Abstract
Although juniper (Juniperus communis) berry essential oil (JEO) has been used in skin care products, research on its biological activity in human skin cells is scarce. In the current study, we explored the biological activity of JEO (with alpha-pinene as the major active component) in pre-inflamed human dermal fibroblasts, which were designed to mimic the disease biology of chronic inflammation and fibrosis. We analyzed the levels of 17 important protein biomarkers pertinent to inflammation and tissue remodeling. JEO exhibited robust antiproliferative activity and significantly inhibited the increased production of the proinflammatory chemokines interferon gamma-induced protein 10 (IP-10) and interferon-inducible T-cell alpha chemoattractant (I-TAC). Additionally, JEO significantly inhibited tissue remodeling biomarkers, namely collagen I, collagen III, and plasminogen activator inhibitor 1 (PAI-I). Macrophage colony-stimulating factor (M-CSF), an immunomodulatory protein molecule, was also significantly downregulated by JEO. Moreover, we found that JEO robustly modulated global gene expression. Ingenuity Pathway Analysis also showed that JEO affected many important signaling pathways that are closely related to metabolism, inflammation, immune response, wound healing, and cancer biology. This study provides the first evidence of the biological activity of JEO in human dermal fibroblasts. Thus, JEO is a promising therapeutic candidate for inflammatory conditions in the skin.
Public Interest Statement
Essential oils have become more popular globally for health reasons. Our study examined the effects of juniper berry essential oil (JEO) in a human skin disease model. These effects of JEO were determined by measuring levels of biomarkers that are linked to inflammation, immune function, and wound healing. The effects of JEO on human genome-wide gene expression were also studied. We found that JEO had strong anti-inflammatory, immune modulatory, and wound healing activities. More interestingly, JEO impacted critical genes and pathways that are associated with metabolism, inflammation, and cancer biology. Findings from this study suggest that JEO may be a good therapeutic candidate for inflammatory, immune, and metabolic diseases. Advanced exploration of the health benefits of JEO may lead to viable options for fighting many of these diseases. Thus, this study provides an important stepping stone for further research on JEO and its health benefits for human beings.
Competing Interest
Xuesheng Han and Tory L. Parker are employees of dōTERRA, where the study agent JEO was manufactured.
1. Introduction
Juniper (Juniperus communis) berry essential oil (JEO) is traditionally used for flavoring and medicinal purposes. JEO and its major active component alpha-pinene have been studied for antimicrobial, antifungal, antiproliferative, anti-inflammatory, and anticancer activities in a variety of settings (Bais, Gill, Rana, & Shandil, Citation2014). In addition, JEO has gained increasing popularity for skin health purposes. However, a literature search conducted by us showed no published studies regarding the biological activity of JEO in human skin cells.
In this study, we investigated the biological activity of a commercially available JEO in an in vitro human skin disease model. First, we studied the effect of JEO on the levels of 17 important biomarkers related to inflammation, immune response, and tissue remodeling processes. Then, using genome-wide analysis of the same cells, we studied the effect of JEO on the expression levels of 21,224 genes. The data provide important evidence of the biological activity of JEO in human dermal fibroblasts. This study may stimulate further research into the mechanisms of action, clinical safety, and efficacy of JEO.
2. Materials and methods
2.1. Model
All experiments were conducted using a Biologically Multiplexed Activity Profiling (BioMAP) system, a cell culture system of human dermal fibroblasts (HDF3CGF) that is designed to model chronic inflammation and fibrosis in a robust and reproducible way. The system consists of three components: a cell type, stimuli to create the disease environment, and a set of biomarker (protein) readouts to examine how treatments affect that disease environment (Berg et al., Citation2010). The methodologies used in this study were essentially the same as those described previously (Berg et al., Citation2010; Kunkel, Dea, et al., Citation2004; Kunkel, Plavec, et al., Citation2004).
2.2. Cell culture
Primary human neonatal fibroblasts were prepared as described previously (Bergamini et al., Citation2012) and were plated under low-serum conditions for 24 h before stimulation with a mixture of interleukin (IL)-1β, tumor necrosis factor (TNF)-α, interferon-ϒ, basic fibroblast growth factor, epidermal growth factor, and platelet-derived growth factor. The cell culture and stimulation conditions for the HDF3CGF assays have been described in detail elsewhere and were performed in a 96-well plate (Bergamini et al., Citation2012; R Development Core Team, Citation2011).
