Abstract
Parkinson’s disease represents one of the most common chronic neurodegenerative diseases in the elderly caused by a reduction of dopamine levels in the brain. The number of people with Parkinson’s disease is expected to grow mostly due to an increase in the aging population. Parkinsonian patients often require complicated dosing or titration schedules and often have multiple comorbidities that necessitate administration of therapies from multiple drug classes. Here we report two cases of parkinsonian patients who suffered acute pancreatitis. The first case presentation covers drug-induced acute pancreatitis in a patient receiving multiple medications, while the second case reveals the complexity of parkinsonian patient treatment in the clinical context of severe recurrent cholecystic acute pancreatitis. Conclusion: We can conclude that the management of Parkinson’s disease and coexistent health problems is extremely sensitive and requires a multidisciplinary team approach. It is therefore important to recognize and address the disease-specific challenges that may affect the optimal hospital care for patients with Parkinson’s disease.
Public Interest Statement
Elderly patients with Parkinson’s disease often require complicated dosing or titration schedules and often have multiple comorbidities that necessitate administration of therapies from multiple drug classes.
Main causes of pancreatitis are gallstones and alcohol abuse, but also important are medications and toxins, high levels of calcium in the blood and hyperparathyroidism, high levels of triglycerides in the blood, infections and other factors.
Here we report two cases of parkinsonian patients who suffered acute pancreatitis. The first case presentation covers drug-induced acute pancreatitis in a patient receiving multiple medications, while the second case reveals the complexity of parkinsonian patient treatment in the clinical context of severe recurrent cholecystic acute pancreatitis. We can conclude that the management of Parkinson’s disease and coexistent health problems is extremely sensitive and requires a multidisciplinary team approach.
Competing Interests
I declare that in the past three years I have not received reimbursements, fees, funding, or salary from an organization that may in any way gain or lose financially from the publication of this manuscript, either now or in the future.
I declare that I do not hold any stocks or shares in an organization that may in any way gain or lose financially from the publication of this manuscript, either now or in the future.
I declare I do not have any other financial competing interests.
1. Introduction
Acute pancreatitis (AP) represents a diffuse systemic immuno-inflammatory response to a localized process of autodigestion within the pancreatic gland, caused by a premature activation of proteolytic digestive enzymes. It can range in intensity from a mild self-limiting disease to a severe disorder with prolonged hospitalization and high morbidity of complications (Harper & Cheslyn-Curtis, Citation2011; Sheu, Furlan, Almusa, Papachristou, Bae, Citation2012). Gallstones and alcohol abuse still represent the two main causes of pancreatitis, accounting for almost 80% of hospital admissions for acute pancreatitis (Sekimoto et al., Citation2006). The list of other causes of pancreatitis is steadily growing, and some of them are nowadays thought to be increasingly important, such as medications and toxins, high levels of calcium in the blood and hyperparathyroidism, high levels of triglycerides in the blood, infections, damage to the pancreas from surgery or endoscopy or from blunt or penetrating injuries, tumors of the pancreas, vascular causes, hereditary pancreatitis, kidney transplantation, abnormalities of the pancreas or intestine, and obesity (Chawla, Atten, & Attar, Citation2011; Federico, Falconi, Zuodar, Falconieri, & Puglisi, Citation2011; Frossard, Steer, & Pastor, Citation2008; Schneider, Büchler, & Werner, Citation2010; Sekimoto et al., Citation2006; Waldthaler, Schütte, & Malfertheiner, Citation2010).
In terms of population aging and progress of medicine the more links in the organism are damaged, the more pharmacological efforts are needed for a productive effect on the damaged system. In this context, drug-related acute pancreatitis is definitely gaining relevance. The estimated incidence of drug-induced acute pancreatitis varies from 1.4% in a retrospective study conducted in Germany and published in 1995 (Lankisch, Droge, & Gottesleben, Citation1995), to 3.4% in a more recent study performed in Australia in 2011 (Badalov et al., Citation2007; Barreto, Tiong, & Williams, Citation2011). According to recent data, a definite causality for acute pancreatitis has been established for 31 drugs, with the highest hazard ratios for mesalazine, azathioprine, and simvastatin (Nitsche, Jamieson, Lerch, & Mayerle, Citation2010).
Neuroleptic malignant syndrome (NMS) is seen in patients treated for Parkinson’s disease (PD) in the setting of withdrawal of L-Dopa or dopamine agonist therapy, as well as with dose reductions and a switch from one agent to another (Shalev, Hermes, & Munitz, Citation1989; Velamoor, Citation1998; Wu, Kan, & Yang, Citation2011). Infection and surgery are also possible precipitants of this potentially fatal disorder (Onofrj & Thomas, Citation2005; Serrano-Dueñas, Citation2003).
