Abstract
Most HIV-1 tropism studies have involved non-A subtypes. Our aim was to study the prevalence of R5- and non-R5-tropic HIV-1 variants and the tropism occurrence relative to the CD4 counts, treatment experiences, transmission routes and other features of infection in Russia, where subtype A is presumably predominant. In this multicenter, single-step, cross-sectional, epidemiologic study, 943 HIV-1-infected patients were enrolled at 12 AIDS centers throughout Russia. Viral tropism was determined using a genotype method-based kit. The V3 loop sequences were analyzed using the geno2pheno resource. The tropism was successfully predicted for 823 (87.3%) patients. Frequencies of R5-tropic and non-R5-tropic viruses in successfully analyzed samples were 70.2% (578) and 29.8% (245), respectively. Co-receptor usage correlated significantly only with the treatment experiences (p = 0.018) and CD4 counts (p = 0.004). But there was no dependence of R5/non-R5 co-receptor usage frequencies on presence/absence of a therapy change (p = 0.664) or HIV infection duration (p = 0.458). According to the env sequences, 457 (83.6%) of the samples in study were subtype A and 70 (12.8%) were subtype B. This indicates a stabilizing of immune system and thus little emergence of X4 viruses. We suggest that CCR5-antagonists could be used in both naïve and experienced patients in Russia after determination of HIV tropism.
Public Interest Statement
HIV infection is a serious problem for Russia. The emergence of a new class of drugs - CCR5 coreceptor antagonist, will enhance the effectiveness of the treatment. This type of drugs is effective only against the variant of the virus tropic to CCR5 co-receptor and it is necessary to perform a tropism test before treatment begins. The epidemic of HIV in Russia is characterized by the fact that about 89% of all newly diagnosed cases of HIV infection are caused by viral subtype A. For this subtype, differences from other subtypes that could affect the effectiveness of the treatment were previously shown. We explored different patient groups in all territory of Russia to examine the features of HIV subtype A. The results of the study showed that despite the revealed features of HIV, the use of CCR5-antagonists for patients in Russia could be effective.
Competing Interests
Evgeniy Bukin and Andrey Polyakov are employees of ViiV Healthcare Russia. Other authors have no competing interests to declare.
1. Introduction
To gain entry into target cell, human immunodeficiency virus type 1 (HIV-1) must bind to the CD4 receptor and in addition to either the CCR5 or to the CXCR4 co-receptor. Viruses that exclusively use the CCR5 or CXCR4 co-receptor are described as R5 or X4 viruses, respectively, whereas those that can use both receptors or mixtures of R5- and X4-variants are described as R5X4 viruses. This difference in selectivity has been used in CCR5 antagonist development. Maraviroc, a CCR5 receptor antagonist, is only effective against the replication of CCR5-tropic HIV-1 variants. Maraviroc was registered in Russian Federation in 2011. This drug was recommended for HIV-infected patients who had previously received antiretroviral therapy (ARV) and who had been found to carry R5-viruses (Kravchenko, Citation2012). Therefore, it is necessary to confirm that the infecting virus only binds CCR5 (Poveda et al., Citation2012) before administering maraviroc-containing therapies.
The phenotypic Trofile assay (OTA) and Enhanced Sensitivity Trofile Assay (ESTA) as an improved version of the Trofile assay are used in clinical studies and diagnostic routine (Adults PoAGf & Adolescents, Citation2009), but these assays are mainly restricted to the US. ESTA for determination of tropism is not practical in Russia for several reasons, including high cost and a legislative ban on the export of biological material abroad. Other methods have been utilized in many other countries because of transport difficulties and long ESTA analysis duration (Gonzalez-Serna et al., Citation2010; Svicher et al., Citation2010, Citation2012). Analysis of the V3 loop sequence according to the genotypic method has been shown to effectively determine viral tropism and predict the success of maraviroc treatment (McGovern et al., Citation2010; Prosperi, Bracciale, Fabbiani, et al., Citation2010; Seclen et al., Citation2010; Svicher et al., Citation2010, Citation2012; Symons et al., Citation2012) and is meanwhile an established procedure in many countries.