2.3. Protein-based readouts
Direct enzyme-linked immunosorbent assay (ELISA) was used to measure the biomarker levels of cell-associated and cell membrane targets. Soluble factors in the supernatants were quantified using either homogeneous time-resolved fluorescence, bead-based multiplex immunoassay, or capture ELISA detection. The adverse effects of the test agents on cell proliferation and viability (cytotoxicity) were measured using the sulforhodamine B assay. For proliferation assays, the cells were cultured and measured after 72 h, which is optimal for the HDF3CGF system, and the detailed procedure has been described in a previous study (Bergamini et al., Citation2012). Measurements were performed in triplicate wells, and a glossary of the biomarkers used in this study is provided in Supplementary Table S1.
Quantitative biomarker data are presented as the mean log10 relative expression level (compared to the respective mean vehicle control value) ± standard deviation of triplicate measurements. Differences in biomarker levels between JEO- and vehicle-treated cultures were tested for significance with the unpaired Student’s t test. A p-value < 0.05, with an effect size of at least 10% (more than 0.05 log10 ratio units), was regarded as statistically significant.
2.4. RNA isolation
Total RNA was isolated from cell lysates using the Zymo Quick-RNA MiniPrep kit (Zymo Research Corp., Irvine, CA, USA), according to the manufacturer’s instructions. The RNA concentration was determined using a NanoDrop ND-2000 system (Thermo Fisher Scientific). RNA quality was assessed using a Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA) and an Agilent RNA 6000 Nano kit. All samples had an A260/A280 ratio between 1.9 and 2.1 and an RNA Integrity Number score >8.0.
2.5. Microarray analysis for genome-wide gene expression
The effect of 0.003% (v/v) JEO on the expression of 21,224 genes was evaluated in the HDF3CGF system after a 24-h treatment. Samples for microarray analysis were processed by Asuragen, Inc. (Austin, TX, USA), according to the company’s standard operating procedures. Biotin-labeled cRNA was prepared from 200 ng of total RNA using an Illumina TotalPrep RNA Amplification kit (Thermo Fisher Scientific, Waltham, MA, USA) and one round of amplification. The cRNA yields were quantified using ultraviolet spectrophotometry, and the distribution of the transcript sizes was assessed using an Agilent Bioanalyzer 2100. Labeled cRNA (750 ng) was used to probe the Illumina human HT-12 v4 expression bead chips (Illumina, Inc., San Diego, CA, USA). Hybridization, washing, staining with streptavidin-conjugated cyanine-3, and scanning of the Illumina arrays were carried out according to the manufacturer’s instructions. Illumina BeadScan software was used to produce the data files for each array; the raw data were extracted using Illumina BeadStudio software.
The raw data were uploaded into R (R Development Core Team, Citation2011) and analyzed for quality-control metrics using the beadarray package (Dunning, Smith, Ritchie, & Tavare, Citation2007). The data were normalized using quantile normalization (Bolstad, Irizarry, Astrand, & Speed, Citation2003), and then re-annotated and filtered to remove probes that were nonspecific or mapped to intronic or intragenic regions (Barbosa-Morais et al., Citation2010). The remaining probe sets comprised the data-set for the remainder of the analysis. The fold-change expression for each set was calculated as the log2 ratio of JEO to the vehicle control. These fold-change values were uploaded into Ingenuity Pathway Analysis (Qiagen, Redwood City, CA, USA, www.qiagen.com/ingenuity) to generate the networks and pathway analyses.
2.6. Reagents
JEO (dōTERRA Intl., Pleasant Grove, UT, USA) was diluted in DMSO to 8 × the specified concentrations (the final DMSO concentration in culture media was no more than 0.1% [v/v]); 25 μL of each 8 × solution was added to the cell culture to a final volume of 200 μL. DMSO (0.1% [v/v]) served as the vehicle control. The gas chromatography–mass spectrometry analysis of JEO indicated that its major chemical constituents (i.e. >5%) were alpha-pinene (36%), myrcene (14%), and sabinene (8%).
3. Results and discussion
In this study, we analyzed the activity of JEO in a dermal fibroblast cell system, HDF3CGF, which features the disease microenvironment of inflamed human skin cells with increased inflammatory and immune responses. None of the four studied concentrations (0.003, 0.001, 0.00033, and 0.00011%, v/v) of JEO was overtly cytotoxic; thus, all concentrations were included for further analyses. Biomarkers were designated as having key activity if their values were significantly different (p < 0.05) after cell treatment with 0.003% JEO, compared to those of vehicle controls, with an effect size of at least 10% (more than 0.05 log ratio units) (Figure ).
Figure 1. The bioactivity profile of juniper berry essential oil (JEO, 0.003%, v/v in DMSO) using the BioMAP System HDF3CGF.