Here we present two distinct cases of acute pancreatitis in parkinsonian patients. The first patient, who had been treated with multiple medications, suffered from drug-induced pancreatitis. The second parkinsonian patient in an attack of severe recurrent cholecystic acute pancreatitis developed neuroleptic malignant-like syndrome caused by unintentional withdrawal of antiparkinsonian medication due to poor intestinal absorption.
2. Case presentation
2.1. Patient 1
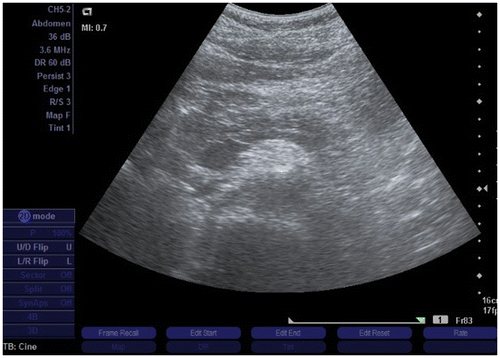
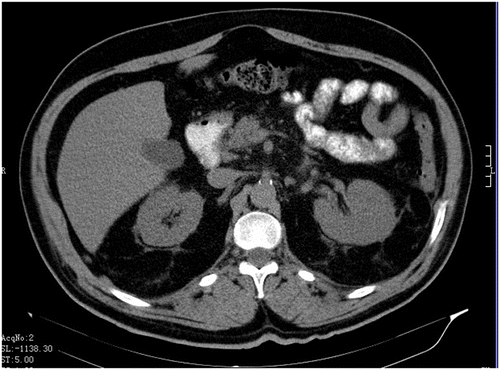
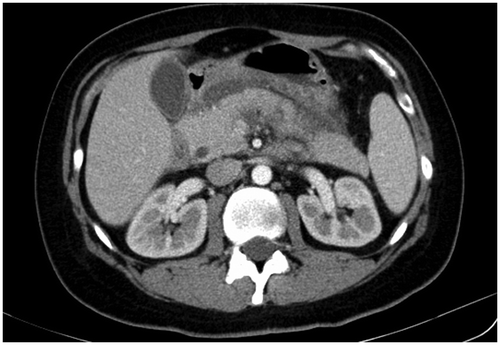
A 76-year-old male parkinsonian patient was admitted to hospital due to a progression of extrapyramidal symptoms. Besides, his medical history included hypertension, diabetes, hyperlipidemia, and ischemic stroke twelve years before. He had been regularly taking levodopa/carbidopa and entacapone for eight years, ropinirole in a dose of 10 mg for more than one year, 100 mg of acetylsalicylic acid daily for more than ten years, perindopril in a dose of 10 mg for two years, amlodipine in a dose of 10 mg for two years, glimepiride in a daily dose of 4 mg for more than 5 years, diclofenac for occasional back pain, as well as simvastatin in a dose of 20 mg for more than 5 years. He had no history of biliary and pancreatic disease or excessive alcohol consumption, and his triglyceride values were within normal limits. On the second day of hospitalization the patient received increased doses of the ropinirole, 12 mg instead of the prior 8-mg dose, in order for a more effective control of the extrapyramidal symptoms. On the fifth day of his stay on the ward the patient received a daily dose of 75 mg of diclofenac intramuscularly due to low back pain. On the seventh day of hospitalization the patient experienced excruciating epigastric pain along with a significant increase of his serum amylase and lipase levels (Table ). Imaging scans that were taken did not show biliary tract dilatation or calculi. The pancreas and surrounding tissue were edematous, but without necrosis or effusions (Figures and ). The patient was diagnosed with presumptive drug-induced pancreatitis and classed as grade 1 using Ranson’s criteria (Ranson et al., Citation1974). With supportive treatment, analgesics, parenteral rehydration, gastric acid suppression, and caloric supplementation, the patient’s symptoms subsided and he had a favorable clinical course consistent with serous pancreatitis. He was discharged home with the same medications with the exception of diclofenac. In ten years subsequent medical monitoring, he has never experienced signs of recurrent pancreatitis.
Table 1. Laboratory data: Patient 1
2.2. Patient 2
The patient was an 80-year-old female diagnosed with PD, who presented with severe abdominal pain and vomiting requiring hospital admission. Her PD symptoms had been treated with levodopa in a dose of 625 mg daily, entacapone in a daily dose of 400 mg, and ropinirole in a dose of 12 mg. She was able to walk and her cognitive functions showed no significant deteriorations. The patient was presented to the emergency department with profuse epigastric abdominal pain. The laboratory investigations are presented in Table . The patient was classed as grade 2 pancreatitis using Ranson’s criteria (Ranson et al., Citation1974). Biliary ultrasound showed acute cholecystitis. The patient refused surgery and was treated conservatively with antibiotics and pain control with a continuation of the antiparkinsonian therapy. Gradual improvement ensued. Two years later, the patient presented to the emergency room again with clinical and biochemical signs of recurrent cholecystic pancreatitis, Ranson grade 3 (Ranson et al., Citation1974). Imaging studies of the abdomen showed signs of cholecystopancreatitis with enlarged gallbladder, thickened wall, and pancreatic and peripancreatic edema. (Figures –). The patient received a nasogastric tube and was admitted to the intensive care unit. She continued to take her usual peroral antiparkinsonian medication. On the third day of hospitalization she showed clinical deterioration. She became somnolent, febrile up to 40°C, with generalized muscular rigidity and profuse diaphoresis. Vital signs monitoring showed tachycardia, with labile blood pressure and tachypnea.