Establishment of alternative phenotypic assays in Russia is not feasible to conduct the routine diagnostic needs. The European guidelines, which were published in 2011 (Vandekerckhove et al., Citation2011), describe applications of various clinical approaches to tropism determination, including genotypic method followed by bioinformatics based interpretation systems. To cover this diagnostic gap, an in vitro diagnostic reagent kit, AmpliSens HIV-Resist-Seq (CRIE, Moscow, Russia), was developed and registered in Russia in 2012 (Lopatukhin et al., Citation2013); this kit is based on a genotypic method to determine HIV-1 tropism and allows physicians to assess patient’s tropism before initiating maraviroc treatment. In Russian Federation, HIV-1 subtype A is predominant and is estimated (Mulinge et al., Citation2013) to account for more than 80% of all newly diagnosed HIV infections in Russian Federation. Therefore, it is important to study the characteristics of HIV-1 subtype A and the prediction of tropism. As differences among nucleotide sequences of env region V3 loop of subtype A and those of other viral subtypes have been previously reported (Thomson et al., Citation2007), the PCR and sequencing needs to be optimized. Additionally we analysed usefulness of the geno2pheno[coreceptor] interpretation system which is widely used for tropism prediction, but used mainly HIV-1 subtypes B for establishing its models (Vasil’ev, Kazennova, & Bobkova, Citation2010).
In this epidemiological multicenter study, we used newly registered diagnostic kit to investigate the prevalence of R5 and non-R5 HIV-1 infected population with respect to HIV infection duration, CD4 lymphocytes and ART experience or naivety.
2. Methods
2.1. Study design
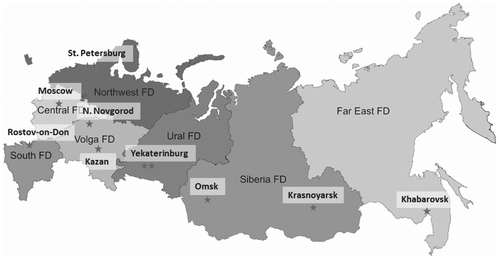
This study was designed as multicenter, single-step study. It consisted of single visit by all patients. During this visit following procedures were performed: receipt of written informed consent, assessment of patient eligibility for the study, stratification of the patient and inclusion of the patient in the study. In addition, detailed information was collected regarding the date of birth, place of residence, date of first HIV + immune blot test, route of infection, all known results of CD4 cell counts and viral load measurements, and all known ARV regimens for treatment-experienced patients. A single blood sample was drawn to study viral tropism and genotype via sequencing. All blood samples were processed and utilized in accordance with the rules of the laboratory. All patients included in the study were older than 18 years of age. A total of 943 patients were enrolled from 12 participating centers located in 10 cities throughout Russia (Figure ). According to the objectives of the study, HIV-1 tropism was analyzed in patients with varying immune condition who had not received ARV therapy as well as those who had experience with ARV therapy.
Figure 1. Locations of HIV/AIDS regional centers that participated in HIV tropism study in Russia, 2014.
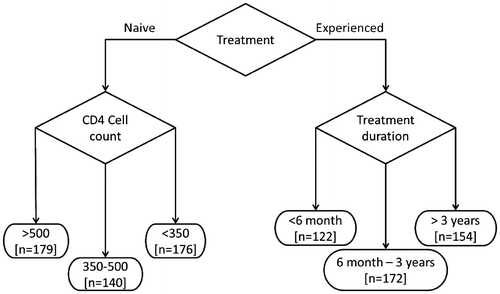
All patients were stratified in two major groups of whom was therapy naive or therapy experienced. Then therapy naive patients were stratified in three groups of 140–179 patients with dependence of their CD4 cell count. And therapy experienced patients were stratified in three of 122–172 patients groups with dependence of the therapy duration (Figure ).
