1. Induction phase
All DS-ALL patients completed the entire course of the treatment. Two DS-ALL patients developed febrile neutropenia and one with Tetralogy of Fallot had methicillin-resistant Staphylococcus epidermidis sepsis and removal of the central venous catheter. This patient also developed a pneumatocele in the intestine and required a 10-day course of oxygen therapy. All DS-ALL patients developed hematological toxicity higher than grade 3.
3.2.2. Central nervous prophylaxis phase
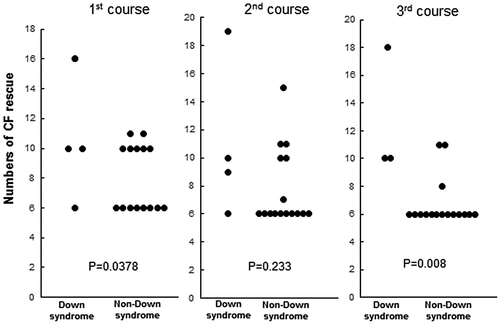
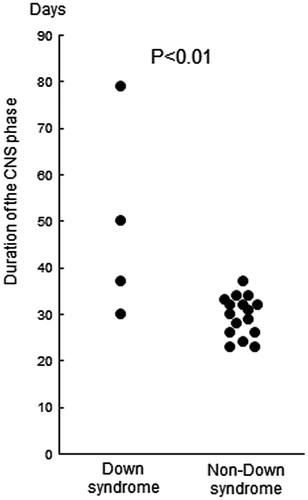
Patient 1, who had reinduction therapy after testicular relapse, received 3 courses of HD-MTX at 3 g/m2 for 24 h and developed SIADH and septic shock after the 3rd course. This patient required 60 days to recover from mucositis and bone marrow suppression. As a consequence, the remaining 3 DS-ALL patients were administered reduced doses of HD-MTX of 2 g/m2 and the infusion time of MTX was shortened to 12 h according to protocol instructions. Furthermore, the 3rd course was omitted for patient 2 because of infection due to high-grade mucositis. All patients developed mucositis and the loss of appetite of higher than grade 3 for a significantly longer duration than NDS-ALL patients (Figure ). In addition to severe mucositis, liver dysfunction of higher than grade 3 was observed in patients 2, 3, and 4. Patient 4 also developed bone marrow suppression of higher than grade 3 (Table ). Due to these adverse effects, the time period of this phase was significantly longer for DS-ALL than for NDS-ALL (Figure ).
Figure 1. Duration of anorexia higher than grade 3.

Table 2. Incidence of toxicitiesTable Footnote*
Figure 2. Duration of the HD-MTX phase.
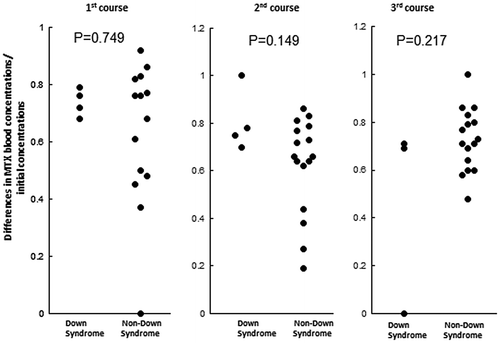
In order to assess the velocity of reductions in MTX blood concentrations, we calculated values for differences in MTX concentrations 72 and 48 h after the initiation of the MTX infusion divided by the MTX concentration at 48 h (Figure ). None of the differences in the courses were significant. The required numbers of leucovorin rescue significantly increased in DS-ALL patients in the 1st and 3rd courses (Figure ).
Figure 3. Ratio of differences in MTX blood concentrations against initial concentrations.
3.2.3. Intermediate dose of the MTX phase
Mucositis of more than Grade 3 was only observed in DS-ALL patients.