Table 2. Laboratory data: Patient 2
Figure 2a. Abdominal ultrasound: image showing signs of cholecystopancreatitis with enlarged gallbladder and thickened wall. Fluid is seen adjacent to the gallbladder. Numerous gallstones are present within, the largest at 6 mm.
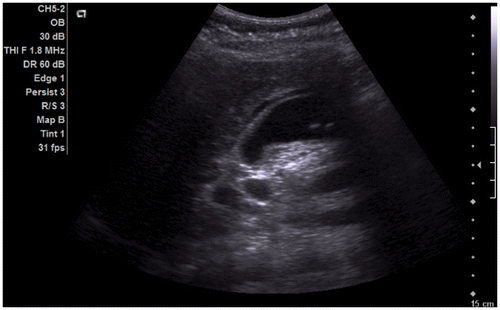
Figure 2b. Abdominal ultrasound: axial image showing the hypoechogenic pancreatic head. The transverse diameter of the pancreatic head is 2.89 cm.
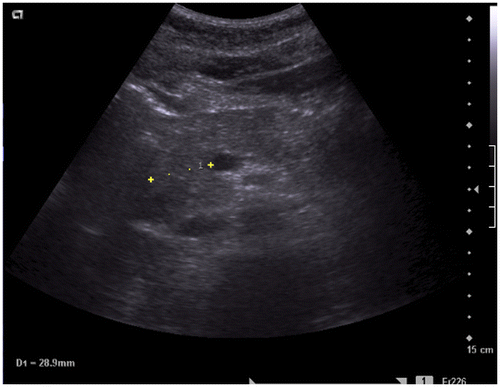
Figure 2c. Multi-slice CT scan of abdomen: the hypoechogenic, enlarged pancreas, particularly its head.
The medical history of antiparkinsonian drug potential withdrawal due to poor absorption with the typical clinical findings and laboratory data (Table ) indicated the development of neuroleptic malignant-like syndrome. Initial treatment was focused on preventing myoglobin precipitation in the urine by inducing and maintaining a brisk diuresis. Immediately administered saline for light myoglobinuria and rhabdomyolysis ameliorated the initial acute kidney injury. The patient showed clinical improvement and at discharge from hospital she was able to walk with the help of another person. She expressed moderate cognitive deterioration on cognitive function tests.
3. Discussion
One of the first reports of drug-induced acute pancreatitis was published in the 1950s, when it was suggested that cortisone could induce acute pancreatitis (Zion, Goldberg, & Suzman, Citation1955). Since that time, the list of drugs associated with acute pancreatitis has been constantly increasing. If we take into account the small number of patients who develop pancreatitis compared to the relatively large number of prescription medications, drug-induced pancreatitis occurs relatively rarely. The proposed pathophysiological mechanisms include hypersensitivity reactions, toxic effects of drug metabolites, and other potential mechanisms in patients with a yet unknown predisposition.
We have reported a case of acute clinically mild pancreatitis in a parkinsonian patient who received multiple medications. First, we ruled out the most common causes of acute pancreatitis, including gallstones, alcohol consumption, hypertriglyceridemia, anatomic anomalies including pancreas divisum, infection, chronic hypercalcemia, and tumors.
According to recent investigations (Badalov et al., Citation2007) drug-induced pancreatitis is usually mild.
Levodopa provides the greatest antiparkinsonian benefit for motor signs and symptoms. Considering acute pancreatitis, there is no clear-cut causative relationship in the present literature between levodopa or carbidopa in Parkinsonian patients (Brooks, Citation2008).
Ropinirole is a selective non-ergoline dopamine D2-like receptor agonist commonly used to treat the symptoms of Parkinson’s disease (Eden et al., Citation1991; Pahwa et al., Citation2007). So far, ropinirole hydrochloride has been administered to 1,599 individuals in clinical trials. In the official US Food and Drug Administration (FDA) information on ropinirole, acute pancreatitis is included neither in the treatment-emergent adverse events list for patients with early Parkinson’s disease, nor for advanced Parkinson disease patients treated with ropinirole hydrochloride (http://www.drugs.com/pro/ropinirole.html). According to published investigations, ropinirole was suspected as a possible cause of pancreatitis only according to a data in the Kaurich review published in 2008 (Kaurich, Citation2008).