2.2. Sequencing and sequence analysis
Tropism determination and subtyping were conducted via sequencing with the AmpliSens HIV-Resist-Seq diagnostic kit. According to the instruction of this kit, analyses can be performed with either viral RNA from blood plasma, or proviral DNA from purified leucocytes. Viral RNA was the preferred sample type if the viral load was ≥1,000 copies/ml. If the viral load was <1,000 copies/ml or undetectable, proviral DNA was used for the analysis. Using DNA to determine HIV tropism shown to be effective in the analysis of samples with low or undetectable viral load (Fabeni et al., Citation2015; Ferrer et al., Citation2013; Lopatukhin et al., Citation2012; Meini et al., Citation2014). Also during the validation of this methodic high concordance of HIV tropism was obtained for viral RNA and proviral DNA (Dauwe et al., Citation2015). Therefore, test results from plasma and leucocytes were merged with each other during the analysis. The analysis comprises viral RNA or proviral DNA extraction, HIV-1 genome fragment amplification of the gp120 V3 loop, population DNA sequencing using ABI genetic analyzer and subsequent analysis using the geno2pheno algorithm according to the European guidelines. For tropism determination, each sample was analyzed in triplicate from the fragment amplification step. Reverse transcription with nested PCR for HIV-1 RNA or one-step PCR amplification for proviral DNA was used to increase sensitivity to minor virus variants (Symons et al., Citation2012). Sequencing analysis was performed on both chains of the gp120 V3 loop.
Subtyping was performed on the env sequences (position 6960 to 7370 of HIV-1 HXB2), as high concordance between env- and pol-based subtyping was shown, shorter sequences were excluded from the subtyping (Neogi et al., Citation2010). DEONA software (MIG, Moscow, Russia), a component of the HIV-Resist-Seq test kit, was used to create and subsequently edit consensus sequence. The COntext-based Modeling for Expeditious Typing (COMET) HIV-1 subtyping tool was used for env sequence analyses.
2.3. Genotypic prediction of viral tropism
According to the AmpliSens HIV-Resist-Seq procedure manual and the European guidelines, the geno2pheno algorithm was used to analyze the V3 loop sequences. If three sequences were amplifiable, the false positive rate (FPR) was offset at 10%. If only one or two sequences were available, the FPR was increased to 20%.
2.4. Statistical analysis
Association between HIV-1 tropism and therapy status, was determined using the χ2 test. Correlations between HIV-1 tropism and continuous variables, including viral load (VL) and CD4-cell counts, were determined using Mann-Whitney test. Correlations between two continuous variables (VL and CD4-cell count) were tested with Spearman correlation test. Data sets were analyzed using statistical software package SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Patient characteristics
This study enrolled 943 white Caucasian patients who had not been treated with CCR5 antagonsts and were grouped according to predefined stratification. The patients’ characteristics are listed in Table . Mean time since first positive HIV for included patients test was 5 years [2.0–9.0]. The viral load and CD4 measurements were analyzed at the time of sample collection.
Table 1. Characteritics of HIV infected patients from 12 HIV/AIDS Centers in Russia, 2014
Patients demographic characteristics in study population. All characteristics represent the value at the time of inclusion in the study.
3.2. Viral load
The blood plasma HIV viral load was determined for all patients enrolled in this study. Viral load was undetectable (<50 copies/ml) for 295 (31.3%) samples; for another 648 (68.7%) samples with detectable viral load its median was 4.23 [3.43–5.06] log copies/ml. The median viral load differed significantly between treatment-naïve patients and treatment-experienced patients (4.31 [3.67–5.07] log copies/ml and 3.83 [2.79–5.04] log copies/ml respectively; p < 0.0001). Additionally, the mean viral load correlated negatively with the CD4 cell count, correlation coefficient −0.188 (p < 0.0001).
