 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Urokinase plasminogen activator (uPA) and its inhibitor (PAI-1) have shown to be of merit as biomarkers for a variety of cancers. The objective of this project was to assay for uPA and PAI-1 in prostate needle biopsy tissue from 217 patients using the FEMTELLE enzyme linked immunosorbent (ELISA) assay, and to examine the robustness of PAI-1 as a candidate marker in benign prostatic hyperplasia (BPH) and prostate cancer (PCa), as previously identified in a different cohort of 111 patients. These results validate the assertion that PAI-1 levels of >4.5 ng mg−1 protein in prostate biopsies are indicative of prostate malignancy in elderly men, but further show that tissue from BPH patients in the 70–80 year age interval express significantly high levels of this marker. To address this anomaly, a malignancy index, derived from the concentrations of prostate-specific antigen (PSA), uPA, and PAI-1, and patient age is proposed. This simple index discriminates prostate tissue from BPH and PCa patients with concordance indices of 0.59 and 0.69 when tissues are taken as biopsy or transurethral resection of the prostate (TURP), respectively. Corresponding indices for PSA as a predictor of prostate disease were 0.67 and 0.73. Further evaluation of the proposed malignancy index using specimens, such as venous blood, could prove valuable in the search for non-invasive predictive assays.
Public Interest Statement
This validation prostate biopsy study confirmed the importance of plasminogen activator inhibitor-1 (PAI-1) as an indicator of malignancy in elderly men. However, PAI-1 expression in tissue from benign prostatic hyperplasia (BPH) patients within the 70–80 year age bracket was around 2-fold higher than the initially suggested cut-off of 4.5 ng mg−1 protein for a diagnosis of prostate cancer (PCa).
This incidence of false-positivity, and therefore lack of specificity, weakens the capacity of PAI-1 to serve as a sole reliable biomarker for PCa. We propose, therefore, the use of a malignancy index, calculated by dividing the product of the concentrations of three popular markers (PSA, PAI-1 and uPA) by patient age. In addition to the clear distinction between the BPH and PCa patient groups in biopsy tissue, the index shows potential in separating benign from malignant tumours in TURP tissue, regardless of age.
Competing Interests
The authors declare no competing interest.
1. Introduction
Urokinase plasminogen activator (uPA) and its inhibitor, plasminogen activator inhibitor-1 (PAI-1), are involved in cancer invasion and metastasis (Duffy, McGowan, Harbeck, Thomssen, & Schmitt, Citation2014). There is consistent proof, validated at level-of-evidence-1, from retrospective studies since the late 1980s, that low levels of both uPA and PAI-1 are associated with excellent prognosis in node-negative breast cancer, whereas high levels of either, or both, are associated with poor outcome (Harbeck et al., Citation2013; Schmitt et al., Citation2011). No definitive role of the involvement of uPA and PAI-1 in prostate cancer (PCa) has emerged. It also remains uncertain whether either of these two components predominates in different stages of disease to highlight a distinguishing feature which could be used for the diagnosis or prognosis of PCa.
Initial research focusing on surgical resections of prostate tissue obtained by transurethral resection of the prostate (TURP) concluded that the uPA/PAI-1 ratio was not merely a numerical phenomenon but separated benign and malignant prostate pathologies, and emerged as a candidate marker for BPH and PCa disease (Akudugu, Serafin, & Böhm, Citation2015; Böhm et al., Citation2013). This was supported by several observations: that the ratios were significantly higher for PCa pathologies than for BPH pathologies; that the ratio was strongly dependent upon the uPA concentration in BPH pathologies, but not in PCa pathologies; and that the ratio for PCa, but not for BPH cases, showed a dependence upon patient age (Akudugu et al., Citation2015; Böhm et al., Citation2013).
In a subsequent study using core needle prostate biopsy tissue, a number of trends emerged in favour of PAI-1 acting, possibly, as a sole PCa biomarker (Serafin, Böhm, Fernandez, Achel, & Akudugu, Citation2016): the separation seen between uPA/PAI-1 ratios for BPH and PCa in the study on TURP tissue was not apparent for biopsy tissue; uPA appeared to play a minor role as a potential marker in prostate biopsy tissue; in men below the age of 60 years, no difference existed in PAI-1 values in biopsies from PCa and BPH patients; persons older than 60 years of age, with PAI-1 values above 4.5 ng mg−1 protein were moderately certain to have PCa; and those below the age of 60 years with PAI-1 values lower than 4.5 ng mg−1 protein were more likely to have BPH.
The goal of the study reported here was to validate findings from an earlier cohort of prostate biopsy samples (Serafin et al., Citation2016). Combined, the results confirm our assertion that PAI-1 may be a candidate prostate biomarker, and further show a significantly raised PAI-1 level in the absence of prostate disease for individuals older than 70 years of age. However, what is not clear about this phenomenon of raised PAI-1 level is whether it is a diagnostic grey zone or due to benign prostatic hyperplasia (BPH) with prostatitis or other pathologies? Because of the semi-random nature of sampling of the prostate during biopsy, urologists and pathologists report that initial prostate biopsy histopathology has a 20 to 30% chance of missing a tumour (Shariat & Roehrborn, Citation2008). To partly address this clinically relevant issue, we propose a “malignancy index” derived from four parameters, namely, the concentrations of three biomarkers (prostate-specific antigen (PSA), PAI-1 and uPA), and patient age. We show that this index displays robustness in distinguishing between BPH and PCa, and may potentially serve as a reliable tool for diagnosing prostate malignancies, especially in individuals above 60 years of age.