Our first patient had a more than 5-year history of using simvastatin in a dose of 20 mg. So far, several hundreds of studies have been conducted on the adverse effects of 3-hydroxy-3 methyl-glutaryl-CoA reductase (HMG-CoA) reductase inhibitors, known as statins, a class of drugs widely used to treat high level of lipids in the blood. Reports of statin-induced acute pancreatitis indicate fluvastatin, rosuvastatin, atorvastatin, lovastatin, simvastatin, and pravastatin as possible causative agents (Anagnostopoulos, Tsiakos, Margantinis, Kostopoulos, & Arvanitidis, Citation2003; Johnson & Loomis, Citation2006; Tysk, Al-Eryani, & Shawabkeh, Citation2002). The Singh and Lokes systematic review of observational studies and spontaneous case reports has showed that pancreatitis can occur at both high and low doses. Statin-induced pancreatitis can occur at any time but seems to be very uncommon early on and more likely to occur after many months of therapy. There is no cumulative dose effect, and increasing age does not appear to be a major susceptibility factor (Singh & Loke, Citation2006).
Our first patient had been receiving a daily dose of 10 mg perindopril, a long-acting angiotensin-converting-enzyme inhibitor (ACE inhibitor), in a fixed-dose combination with the antihypertensive drug amlodipin in a 10 mg dosage. The duration of therapy with this dosage combination was two years. The case reports of ACE inhibitor-induced AP involve perindopril, benazepril, captopril, cilazapril, enalapril, lisinopril, fosinopril, quinapril, and ramipril (Eland et al., Citation2006; Famularo, Minisola, Nicotra, & De Simone, Citation2005; Iliopoulou, Giannakopoulos, Pagoy, Christos, & Theodore, Citation2001; Kaurich, Citation2008; Muchnick & Mehta, Citation1999). The results of a European case-control study that included 724 patients with acute pancreatitis indicated that ACE inhibitors were associated with an increased risk of developing AP and that a higher risk of AP occurred within the first 6 months of initiating therapy and with higher doses of ACE inhibitors (Eland et al., Citation2006). The results of the European case-control study (Eland et al., Citation2006) indicated that, in addition to ACE inhibitors, calcium channel blockers are associated with an elevated risk of acute pancreatitis. The study did not prove an apparent dose response or duration response.
The most common treatment approach in type 2 diabetes is administration of oral sulfonylureas, such as glibenclamide and glimepiride. The use of glimepiride was associated with a raised risk of acute pancreatitis in several reports (Duboeuf, De Widerspach-Thor, Scotto, & Bacq, Citation2004; Werth, Kuhn, Hartmann, & Reinhart, Citation1995).
In addition to increased doses of ropinirole, during his hospital stay our first patient received diclofenac regularly, unlike to previous periodic sampling. Although pancreatitis is listed as a rare complication of chronic nonsteroidal anti-inflammatory drug (NSAID) use, there are several evidences of a possible etiological connection between salicylate and pancreatitis (Antonopoulos et al., Citation2005; Khan & Edward, Citation1993; Miltiadous, Anthopoulou, & Elisaf, Citation2003; Trivedi & Pitchumoni, Citation2005). In the work of Khan and Edward (Khan & Edward, Citation1993) it has been suggested that the incidence of NSAID-caused pancreatitis is probably underestimated because patients with less severe symptoms are not tested routinely for raised serum amylase. The authors argue that abdominal pain, vomiting, and nausea are often reported by patients using NSAIDs so that these symptoms are often regarded as manifestations of gastritis, or peptic ulceration, and not as pancreatitis.
Drug-induced pancreatitis is a clinical entity generally difficult to establish due to common polypragmatism, absence of specific statistical and experimental data concerning drug involvement in pancreatic inflammation, and excluding other possible causes and reporting the event. Parkinsonian patients are distinctive in multidrug treatment for at least two reasons. One reason is that most parkinsonian patients are diagnosed at 60 years of age or older, which makes it common for many of them to have multiple co-morbidities and consequently be on multiple medications. Furthermore, despite successful treatment the symptoms progressively worsen over time, so the patient will receive more antiparkinsonian drugs. In such conditions it is normally difficult to establish which particular drug in a caused a drug-induced acute pancreatitis episode.
In our first patient, probably except for levodopa, almost all the drugs he was taking could have theoretically been the cause of iatrogenic pancreatitis.
Physicians treating patients with PD and pancreatitis face another distinct problem. In some protocols nasogastric decompression is essential, oral intake is prohibited, and antisecretory drugs are used in attempts to reduce gastrointestinal and pancreatic secretion (Rünzi & Layer, Citation1999; UK Guidelines, Citation2005). People with PD undergoing acute pancreatitis management and surgery are at increased risk owing to their condition and to potential omission of medication. There is a spectrum of complications associated with the withdrawal of dopamine agonist and levodopa medications. The most serious of these is neuroleptic malignant-like syndrome (NMLS), associated with fever, confusion, raised concentrations of muscle enzyme, and even death (Adnet, Lestavel, & Krivosic-Horber, Citation2000; Gordon & Frucht, Citation2001). In this context, the condition has been termed the parkinsonism-hyperpyrexia syndrome (Brennan & Genever, Citation2010; Factor, Citation2007; Newman, Grosset, & Kennedy, Citation2009).