Among patients with undetectable viral load, 238 were therapy experienced patients with undetectable viral load and only 57 were therapy naive. There were no significant differences in viral load between patients with detectable viral load. For 378 patients infected with R5 mean viral load was 0.28 [3.50–5.11] log copies/ml and 4.30 [3.49–5.17] log copies/ml for 143 non-R5.
3.3. Subtype analysis
The sequence of env gene was used to determine subtypes in 546 samples. Sequences of another 397 samples were too short for reliable subtype determination. The overall distribution of subtypes was subtype A, 457(83.6%); subtype B, 70 (12.8%); subtype G, 11 (2.0%); subtype C, 7 (1.2%) and CRF01_AE, 3 (0.5%). There were small variations associated with the geographical region, but these associations were not significant (83.6 ± 5.2% for subtype A and 12.8 ± 5.4% for subtype B).
3.4. Performance efficiency of tropism testing
HIV tropism was determined in all patients in the study. The tropism was successfully predicted for 823 (87.3%) patients. The effectiveness of the HIV tropism testing depended on the patients’ viral loads at the time of enrollment. Nevertheless there was no significant difference in testing effectiveness between ART-naïve (88.1%) and ART-experienced (86.4%) patients.
For samples with a low viral load, proviral DNA was used for the analysis; the performance efficiency varied from 83.0% in the group with a VL <500 copies/ml (including undetectable VL) to 92.1% in the group with 500–1,000 copies/ml, to 88.6% for the group with a VL of 1,000–10,000 copies/ml and 92.4% for the group with VLs >10,000,000 copies/ml. Therefore, the efficiency of the analysis increased as the viral load increased. However, the efficiency of the analysis was sufficiently high even for samples with undetectable viral loads, which is typical for ART-experienced patients.
3.5. Co-receptor usage
Overall, frequencies of R5-tropic and non-R5-tropic viruses in successfully analyzed samples were 70.2% (578) and 29.8% (245), respectively. These results are in accordance with those of previous publications (Ataher et al., Citation2012; Brumme et al., Citation2005; Gonzalez-Serna et al., Citation2010; Neogi et al., Citation2010; Svicher et al., Citation2010; Symons et al., Citation2012). Further, co-receptor usage was studied depending on various factors (Table ).
Table 2. Co-receptor usage frequencies in dependence geographical region and treatment expirience
3.5.1. Co-receptor usage by geographical region
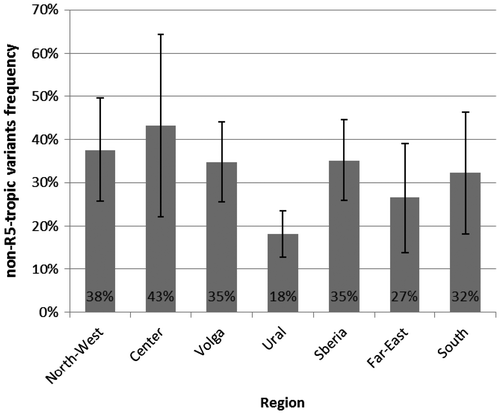
R5/non-R5 co-receptor usage frequencies distribution according to its geographical origin is shown on Figure . Frequency of non-R5-tropic HIV variants in different regions varied from 18 to 43%. Prevalence of non-R5-torpic variants did not differ significantly depending on the region, with the exception of the Ural region, where it is lower at 18% [13–24%] (p = 0.05).
3.5.2. Co-receptor usage by treatment experience
After stratifying by treatment experience, we observed significant differences in R5/non-R5 frequencies (p = 0.018) between naïve (73.9/26.1%; 322/114) and experienced patients (66.1/33.9%; 256/131). However, these differences were not as large as those previously observed (Ataher et al., Citation2012). We did not observe significant differences among treatment-experienced patients after stratifying by therapy change (p = 0.664). R5/non-R5 frequencies in patients who experienced therapy change were 67.1/32.9% (151/74) and 64.8/35.2% (105/57) in patients who had not had a therapy change.