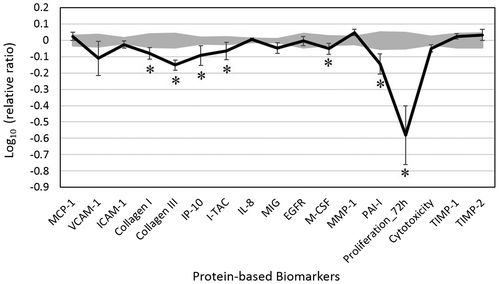
JEO treatment inhibited all 17 of the biomarkers studied. It showed significant antiproliferative activity in dermal fibroblast cells. In particular, JEO significantly inhibited the increased production of two proinflammatory chemokines: interferon gamma-induced protein 10 (IP-10) and interferon-inducible T-cell alpha chemoattractant (I-TAC). Three tissue remodeling biomarkers, namely collagen I, collagen III, and plasminogen activator inhibitor 1 (PAI-I), were also significantly decreased by JEO treatment. In addition, it significantly inhibited the production of macrophage colony-stimulating factor (M-CSF), an immunomodulatory molecule. JEO did not significantly impact other protein readouts. The inhibitory effect of JEO on these proteins suggests that it may possess anti-inflammatory, tissue remodeling, and immunomodulatory properties; thus, it may promote better wound healing in human skin.
Alpha-pinene, the principal constituent of JEO, is widely recognized as the major anti-inflammatory component of JEO. Gayathri, Manjula, Vinaykumar, Lakshmi, and Balakrishnan (Citation2007) have shown that alpha-pinene possesses anti-inflammatory properties in human peripheral blood mononuclear cells and mouse macrophages through inhibition of TNF-α, IL-1β, nitric oxide, and mitogen-activated protein kinases. Another study has found that alpha-pinene inhibits the nuclear translocation of nuclear factor (NF)-κB induced by lipopolysaccharides in THP-1 cells (Zhou, Tang, Mao, & Bian, Citation2004).
More recently, alpha-pinene has been found to inhibit the production of TNF-α, IL-1β, and IL-6 during cerulean-induced acute pancreatitis in mice (Bae et al., Citation2012). Furthermore, alpha-pinene has been reported to inhibit TNF-α, ICAM-1, and NF-κB in a mouse model of allergic rhinitis (Nam et al., Citation2014). Moreover, Kim et al. (Citation2015) have demonstrated that alpha-pinene significantly decreases the increased production of IL-6, TNF-α, nitric oxide, inducible nitric oxide synthase, and cyclooxygenase-2 in lipopolysaccharide-treated macrophages of mice. Alpha-pinene also has been reported to suppress inflammation through inhibiting COX-2 overexpression (Li, Yang, Li, Zhang, & Tang, Citation2016). In addition, a methanol extract of Juniperus Communis (with alpha-pinene reported to be the top active component) has been demonstrated to exhibit anti-inflammatory activity in an adjuvant-induced arthritic rat model (Bais, Abrol, Prashar, & Kumari, Citation2016). Alpha-pinene also has been shown to be antiproliferative and proapoptotic toward several types of cancer cells (Chen et al., Citation2015; Kusuhara et al., Citation2012; Matsuo et al., Citation2011), suggesting its anticancer potential. Collectively, our findings along with existing research suggest that JEO (with alpha-pinene as the major active component) may possess anti-inflammatory, tissue remodeling, pro-wound healing, and anticancer properties; thus, it may be a promising candidate for treating inflammatory conditions.
Next, we analyzed the effect of 0.003% (v/v) JEO (the highest analyzed concentration that was noncytotoxic to these cells) on the RNA expression of 21,224 genes in the HDF3CGF system. The results showed a fairly diverse effect of JEO on regulating human genes, with many downregulated genes and many upregulated genes. Among the 86 most-impacted genes (with a fold-change ratio of expression over vehicle control ≥ |1.5|) by JEO, most of them (52 out of 86 genes) were significantly upregulated, and the remaining were downregulated (Table S2).
Ingenuity Pathway Analysis showed that the bioactivity of JEO significantly matched with many canonical signaling pathways from the literature-validated database (Figure ). Many of these pathways are critically involved in a wide range of biological and physiological processes, including metabolism, inflammation, immune response, cell cycle control, and cancer signaling. For instance, the four most-matched signaling pathways were salvage pathways of pyrimidine ribonucleotides, the pyridoxal 5’-phosphate salvage pathway, mitotic roles of polo-like kinase, and thyroid cancer signaling. Overall, it was observed that JEO inhibited critical genes in these pathways, suggesting its potential roles in modulating metabolism, immune responses, and cancer biology. These findings largely support the promising properties of JEO, including anti-inflammatory, tissue remodeling, antiproliferative, and anticancer properties.