Several investigative groups have demonstrated that nasogastric decompression does not appear to alter the course or outcome of a pancreatitis attack, although it may provide for greater patient comfort during the early stages when nausea and vomiting are common (Case, Citation1998; Eatock et al., Citation2005; Gerlach, Winogrodzka, & Weber, Citation2011). Recent studies have demonstrated that most patients with pancreatitis, including those with severe pancreatitis, can actually tolerate small amounts of enterally administered nutrients (Modena, Cevasco, Basto, Vicuna, & Ramirez, Citation2006). Pancreatitis infections are believed to occur because gut bacteria are translocated across the injured bowel wall adjacent to areas of the pancreatic injury. Theoretically, enteral nutrition exerts a trophic effect on the injured bowel wall that could reduce this translocation and, thus, reduce the incidence of pancreatitis infections. Studies evaluating this concept are currently underway, but even in the absence of definitive results, a growing number of specialists prefer administration of trophic amounts of nutrients to patients with severe pancreatitis and begin that treatment within 72 h of hospitalization (Marik & Zaloga, Citation2004). However, we still need to take particular care of possible changes in the level of intestinal drug absorption, which have been proven to be very important in treating patients with PD. Normally, levodopa is rapidly absorbed from the proximal small intestine (Nutt & Fellman, Citation1984). Today the most commonly used dopamine agonists, pramipexole and ropinirole in conventional peroral formulations, are quickly absorbed from the gastrointestinal tract and peak plasma concentrations are reached within about 2 h in fasting patients and in about 3 h when given with food (Kvemmo, Härtter, & Burger, Citation2006). Some new formulations of known dopamine agonist molecules in the form of prolonged-release tablets must be swallowed whole and must not be chewed, crushed, or divided. Following oral administration of ropinirole prolonged-release tablets, plasma concentrations increase slowly, with a median time to Cmax generally achieved between 6 and 10 h (Nashatizadeh, Lyons, & Pahwa, Citation2009). If patients receive medication through a nasogastric tube, they should be switched from prolonged-release ropinirole tablets to immediate-release tablets according to the scheme described by the manufacturer. In circumstances where the bowel may cause problems with drug absorption and thus lead to a deterioration in the motor symptoms of PD, a better option might be careful and accurate switching to an available alternative route of drug intake. Levodopa can be given intravenously if both oral and nasogastric feeding are contraindicated (Quinn, Marsden, & Parkes, Citation1982; Rosin, Devereux, Eng, Calne, Citation1979); options for dopamine agonists include transdermal rotigotine and apomorphine by injection or continuous infusion (Chen, Swope, Dashtipour, & Lyons, Citation2009; Contin, Riva, Albani, & Baruzzi, Citation1996; LeWitt, Boroojerdi, & MacMahon, Citation2007).
4. Clinical implications
Optimizing drug therapy is an essential part of caring for a parkinsonian patient. In the treatment of elderly patients with Parkinson’s disease, physicians are often faced with at least two challenges, the first of which is the problem of polypharmacy, of particular concern in older people who, compared to younger individuals, tend to have more disease conditions for which therapies are prescribed. The second important problem are co-morbidities in older individuals that may significantly change the conditions of PD treatment. We can conclude that parkinsonian patients require input from a wide range of professionals and only the early involvement of a multidisciplinary team has physical and psychological benefits.
| List of abbreviations | ||
| AP | = | Acute pancreatitis |
| NMS | = | Neuroleptic malignant syndrome |
| PD | = | Parkinson’s disease |
| CK | = | Creatine kinase |
| FDA | = | Food and drug administration |
| WHO | = | World Health Organisation |
| WHOART | = | World Health Organisation adverse reactions terminology |
| HMG-CoA | = | 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitors |
| ACE inhibitor | = | Angiotensin-converting-enzyme Inhibitor |
| NSAID | = | Nonsteroidal anti-inflammatory drug |
| WBC | = | White blood cells |
| BUN | = | Blood urea nitrogen |
| AST | = | Aspartate aminotransferase |
| ALT | = | Alanine transaminase |
| ALP | = | Alkaline phosphatase |
| GGT | = | Gamma-glutamyl transferase |
| LDH | = | Lactate dehydrogenase |
Consent
Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Authors’ contributions
Sanja Kovacic has made substantial contributions to conception and design.
Sanja Kovacic, Sinisa Roginic, Johann Nemrava, Maida Seferovic Saric and Ksenija Gospocic have made substantial contributions acquisition of data, analysis and interpretation of data (Sanja Kovacic, Sinisa Roginic, Johann Nemrava and Maida Seferovic Saric for clinical data, and Ksenija Gospocic for radiological data).
Kresimir Luetic have been involved in drafting the manuscript or revising it critically for important intellectual content.