3.5.3. Co-receptor usage and CD4 cell counts
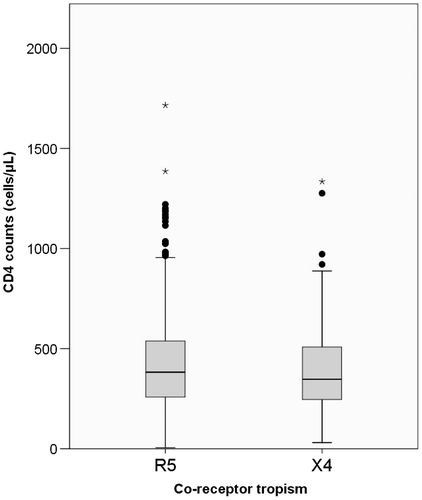
A positive correlation was observed between an increasing proportion of R5 variants and CD4 cell counts (Figure ). X4-tropic variants are characterized by lower levels of CD4 counts (p = 0.037). This trend continues in the analysis only naive patients (p = 0.037). However, analysis of differences in therapy-experienced patients shows no differences between R5 and X4 frequencies (p = 0.833).
3.5.4. Co-receptor usage by infection route
Only a trend was observed between R5/non-R5 co-receptor usage frequencies and infection route. The R5 co-receptor usage in the group with a homosexual infection route was considerably lower (60%; 18) that of the two major groups and considerably higher (81.8%; 9) with a mother-to-child transmission group if compare it to overall R5 co-receptor usage frequency (70.2%).
3.5.5. Co-receptor usage and infection duration
We did not find an association between viral tropism and infection duration after diagnosis (p = 0.458). The median durations were 72 months for patients with R5-viruses and 76 months for non-R5- viruses. Additionally, there was no association between the viral tropism and viral load among naïve patients.
3.5.6. Co-receptor usage by viral subtype
One of the goals of this study was to estimate features of tropism among the subtype A variants circulating in Russia. Therefore, the R5/non-R5 co-receptor usage frequencies were calculated after stratifying patients by viral subtype. There was no significant difference between the two major groups (subtypes A and B). The differences between subtype A and the other subtypes or CRF were not significant because of the small group numbers.
3.6. Factors affecting viral tropism
After all we perform a bivariate logistic regression test for outcomes being R5 or non-R5 tropic to find the factors that are relevant for the two possible outcomes. In statistical model we include such factor as therapy experience, therapy change experience, CD4 cell count, route of infection and viral subtype.
The logistic regression model analysis shows the only factor that is significantly relevant for outcomes being R5 or non-R5 tropic is CD4 cell count (Table ).
Table 3. Factors associated with co-receptor usage
4. Discussion
The results of this study demonstrate that in Russian Federation most HIV-infected individuals carry R5 variants. In comparison with previous studies (Ataher et al., Citation2012; Brumme et al., Citation2005; Neogi et al., Citation2010), there is a high prevalence of non-R5-tropic HIV variants among the main subtypes present in Russia. This trend was observed in both naïve and ART-experienced patients. Because a genotypic, non-Trofile or ESTA-supported methodology was used in this study, the observed differences might have been due to circulating in Russia HIV-1 subtype A, which is genetically distinct from subtype A widespread in the rest of the world (Bobkova, Citation2013). R5-tropic variants of HIV prevailed over non-R5-tropic variants also for subtype B which is the second prevalent subtype in Russia (12.8%). Furthermore there is no significant differences in R5/non-R5- co-receptor usage frequencies between these subtypes.
The strongest association (p = 0.04) was found between viral tropism and the CD4 cell count; specifically, lower CD4 cell counts corresponded to a higher incidence of non-R5-tropic HIV variants. However, this relationship was not as strong as that reported in previous published papers (p ≤ 0.0001) (Gonzalez-Serna et al., Citation2010; Prosperi et al., Citation2010; Seclen et al., Citation2010; Svicher et al., Citation2010, Citation2012; Symons et al., Citation2012), and the differences between the groups were not significant. For example, in a study in Canada (Brumme et al., Citation2005), mean CD4 cell count for patients with R5-tropic variant were 290 (160–430) compared to 110 (30–260) non-R5-infected patients.