3.2.4. Maintenance phase
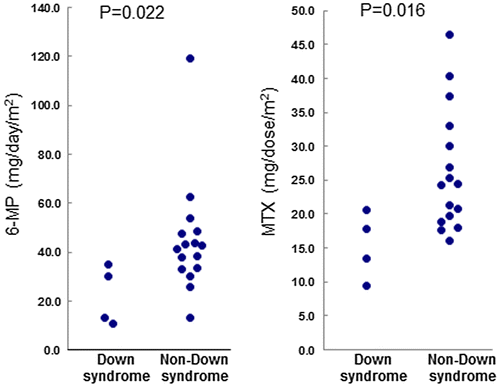
Although severe toxicity requiring the interruption of maintenance therapy was not observed in DS-ALL or NDS-ALL patients, the dosages of 6-MP and MTX had to be reduced in 50% of DS-ALL patients (Figure ). The mean dosages of 6-MP were 22.1 ± 9.9 mg/m2 for DS-ALL patients and 43.5 ± 23.1 mg/m2 for NDS-ALL patients, while the mean dosages of MTX were 16.1 ± 4.5 mg/m2 for DS-ALL patients and 25.2 ± 8.8 mg/m2 for NDS-ALL patients. All DS-ALL patients are alive and have maintained complete remission for a median of 10 years 0 months (range: 8 years 10 months-14 years 1 month) as of 1 January 2017.
4. Discussion
Although DS-ALL generally has few adverse prognostic features, its treatment outcome has been reported to be poorer than that of NDS-ALL (James et al., Citation2008; Lundin et al., Citation2014; Whitlock, Citation2006; Xavier et al., Citation2009). This poorer outcome has been attributed to excessive sensitivity to drugs, particularly MTX (Buitenkamp, Mathot, de Haas, Pieters, & Zwaan, Citation2010; Patrick et al., Citation2014; Xavier et al., Citation2009). Although higher relapse rate may be play a role in the poorer outcomes observed, this higher relapse rate may be related more to inadequate treatment intensity due to poorer tolerance to anti-leukemic drugs because DS-ALL cells are not necessarily more resistant to anti-leukemic drugs than NDS-ALL cells (Zwaan et al., Citation2002).
Since excessive sensitivity to MTX appears to be related to its pharmacodynamics rather than its pharmacokinetics (Buitenkamp et al., Citation2010), difficulties are associated with theoretically predicting dose adjustments for MTX. Therefore, some treatment protocols for ALL, including our protocols, exclude the enrollment of DS-ALL (Arico et al., Citation2008). However, since it is challenging to individualize treatments and these protocols have dosage adjustment and scheduling rules, we decided to use this treatment protocol. Accordingly, we completed the planned treatment with appropriate adjustments in dosages and scheduling with 2 exceptions in the HD-MTX phase. One exception was a dose reduction in MTX for HD-MTX. A similar dose reduction was previously performed in BFM trials (Dördelmann et al., Citation1998). Another exception was the omission of the 3rd course of HD-MTX in 1 DS-ALL patient. The HD-MTX phase required a longer duration and increased numbers of leucovorin rescue, which were mainly due to more severe mucositis accompanied by severe infection in 1 DS-ALL patient. DS-ALL patients had a longer duration of anorexia during the HD-MTX phase than NDS-ALL patients. Although reductions in the dosage and number of HD-MTX courses have been reported to result in inferior 5-year survivals (Goto et al., Citation2011), all of our DS-ALL patients have remained alive in complete remission for more than 8 years and 10 months as of 1 January 2017. The relapse rate appears to be related to the degree of myelosuppression by MTX and 6-MP during the maintenance phase (Bohnstedt et al., Citation2013), and appropriate dose adjustments causing adequate myelosuppression without increases in infectious risks appear to be important. As a result of dose adjustments based on the instructions of the protocols, the maintenance phase required marked reductions in the dosages of 6-MP and MTX, as reported in the literature (Bohnstedt et al., Citation2013). We have not yet encountered a case of induction failure or death during the induction and maintenance phases. Although our DS-ALL patients developed severe myelosuppression and infectious complications, they overcame these complications. As commonly the case in Japan, intensive treatment courses were typically provided at in-patient wards. Therefore, the early detection of adverse reactions and early appropriate treatment were possible. These treatment policies may be appropriate for managing DS-ALL; however, this may not be the case for NDS-ALL. Our results indicate that the same treatment protocol for NDS-ALL is applicable to DS-ALL if more careful supportive care is provided without excessive adverse effects or the possibility of therapy-related death or excessive relapse. Although the number of DS-ALL patients in our study was too small to reach concrete conclusions, previous findings by several study groups reached a similar conclusion. The BFM group showed that most of their patients were successfully treated with 4 consecutive protocols and concluded that it is possible to treat DS-ALL receiving intensive anti-leukemic treatment with no major modifications and the more sophisticated management of toxicity (Dördelmann et al., Citation1998). The Italian group also reached similar conclusions, albeit based on indirect evidence, that more recent treatment protocols achieved better results due to treatment intensification and the accumulation of experience in the care of patients (Arico et al., Citation2008) who require special care. The Nordic group showed that insufficient treatment intensity during maintenance therapy may contribute to a poor prognosis. They showed that it was important to maintain WBC at less than 3,500/μl by adjusting the dosages of 6-MP and MTX during maintenance therapy (Bohnstedt et al., Citation2013). The Children’s Oncology Group in U.S.A employed a different, but highly successful strategy for the treatment of ALL, which included interim maintenance therapy consisting of intravenous MTX at a dosage that was escalated until toxicity (Matloub et al., Citation2011). Further studies are warranted to establish whether this strategy may be employed to treat DS-ALL and is useful for avoiding excessive MTX toxicity. Furthermore, the recent discovery of several important genetic changes in DS-ALL (Lee, Bhansali, Izraeli, Hijiya, & Crispino, Citation2016) may make novel targeted therapy for the treatment of DS-ALL possible.