All authors have given final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Each author have participated sufficiently in the work to take public responsibility for appropriate portions of the content.
Additional information
Funding
Notes on contributors
Sanja Kovacic
Sanja Kovacic graduated in 1995, along with clinical work with patients, She has been involved in ongoing basic and clinical research work. Long-standing commitment to scientific work has led her to become the head of the Scientific Research Unit in the General Hospital Zabok in 2010. In this position, her general task is to help young colleagues in the design and implementation of research. In her research career, she has published a series of papers and participated in conferences and courses as an invited lecturer. In 2011, she acquired her PhD degree. Alongside her research work, she has never given up on clinical work and patients have always been her main subject of interest. Every patient in some way represented a scientific challenge.
References
- Adnet, P., Lestavel, P., & Krivosic-Horber, R. (2000). Neuroleptic malignant syndrome. British Journal of Anaesthesia, 85, 129–135.10.1093/bja/85.1.129
- Anagnostopoulos, G. K., Tsiakos, S., Margantinis, G., Kostopoulos, P., & Arvanitidis, D. (2003). Acute pancreatitis due to pravastatin therapy. JOP, 4, 129–132.
- Antonopoulos, S., Mikros, S., Kokkoris, S., Protopsaltis, J., Filioti, K., Karamanolis, D., & Giannoulis, G. (2005). A case of acute pancreatitis possibly associated with combined salicylate and simvastatin treatment. JOP, 6, 264–268.
- Badalov, N., Baradarian, R., Iswara, K., Li, J., Steinburg, W., & Tenner, S. (2007). Drug-induced acute pancreatitis: An evidence-based review. Clinical Gastroenterology and Hepatology, 5, 648–661.10.1016/j.cgh.2006.11.023
- Barreto, S. G., Tiong, L., & Williams, R. (2011). Drug-induced acute pancreatitis in a cohort of 328 patients. A single-centre experience from Australia. JOP, 12, 581–585.
- Brennan, K. A., & Genever, R. W. (2010). Managing Parkinson’s disease during surgery. BMJ, 341, c5718.10.1136/bmj.c5718
- Brooks, D. J. (2008). Optimizing levodopa therapy for Parkinson’s disease with levodopa/carbidopa/entacapone: Implications from a clinical and patient perspective. Neuropsychiatric Disease and Treatment, 4, 39–47.10.2147/NDT
- Case, R. M. (1998). Pancreatic exocrine secretion: Mechanisms and control. In H. G. Beger, A. W. Warshaw, & M. W. Buchler (Eds.), The pancreas (pp. 63–100). Oxford: Blackwell Science.
- Chawla, S., Atten, M. J., & Attar, B. M. (2011). Acute pancreatitis as a rare initial manifestation of Wegener’s granulomatosis. A case based review of literature. JOP, 12, 167–169.
- Chen, J. J., Swope, D. M., Dashtipour, K., & Lyons, K. E. (2009). Transdermal rotigotine: A clinically innovative dopamine-receptor agonist for the management of Parkinson’s disease. Pharmacotherapy, 29, 1452–1467.
- Contin, M., Riva, R., Albani, F., & Baruzzi, A. (1996). Pharmacokinetic optimisation in the treatment of Parkinson’s disease. Clinical Pharmacokinetics, 30, 463–481.10.2165/00003088-199630060-00004
- Duboeuf, T., De Widerspach-Thor, A., Scotto, B., & Bacq, Y. (2004). Acute glimepiride-induced pancreatitis. Gastroentérologie Clinique et Biologique, 28, 409–410.10.1016/S0399-8320(04)94947-0
- Eatock, F. C., Chong, P., Menezes, N., Murray, L., McKay, C. J., Carter, C. R., & Imrie, C. W. (2005). A randomized study of early nasogastric versus nasojejunal feeding in severe acute pancreatitis. The American Journal of Gastroenterology, 100, 432–439.10.1111/ajg.2005.100.issue-2
- Eden, R. J., Costall, B., Domeney, A. M., Gerrard, P. A., Harvey, C. A., Kelly, M. E., ... Wright, A. (1991). Preclinical pharmacology of ropinirole (SK&F 101468-A) a novel dopamine D2 agonist. Pharmacology Biochemistry and Behavior, 38, 147–154.10.1016/0091-3057(91)90603-Y
- Eland, I. A., Sundström, A., Velo, G. P., Andersen, M., Sturkenboom, M. C., Langman, M. J., … For the EDIP Study Group of the European Pharmacovigilance Research Group. (2006). Antihypertensive medication and the risk of acute pancreatitis: The European case-control study on drug-induced acute pancreatitis (EDIP). Scandinavian Journal of Gastroenterology, 41, 1484–1490.10.1080/00365520600761676
- Factor, S. A. (2007). Fatal Parkinsonism-hyperpyrexia syndrome in a Parkinson’s disease patient while actively treated with deep brain stimulation. Movement Disorders, 22, 148–149.10.1002/(ISSN)1531-8257
- Famularo, G., Minisola, G., Nicotra, G. C., & De Simone, C. (2005). Diosyncratic pancreatitis associated with perindopril. JOP, 6, 605–607.