It should be noted that a lower CD4 cell count indicates a later HIV infection stage and longer infection duration (Levy, Citation1993). It can therefore be expected that the proportion of R5-tropic variants will decrease as the infection duration increases. Despite the correlation with CD4 cell counts, there was no correlation between the R5/non-R5 co-receptor usage frequencies and duration since first positive screening test. The absence of a significant correlation between the frequency of non-R5 variants and the duration since HIV diagnosis, it could be explained by the fact that the date of HIV diagnosis does not reflect the actual duration of infection. The studied cohort included 176 of 495 ART patients with CD4 350 ml (late presenters), which is characteristic of the HIV epidemic in Russia (Pokrovskaya et al., Citation2015). Furthermore it implies that emergence of X4-variants is related to damage of immune system (Berger, Murphy, & Farber, Citation1999), visible in low CD4 cell counts.
5. Conclusions
During patient enrollment in the study, treatment-experienced HIV-infected patients were stratified by treatment duration. Additionally, information was collected from the patients regarding changes in their courses of therapy, HIV infection duration and CD4 lymphocytes counts. Newly registered diagnostic kit used in the study, showed high efficiency for tropism determination. The results showed positive correlation of non-R5 variants and CD4 cell counts. But there was no dependence of R5/non-R5 co-receptor usage frequencies on presence/absence of a therapy change or HIV infection duration. This indicates a stabilizing of immune system and thus little emergence of X4 viruses. We found that 70.2% of HIV-1 strains were CCR5-tropic, and we suggest that CCR5-antagonists could be used in both naïve and experienced patients in Russia after determination of HIV tropism.
Funding
This study was supported by grant from viiv healthcare Russia [grant number WS2041679].
Ethical Approval
This study was approved by the institutional review board and the local ethics committee of the Central Research Institute for Epidemiology (№29 at 12/14/2011) and registered at clinicaltrials.gov (NCT01823614). All the patients signed an informed consent to participate in the study.
Acknowledgements
The authors thank Anna V. Valdokhina and Viktoria P. Bulanenko for their support in samples sequencing. The authors also thank the American Journal Experts team for their editing and formatting services.
Supplemental data
Supplemental data for this article can be accessed at http://dx.doi.org/10.1080/2331205X.2017.1311470.
OAMD_1311470_Supplementary_Material.pdf
Download PDF (225.1 KB)Additional information
Notes on contributors
Alexey Lopatukhin
The research activities of HIV research group in Central Research Institute of Epidemiology are focused on molecular epidemiology of HIV and include: analysis of HIV subtypes circulating in Russia, the identification of new HIV recombinant forms, HIV forensics using phylogenetic analysis, features of HIV epidemic in Russia. Research group are also involved in the development of national guidelines and recommendations on the provision of medical care to HIV infected persons. Presented study of HIV tropism, helped to determine the prospects of using CCR5 co-receptor antagonist drugs in Russia. To make our patients sample more representative and include the variants of HIV that circulates in different regions of Russia, our team was joined by experts from regional AIDS centers.
References
- Adults PoAGf and Adolescents. (2009). Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents (pp. 1–161). Bethesda, MD: Department of Health and Human Services.
- Ataher, Q., Portsmouth, S., Napolitano, L. A., Eng, S., Greenacre, A., Kambugu, A., … Tressler, R. (2012). The epidemiology and clinical correlates of HIV-1 co-receptor tropism in non-subtype B infections from India, Uganda and South Africa. Journal of the International AIDS Society, 15, 1–9.10.1186/1758-2652-15-2
- Berger, E. A., Murphy, P. M., Farber, J. M. (1999). Chemokine receptors as HIV-1 coreceptors: Roles in viral entry, tropism, and disease. Annual Review of Immunology, 17, 657–700.10.1146/annurev.immunol.17.1.657
- Bobkova, M. (2013). Current status of HIV-1 diversity and drug resistance monitoring in the former USSR. AIDS Reviews, 15, 204–212.