Figure 2. The top 20 canonical pathways matching the gene expression bioactivity profile of juniper berry essential oil (JEO) in the HDF3CGF system, produced via Ingenuity Pathway Analysis (Qiagen, www.qiagen.com/ingenuity).
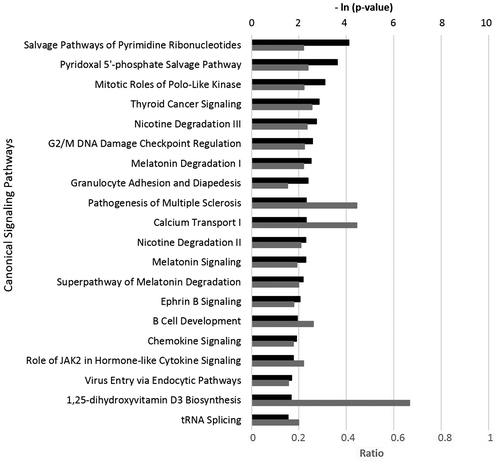
Of note, a literature search conducted by us revealed no published study regarding the impact of JEO on metabolism in human cells. The current gene expression data indicated that JEO may potentially regulate metabolism; thus, it may be a possible option for fighting metabolic diseases. Further research into the biological and physiological mechanism of action of JEO is recommended.
4. Conclusions
To the best of our knowledge, this is the first study to determine the biological activity of JEO in human dermal fibroblasts. JEO demonstrated significant antiproliferative properties and significantly inhibited the increased production of IP-10, I-TAC, collagen I, collagen III, M-CSF, and PAI-I. Genome-wide gene expression analysis also showed that JEO modulated global gene expression. Moreover, it robustly affected signaling pathways that are relevant to metabolism, inflammation, and cancer biology. Therefore, the data suggest that JEO (with alpha-pinene as the major active component) is a promising therapeutic candidate for inflammatory conditions in the skin. Further research into the mechanisms of action, clinical safety, and efficacy of JEO are recommended. The impact of JEO on metabolism is also worth further investigation.
Funding
This study was funded by dōTERRA (Pleasant Grove, UT, USA) and conducted at DiscoverX (Fremont, CA, USA).
JEO_SI_030917.docx
Download MS Word (19 KB)Additional information
Notes on contributors
Xuesheng Han
Our group primarily studies the health benefits of essential oils. We are specifically interested in the efficacy and safety of essential oils and their active components. Our studies of essential oils in both in vitro and clinical settings utilize a variety of experimental approaches, including analytical, biological, biochemical, and biomedical methodologies. We work closely with hospitals, clinics, and research institutes towards developing quality essential oils with therapeutic benefits. The research work discussed in this paper represents one part of a large research project, which was designed to extensively examine the impact of essential oils on human cells. This study, along with others, will further the understanding of the health benefits of essential oils for a wide research audience. We believe that a full understanding of these health benefits will ultimately lead to the evaluation and use of essential oils as an adjunctive therapy for a variety of diseases.
References
- Bae, G.-S., Park, K.-C., Choi, S. B., Jo, I.-J., Choi, M.-O., Hong, S.-H., ... Park, S.-J. (2012). Protective effects of alpha-pinene in mice with cerulein-induced acute pancreatitis. Life Sciences, 91, 866–871. doi:10.1016/j.lfs.2012.08.035
- Bais, S., Gill, N. S., Rana, N., & Shandil, S. (2014). A phytopharmacological review on a medicinal plant: Juniperus communis. International Scholarly Research Notices, 2014, 634723. doi:10.1155/2014/634723
- Bais, S., Abrol, N., Prashar, Y., & Kumari, R. (2016). Modulatory effect of standardised amentoflavone isolated from Juniperus communis L. agianst Freund’s adjuvant induced arthritis in rats (histopathological and X Ray anaysis). Biomedicine & Pharmacotherapy, 86, 381–392. doi:10.1016/j.biopha.2016.12.027
- Barbosa-Morais, N. L., Dunning, M. J., Samarajiwa, S. A., Darot, J. F. J., Ritchie, M. E., Lynch, A. G., & Tavare, S. (2010). A re-annotation pipeline for Illumina BeadArrays: Improving the interpretation of gene expression data. Nucleic Acids Research, 38, e17. doi:10.1093/nar/gkp942