5. Conclusion
DS-ALL may be treated using the same treatment protocol as that for NDS-ALL with adequate care and few modifications. We propose that DS-ALL be incorporated into the same treatment protocol as that for NDS-ALL under adequate supportive care with as few modifications as possible, if necessary. Based on this approach, the careful evaluation of modifications may lead to the expansion of treatment protocols suitable for DS-ALL.
Additional information
Funding
Notes on contributors
Yuki Gemma
Authors of this paper belong to Tokyo Children’s Cancer Study Group (TCCSG) and work at the University Hospital, where number of staff belonging to each specialty is very few. TCCSG consists of more than 30 institutes. Each institute can take care of only small number of patients and experience of the staff at each institute is limited and it is difficult to provide quality care. However, there are some merits, too, in this situation. The staff are obliged to care not only children with diseases in the field of their specialty, that is, children’s cancers in this case, but also children with diseases of almost all fields. Under this situation, the staff become familiar to many kinds of children’s diseases even out of their specialties and thus they can provide comprehensive care to their limited number of patients.
References
- Arico, M., Ziino, O., Valsecchi, M. G., Cazzaniga, G., Baronci, C., Messina, C., ... Conter, V. (2008). Acute lymphoblastic leukemia and Down syndrome: Presenting features and treatment outcome in the experience of the Italian Association of Pediatric Hematology and Oncology (AIEOP). Cancer, 113, 515–521.10.1002/cncr.v113:3
- Bohnstedt, C., Levinsen, M., Rosthøj, S., Zeller, B., Taskinen, M., Hafsteinsdottir, S., ... Schmiegelow, K. (2013). Physicians compliance during maintenance therapy in children with Down syndrome and acute lymphoblastic leukemia. Leukemia, 27, 866–870.10.1038/leu.2012.325
- Buitenkamp, T. D., Mathôt, R. A., de Haas, V., Pieters, R., & Zwaan, C. M. (2010). Methotrexate-induced side effects are not due to differences in pharmacokinetics in children with Down syndrome and acute lymphoblastic leukemia. Haematologica, 95, 1106–1113.10.3324/haematol.2009.019778
- Buitenkamp, T. D., Izraeli, S., Zimmermann, M., Forestier, E., Heerema, N. A., van den Heuvel-Eibrink, M. M., ...Zwaan, C. M. (2014). Acute lymphoblastic leukemia in children with Down syndrome: A retrospective analysis from the Ponte di Legno study group. Blood, 123, 70–77.10.1182/blood-2013-06-509463
- Burgio, G. R., Lanzavecchia, A., Maccario, R., Vitiello, A., Plebani, A., & Ugazio, A. G. (1978). Immunodeficiency in Down’s syndrome: T-lymphocyte subset imbalance in trisomic children. Clinical & Experimental Immunology, 33, 298–301.
- Carsetti, R., Valentini, D., Marcellini, V., Scarsella, M., Marasco, E., Giustini, F., ... Ugazio, A. G. (2015). Reduced numbers of switched memory B cells with high terminal differentiation potential in Down syndrome. European Journal of Immunology, 45, 903–914.10.1002/eji.201445049
- Creutzig, U. (2005). AML patients with Down syndrome have a high cure rate with AML-BFM therapy with reduced dose intensity. Leukemia, 19, 1355–1360.10.1038/sj.leu.2403814
- Dördelmann, M., Schrappe, M., Reiter, A., Zimmermann, M., Graf, N., Schott, G., ... Riehm, H. (1998). Down’s syndrome in childhood acute lymphoblastic leukemia: Clinical characteristics and treatment outcome in four consecutive BFM trials. Berlin-Frankfurt-Münster Group. Leukemia, 12, 645–651.