- Brumme, Z. L., Goodrich, J., Mayer, H. B., Brumme, C. J., Henrick, B. M., Wynhoven, B., … Harrigan, P. R. (2005). Molecular and clinical epidemiology of CXCR4‐using HIV‐1 in a large population of antiretroviral‐naive individuals. The Journal of Infectious Diseases, 192, 466–474.10.1086/jid.2005.192.issue-3
- Dauwe, K., Mortier, V., Schauvliege, M., Van Den Heuvel, A., Fransen, K., Servais, J. Y., … Verhofstede, C. (2015). Characteristics and spread to the native population of HIV-1 non-B subtypes in two European countries with high migration rate. BMC Infectious Diseases, 15, 1–524.
- Fabeni, L., Berno, G., Svicher, V., Ceccherini-Silberstein, F., Gori, C., Bertoli, A., … Pinnetti, C. (2015). Genotypic tropism testing in HIV-1 proviral DNA can provide useful information at low-level viremia. Journal of Clinical Microbiology, 53, 2935–2941.10.1128/JCM.00893-15
- Ferrer, P., Montecinos, L., Tello, M., Tordecilla, R., Rodríguez, C., Ferrés, M., … Afani, A. (2013). HIV-1 tropism: A comparison between RNA and proviral DNA in routine clinical samples from Chilean patients. Virology Journal, 10, 1.
- Gonzalez-Serna, A., Leal, M., Genebat, M., Abad, M. A., Garcia-Perganeda, A., Ferrando-Martinez, S., & Ruiz-Mateos, E. (2010). TROCAI (tropism coreceptor assay information): A new phenotypic tropism test and its correlation with trofile enhanced sensitivity and genotypic approaches. Journal of Clinical Microbiology, 48, 4453–4458.10.1128/JCM.00953-10
- Kravchenko, A. (2012). Use of maraviroc, the first CCR5 receptor antagonist, in HIV treatment regimens. Terapevticheskii arkhiv, 85, 125–129.
- Levy, J. A. (1993). HIV pathogenesis and long-term survival. AIDS, 7, 1401–1410.10.1097/00002030-199311000-00001
- Lopatukhin, A., Kireev, D., Kuevda, D., Shipulin, G., Luebke, N., & Kaiser, R. (2012, March 28–30), Validation of genotypic HIV-1 coreceptor tropism determination method in Russia and results comparison of two diverse labs. Abstracts in Reviews in Antiviral Therapy & Infectious Diseases, V.2:2012, Abstract P_62, p. 78. In 10th European Meeting on HIV & Hepatitis, Barcelona.
- Lopatukhin, A, Kireev, D, Poliakov, A, Bukin, E. K., Kaiser, R., Luebke, N., … Shipulin, G. A. (2013). Klinicheskaia laboratornaia diagnostika [The first experience of application of standardized genotype technique of identification of HIV-tropism]. 46–48.