- Berg, E. L., Yang, J., Melrose, J., Nguyen, D., Privat, S., Rosler, E., ... Ekins, S. (2010). Chemical target and pathway toxicity mechanisms defined in primary human cell systems. Journal of Pharmacological and Toxicological Methods, 61(1), 3–15. doi:10.1016/j.vascn.2009.10.001
- Bergamini, G., Bell, K., Shimamura, S., Werner, T., Cansfield, A., Müller, K., ... Neubauer, G. (2012). A selective inhibitor reveals PI3Kγ dependence of TH17 cell differentiation. Nature Chemical Biology, 8, 576–582. doi:10.1038/nchembio.957
- Bolstad, B. M., Irizarry, R. A., Astrand, M., & Speed, T. P. (2003). A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics, 19, 185–193.10.1093/bioinformatics/19.2.185
- Chen, W., Liu, Y., Li, M., Mao, J., Zhang, L., Huang, R., ... Ye, L. (2015). Anti-tumor effect of α-pinene on human hepatoma cell lines through inducing G2/M cell cycle arrest. Journal of Pharmacological Sciences, 127, 332–338. doi:10.1016/j.jphs.2015.01.008
- Dunning, M. J., Smith, M. L., Ritchie, M. E., & Tavare, S. (2007). beadarray: R classes and methods for Illumina bead-based data. Bioinformatics, 23, 2183–2184. doi:10.1093/bioinformatics/btm311
- Gayathri, B., Manjula, N., Vinaykumar, K. S., Lakshmi, B. S., & Balakrishnan, A. (2007). Pure compound from Boswellia serrata extract exhibits anti-inflammatory property in human PBMCs and mouse macrophages through inhibition of TNFα, IL-1β, NO and MAP kinases. International Immunopharmacology, 7, 473–482. doi:10.1016/j.intimp.2006.12.003
- Kim, D.-S., Lee, H.-J., Jeon, Y.-D., Han, Y.-H., Kee, J.-Y., Kim, H.-J., & Hong, S.-H. (2015). Alpha-pinene exhibits anti-inflammatory activity through the suppression of MAPKs and the NF-κB pathway in mouse peritoneal macrophages. The American Journal of Chinese Medicine, 43, 731–742. doi:10.1142/S0192415X15500457.
- Kunkel, E. J., Dea, M., Ebens, A., Hytopoulos, E., Melrose, J., Nguyen, D., & Berg, E. L. (2004). An integrative biology approach for analysis of drug action in models of human vascular inflammation. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 18, 1279–1281. doi:10.1096/fj.04-1538fje
- Kunkel, E. J., Plavec, I., Nguyen, D., Melrose, J., Rosler, E. S., Kao, L. T., ... Berg, E. L. (2004). Rapid structure-activity and selectivity analysis of kinase inhibitors by bioMAP analysis in complex human primary cell-based models. ASSAY and Drug Development Technologies, 2, 431–442. doi:10.1089/adt.2004.2.431
- Kusuhara, M., Urakami, K., Masuda, Y., Zangiacomi, V., Ishii, H., Tai, S., ... Yamaguchi, K. (2012). Fragrant environment with α-pinene decreases tumor growth in mice. Biomedical Research, 33, 57–61.10.2220/biomedres.33.57
- Li, X.-J., Yang, Y.-J., Li, Y.-S., Zhang, W. K., & Tang, H.-B. (2016). α-Pinene, linalool, and 1-octanol contribute to the topical anti-inflammatory and analgesic activities of frankincense by inhibiting COX-2. Journal of Ethnopharmacology, 179, 22–26. doi:10.1016/j.jep.2015.12.039
- Matsuo, A. L., Figueiredo, C. R., Arruda, D. C., Pereira, F. V., Scutti, J. A. B., Massaoka, M. H., ... Lago, J. H. G. (2011). α-Pinene isolated from Schinus terebinthifolius Raddi (Anacardiaceae) induces apoptosis and confers antimetastatic protection in a melanoma model. Biochemical and Biophysical Research Communications, 411, 449–454. doi:10.1016/j.bbrc.2011.06.176
- Nam, S.-Y., Chung, C., Seo, J.-H., Rah, S.-Y., Kim, H.-M., & Jeong, H.-J. (2014). The therapeutic efficacy of α-pinene in an experimental mouse model of allergic rhinitis. International Immunopharmacology, 23, 273–282. doi:10.1016/j.intimp.2014.09.010
- R Development Core Team. (2011). R: A language and environment for statistical computing. Vienna: the R Foundation for Statistical Computing. Retrieved from http://www.R-project.org/
- Zhou, J., Tang, F., Mao, G., & Bian, R. (2004). Effect of alpha-pinene on nuclear translocation of NF-kappa B in THP-1 cells. Acta Pharmacologica Sinica, 25, 480–484.