- Federico, E., Falconi, M., Zuodar, G., Falconieri, G., & Puglisi, F. (2011). B-cell lymphoma presenting as acute pancreatitis. Pancreatology, 11, 553–556.10.1159/000332038
- Frossard, J. L., Steer, M. L., & Pastor, C. M. (2008). Acute pancreatitis. The Lancet, 371, 143–152.10.1016/S0140-6736(08)60107-5
- Gerlach, O. H., Winogrodzka, A., & Weber, W. E. (2011). Clinical problems in the hospitalized Parkinson’s disease patient: Systematic review. Movement Disorders, 26, 197–208.
- Gordon, P. H., & Frucht, S. J. (2001). Neuroleptic malignant syndrome in advanced Parkinson’s disease. Movement Disorders, 16, 960–962.10.1002/mds.v16:5
- Harper, S. J., & Cheslyn-Curtis, S. (2011). Acute pancreatitis. Annals of Clinical Biochemistry, 48, 23–37.10.1258/acb.2010.010196
- Iliopoulou, A., Giannakopoulos, G., Pagoy, H., Christos, T., & Theodore, S. (2001). Case report: Acute pancreatitis due to captopril treatment. Digestive Diseases and Sciences, 46, 1882–1883.10.1023/A:1010674812573
- Johnson, J. L., & Loomis, I. B. (2006). A case of simvastatin-associated pancreatitis and review of statin-associated pancreatitis. Pharmacotherapy, 26, 414–422.10.1592/phco.26.3.414
- Kaurich, T. (2008). Drug-induced acute pancreatitis. Proceedings of the Baylor University Medical Center, 21, 77–81.
- Khan, I. H., & Edward, N. (1993). Pancreatitis associated with diclofenac. Postgraduate Medical Journal, 69, 486–487.10.1136/pgmj.69.812.486
- Kvemmo, T., Härtter, S., & Burger, E. (2006). A review of the receptor-binding and pharmacokinetic properties of dopamine agonists. Clinical Therapeutics, 28, 1065–1078.
- Lankisch, P. G., Droge, M., & Gottesleben, F. (1995). Drug induced acute pancreatitis: Incidence and severity. Gut, 37, 565–567.10.1136/gut.37.4.565
- LeWitt, P. A., Boroojerdi, B., & MacMahon, D. (2007). Overnight switch from oral dopaminergic agonists to transdermal rotigotine patch in subjects with Parkinson’s disease. Clinical Neuropharmacology, 30, 256–265.10.1097/wnf.0b013e318154c7c4
- Marik, P. E., & Zaloga, G. P. (2004). Meta-analysis of parenteral nutrition versus enteral nutrition in patients with acute pancreatitis. BMJ, 328, 1407–1412.10.1136/bmj.38118.593900.55
- Miltiadous, G., Anthopoulou, A., & Elisaf, M. (2003). Acute pancreatitis possibly associated with combined salicylate and atorvastatin therapy. JOP, 4, 20–21.
- Modena, J. T., Cevasco, L. B., Basto, C. A., Vicuna, A. O., & Ramirez, M. P. (2006). Total enteral nutrition as prophylactic therapy for pancreatic necrosis infection in severe acute pancreatitis. Pancreatology, 6, 58–64.10.1159/000090024
- Muchnick, J. S., & Mehta, J. L. (1999). Angiotensin-converting enzyme inhibitor-induced pancreatitis. Clinical Cardiology, 22, 50–51.10.1002/clc.v22:1
- Nashatizadeh, M. M., Lyons, K. E., & Pahwa, R. (2009). A review of ropinirole prolonged release in Parkinson’s disease. Clinical Interventions in Aging, 4, 179–186.
- Newman, E. J., Grosset, D. G., & Kennedy, P. G. (2009). The Parkinsonism-hyperpyrexia syndrome. Neurocritical Care, 10, 136–140.10.1007/s12028-008-9125-4
- Nitsche, C. J., Jamieson, N., Lerch, M. M., & Mayerle, J. V. (2010). Drug induced pancreatitis. Best Practice & Research Clinical Gastroenterology, 24, 143–155.10.1016/j.bpg.2010.02.002
- Nutt, J. G., & Fellman, J. H. (1984). Pharmacokinetics of levodopa. Clin Neuropharmacol, 7, 35–39.