- McGovern, R. A., Thielen, A., Mo, T., Dong, W., Woods, C. K., Chapman, D., … Harrigan, P. R. (2010). Population-based V3 genotypic tropism assay: a retrospective analysis using screening samples from the A4001029 and MOTIVATE studies. AIDS, 24, 2517–2525.10.1097/QAD.0b013e32833e6cfb
- Meini, G., Rossetti, B., Bianco, C., Ceccherini-Silberstein, F., Di Giambenedetto, S., Sighinolfi, L., … Zazzi, M. (2014). Longitudinal analysis of HIV-1 coreceptor tropism by single and triplicate HIV-1 RNA and DNA sequencing in patients undergoing successful first-line antiretroviral therapy. Journal of Antimicrobial Chemotherapy, 69, 735–741.10.1093/jac/dkt426
- Mulinge, M., Lemaire, M., Servais, J.-Y., Rybicki, A., Struck, D., da Silva, E. S., … Bercoff, D. P. (2013). HIV-1 tropism determination using a phenotypic env recombinant viral assay highlights overestimation of CXCR4-usage by genotypic prediction algorithms for CRRF01_AE and CRF02_AG. PLoS ONE, 8, e60566.10.1371/journal.pone.0060566
- Neogi, U., Prarthana, S. B., D’Souza, G., DeCosta, A., Kuttiatt, V. S., Ranga, U., & Shet, A. (2010). Co-receptor tropism prediction among 1045 Indian HIV-1 subtype C sequences: Therapeutic implications for India. AIDS Research and Therapy, 7, 24.10.1186/1742-6405-7-24
- Pokrovskaya, A. V., Kozyrina, N. V., Guschina, U. S., Yurin, O. G., Suvorova, Z. K., & Pokrovsky V. V. (2015, October 21–24). Portrait of the Patient study group. Lost opportunities for early ART. 15th European AIDS Conference, Barcelona.
- Poveda, E., Paredes, R., Moreno, S., Alcamí, J., Córdoba, J., Delgado, R., … Pulido, F. (2012). Update on clinical and methodological recommendations for genotypic determination of HIV tropism to guide the usage of CCR5 antagonists. AIDS Reviews, 14, 208–217.
- Prosperi, M. C., Bracciale, L., Fabbiani, M., Di Giambenedetto, S., Razzolini, F., Meini, G., … De Luca, A. (2010). Research Comparative determination of HIV-1 co-receptor tropism by Enhanced Sensitivity Trofile, gp120 V3-loop RNA and DNA genotyping.
- Seclen, E., Garrido, C., Gonzalez, M. D. M., González-Lahoz, J., de Mendoza, C., Soriano, V., & Poveda, E. (2010). High sensitivity of specific genotypic tools for detection of X4 variants in antiretroviral-experienced patients suitable to be treated with CCR5 antagonists. Journal of Antimicrobial Chemotherapy, 65, 1486–1492.10.1093/jac/dkq137
- Svicher, V., Alteri, C., Montano, M., D'Arrigo, R., Andreoni, M., Angarano, G., … Baldanti, F. (2012). Performance of genotypic tropism testing on proviral DNA in clinical practice: Results from the DIVA study group. New Microbiologica, 35, 17–25.
- Svicher, V., D’Arrigo, R., Alteri, C., Andreoni, M., Angarano, G., Antinori, A., … Borderi, M. (2010). Performance of genotypic tropism testing in clinical practice using the enhanced sensitivity version of Trofile as reference assay: Results from the OSCAR Study Group. New Microbiologica, 33, 195–206.
- Symons, J., Vandekerckhove, L., Paredes, R., Verhofstede, C., Bellido, R., Demecheleer, E., … Nijhuis, M. (2012). Impact of triplicate testing on HIV genotypic tropism prediction in routine clinical practice. Clinical Microbiology and Infection, 18, 606–612.10.1111/j.1469-0691.2011.03631.x
- Thomson, M. M., de Parga, E. V., Vinogradova, A., Sierra, M., Yakovlev, A., Rakhmanova, A., … Vega, Y. (2007). New Insights into the origin of the HIV type 1 subtype a epidemic in former soviet union's countries derived from sequence analyses of preepidemically transmitted viruses. AIDS Research and Human Retroviruses, 23, 1599–1604.10.1089/aid.2007.0166
- Vandekerckhove, L., Wensing, A., Kaiser, R., Brun-Vezinet, F., Clotet, B., De Luca, A., … Korn, K. (2011). European guidelines on the clinical management of HIV-1 tropism testing. The Lancet Infectious Diseases, 11, 394–407.10.1016/S1473-3099(10)70319-4
- Vasil’ev A., Kazennova E., & Bobkova M. (2010). Analysis of the prevalence of CCR5 coreceptor antagonist resistance mutations among HIV-1 variants in Russia. Voprosy virusologii, 56, 32–37.