- Goto, H., Inukai, T., Inoue, H., Ogawa, C., Fukushima, T., Yabe, M., ... Tsuchida, M. (2011). Acute lymphoblastic leukemia and Down syndrome: The collaborative study of the Tokyo Children’s Cancer Study Group and the Kyushu Yamaguchi Children’s Cancer Study Group. International Journal of Hematology, 93, 192–198.10.1007/s12185-011-0765-3
- Izraeli, S. (2014). How I treat ALL in Down’s syndrome: Pathobiology and management. Blood, 123, 35–40.10.1182/blood-2013-07-453480
- James, R., Lightfoot, T., Simpson, J., Moorman, A. V., Roman, E., & Kinsey, S. (2008). Acute leukemia in children with Down’s syndrome: The importance of population based study. Haematologica, 93, 1262–1263.10.3324/haematol.12831
- Kanda, Y. (2013). Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplantation, 48, 452–458.10.1038/bmt.2012.244
- Kojima, S. (2000). An effective chemotherapeutic regimen for acute myeloid leukemia and myelodysplastic syndrome in children with Down’s syndrome. Leukemia, 14, 786–791.10.1038/sj.leu.2401754
- Lee, P., Bhansali, R., Izraeli, S., Hijiya, N., & Crispino, J. D. (2016). The biology, pathogenesis and clinical aspects of acute lymphoblastic leukemia in children with Down syndrome. Leukemia, 30, 1816–1823.10.1038/leu.2016.164
- Lundin, C., Forestier, E., Klarskov Andersen, M., Autio, K., Barbany, G., Cavelier, L., ... Johansson, B. (2014). Clinical and genetic features of pediatric acute lymphoblastic leukemia in Down syndrome in the Nordic countries. Journal of Hematology & Oncology, 7, 32. doi:10.1186/1756-8722-7-32
- Manabe, A., Ohara, A., Hasegawa, D., Koh, K., Saito, T., Kiyokawa, N., ... Tsuchida, M. (2008). Significance of the complete clearance of peripheral blasts after 7 days of prednisolone treatment in children with acute lymphoblastic leukemia: The Tokyo Children’s Cancer Study Group Study L99-15. Haematologica, 93, 1155–1160.10.3324/haematol.12365
- Matloub, Y., Bostrom, B. C., Hunger, S. P., Stork, L. C., Angiolillo, A., Sather, H., ... Gaynon, P. S. (2011). Escalating intravenous methotrexate improves event-free survival in children with standard-risk acute lymphoblastic leukemia: A report from the Children’s Oncology Group. Blood, 118, 243–251.10.1182/blood-2010-12-322909
- Patrick, K., Wade, R., Goulden, N., Rowntree, C., Hough, R., Moorman, A. V., ... Vora, A. (2014). Outcome of Down syndrome associated acute lymphoblastic leukaemia treated on a contemporary protocol. British Journal of Haematology, 165, 552–555.10.1111/bjh.2014.165.issue-4
- Ram, G., & Chinen, J. (2011). Infections and immunodeficiency in Down syndrome. Clinical & Experimental Immunology, 164, 9–16.10.1111/cei.2011.164.issue-1
- Ross, J. A. (2005). Epidemiology of leukemia in children with Down syndrome. Pediatric Blood & Cancer, 44, 8–12.10.1002/(ISSN)1545-5017
- Schmiegelow, K. (2006). How to adapt drug doses in maintenance therapy of ALL. SIOP Education Book, 17–28.
- Whitlock, J. A. (2006). Down syndrome and acute lymphoblastic leukaemia. British Journal of Haematology, 135, 595–602.10.1111/bjh.2006.135.issue-5
- Xavier, A. C., Ge, Y., & Taub, J. W. (2009). Down syndrome and malignancies: A unique clinical relationship. The Journal of Molecular Diagnostics, 11, 371–380.10.2353/jmoldx.2009.080132
- Zwaan, C. M., Kaspers, G. J., Pieters, R., Hählen, K., Janka-Schaub, G. E., van Zantwijk, C. H., ... Veerman, A. J. (2002). Different drug sensitivity profiles of acute myeloid and lymphoblastic leukemia and normal peripheral blood mononuclear cells in children with and without Down syndrome. Blood, 99, 245–251.10.1182/blood.V99.1.245