- Onofrj, M., & Thomas, A. (2005). Acute akinesia in Parkinson disease. Neurology, 64, 1162–1169.10.1212/01.WNL.0000157058.17871.7B
- Pahwa, R., Stacy, M. A., Factor, S. A., Lyons, K. E., Stocchi, F., Hersh, B. P., & EASE-PD Adjunct Study Investigators. (2007). Ropinirole 24-h prolonged release: Randomized, controlled study in advanced Parkinson disease. Neurology, 68, 1108–1115.10.1212/01.wnl.0000258660.74391.c1
- Quinn, N., Marsden, C. D., & Parkes, J. D. (1982). Complicated response fluctuations in Parkinson’s disease: Response to intravenous infusion of levodopa. The Lancet, 320, 412–415.10.1016/S0140-6736(82)90442-1
- Ranson, J. H., Rifkind, K. M., Roses, D. F., Fink, S. D., Eng, K., & Spencer, F. C. (1974). Prognostic signs and the role of operative management in acute pancreatitis. Surgery, Gynecology & Obstetrics, 139, 69–81.
- Rosin, A. J., Devereux, D., Eng, N., Calne, D. B. (1979). Parkinsonism with ‘on-off’ phenomena. Intravenous treatment with levodopa after major abdominal surgery. Archives of Neurology, 36, 32–34.10.1001/archneur.1979.00500370062014
- Rünzi, M., & Layer, P. (1999). Nonsurgical management of acute pancreatitis: Use of antibiotics. Surgical Clinics of North America, 79, 759–765.
- Schneider, L., Büchler, M. W., & Werner, J. (2010). Acute pancreatitis with an emphasis on infection. Infectious Disease Clinics of North America, 24, 921–941.
- Sekimoto, M., Takada, T., Kawarada, Y., Hirata, K., Mayumi, T., Yoshida, M., ... Koizumi, S. (2006). JPN Guidelines for the management of acute pancreatitis: Epidemiology, etiology, natural history, and outcome predictors in acute pancreatitis. Journal of Hepato-Biliary-Pancreatic Surgery, 13, 10–24.10.1007/s00534-005-1047-3
- Serrano-Dueñas, M. (2003). Neuroleptic malignant syndrome-like, or – dopaminergic malignant syndrome – to levodopa therapy withdrawal. Clinical features in 11 patients. Parkinsonism & Related Disorders, 9, 175–178.10.1016/S1353-8020(02)00035-4
- Shalev, A., Hermes, H., & Munitz, H. (1989). Mortality from neuroleptic malignant syndrome. The Journal of Clinical Psychiatry, 50, 18–25.
- Sheu, Y., Furlan, A., Almusa, O., Papachristou, G., Bae, K. T. (2012). The revised Atlanta classification for acute pancreatitis: A CT imaging guide for radiologists. Emergency Radiology, 19, 237–243.10.1007/s10140-011-1001-4
- Singh, S., & Loke, Y. K. (2006). Statins and pancreatitis: A systematic review of observational studies and spontaneous case reports. Drug Safety, 29, 1123–1132.10.2165/00002018-200629120-00004
- Singh, S., Nautiyal, A., & Dolan, J. G. (2004). Recurrent acute pancreatitis possibly induced by atorvastatin and rosuvastatin. Is statin induced pancreatitis a class effect? JOP, 5, 502–504.
- Trivedi, C. D., & Pitchumoni, C. S. (2005). Drug-induced pancreatitis: An update. Journal of Clinical Gastroenterology, 39, 709–716.10.1097/01.mcg.0000173929.60115.b4
- Tysk, C., Al-Eryani, A. Y., & Shawabkeh, A. A. (2002). Acute pancreatitis induced by fluvastatin therapy. Journal of Clinical Gastroenterology, 35, 406–408.10.1097/00004836-200211000-00010
- UK Guidelines for the Management of Acute Pancreatitis. (2005). Working party of the British society of gastroenterology, association of surgeons of Great Britain and Ireland, pancreatic society of Great Britain and Ireland, association of Upper GI surgeons of Great Britain and Ireland. Gut, 54, 1–9.
- Velamoor, V. R. (1998). Neuroleptic malignant syndrome. Recognition, prevention and management. Drug Safety, 19, 73–82.10.2165/00002018-199819010-00006
- Waldthaler, A., Schütte, K., & Malfertheiner, P. (2010). Causes and mechanisms in acute pancreatitis. Digestive Diseases, 28, 364–372.10.1159/000319416
- Werth, B., Kuhn, M., Hartmann, K., & Reinhart, W. H. (1995). Medikamentos induzierte Pankreatitiden: Erfahrungen der Schweizerischen Arzneimittel-Nebenwirkungszentrale (SANZ) 1981–1993. Schweiz Med Wochenschr, 125, 731–734.
- Wu, Y. F., Kan, Y. S., & Yang, C. H. (2011). Neuroleptic malignant syndrome associated with bromocriptine withdrawal in Parkinson’s disease - a case report. General Hospital Psychiatry, 33, 301.e7–301.e8.10.1016/j.genhosppsych.2010.11.013
- Zion, M. M., Goldberg, B., & Suzman, M. M. (1955). Corticotrophin and cortisone in the treatment of scleroderma. QJM, 24, 215–227.