2. Materials and methods
2.1. Patients and samples
Prostate biopsy tissue was obtained from 217 patients after signed consent, and according to ethical guidelines. Patients were recruited from Tygerberg Academic Hospital and the Gatesville Medical Centre (Cape Town, South Africa). One hundred and fourteen patients were diagnosed as PCa and 103 as BPH, based on digital rectal examination (DRE), PSA, Gleason score and histopathology, as described elsewhere (Böhm et al., Citation2013; Serafin et al., Citation2016). An abnormal PSA and/or DRE finding resulted in an 8 core transrectal prostate biopsy. A histology positive score was added to the PSA and DRE scores to obtain a 10-point final score. For example, patients with a negative DRE, a PSA value of 4 μg L−1 and one positive core (cT1) were given a rating of 1, indicating a low probability of PCa and a high probability of BPH. A distinctly abnormal DRE with a PSA value of 100 μg L−1, and one or more positive cores received a rating of 10, indicating a high probability of PCa and a low probability of BPH. Due to an unvalidated scoring system only patients with a score of 8 or higher, in the PCa category, were included. In order to reduce errors, a second urologist reviewed the clinical data and scores of patients. The objective of the scoring system was to obtain a high level of certainty of PCa identification with a low probability of missing same.
The average ages of the PCa and BPH groups were 66.3 years (range: 46–86 years) and 64.0 years (range: 41–85 years), respectively. Corresponding PSA concentrations for the patient groups emerged as 77 ng mL−1 (range: 0.67–1526 ng mL−1) and 11 ng mL−1 (range: 0.40–69 ng mL−1). The project was approved (reference N09/11/330) by the Ethics Committee of the Faculty of Medicine and Health Sciences at the University of Stellenbosch (South Africa), and conducted in accordance with the Helsinki Declaration of 1975.
2.2. Sample preparation and measurement of PAI-1 and uPA content
For protein extraction, prostate biopsy tissue samples were placed in cold extraction buffer, composed of 240 μL pH 8.5 Tris-buffered saline and 10 μL of 25% Triton X-100 (Sekisui Diagnostics Product R22), at 4°C for 24 h on a rotating roller, followed by centrifugation at 20,000×g for 20 min at 4°C. The total protein concentration of the extracts was measured by means of the Pierce Bicinchoninic acid (BCA) assay (Thermo Scientific, Rockford, USA). Briefly, the total protein concentration is manifest by a colour change in the sample solution from green to purple in proportion to protein concentration, which is then measured using a colorimetric technique. Determination of the PAI-1 and uPA content in the biopsy tissue samples was by the FEMTELLE enzyme linked immunosorbent (ELISA) assay (Sekisui Diagnostics, LLC, Lexington, MA, USA), as described elsewhere (Jänicke et al., Citation1994). Total protein was expressed as mg mL−1, while the content of PAI-1 and uPA was expressed in ng mg−1 total protein. These data were used to test the robustness of PAI-1 as a predictor of prostate disease as suggested previously (Serafin et al., Citation2016). The results showed that some of the BPH patients had surprisingly high levels of PAI-1, in contrast to earlier findings (Serafin et al., Citation2016). Given that we did not have access to prostate volumes for this cohort of patients, we could not attribute this anomaly to prostate size, as suggested elsewhere (Gondek et al., Citation2014). Therefore, we propose a new multi-parameter approach for distinguishing between BPH and PCa. For the latter, a malignancy index (in units of mal: 1 mal = 1 ng3 (mg protein)−2 ml−1 y−1) was derived for each patient from the concentrations of PSA, uPA and PAI-1, as follows:
3. Statistical analysis
Statistical analyses were performed using the GraphPad Prism computer program (GraphPad Software, San Diego, CA, USA). To compare two data-sets, the unpaired t test was used. The p-values were calculated from two-sided tests. A p-value of <0.05 indicates a statistically significant difference between the data sets. The predictive powers of the proposed malignancy index and PSA level were assessed using receiver operating characteristic (ROC) analysis. Briefly, cut-off points were chosen within the ranges of indices and PSA levels above which patients would have PCa. By moving the cut-off points across the data ranges, an array of true positive rates (sensitivity) and corresponding false positive rates (1-specificity) were generated from which the ROC curves were constructed. The accuracy of prediction was measured by the concordance statistic (c-index) representing the area under the ROC curve (AUC). An area of 1.0 represents a perfect prediction, while an area of 0.5 represents a non-discriminating test.
4. Results
4.1. PAI-1 expression in core needle biopsies of the prostate
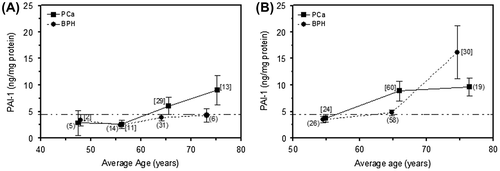
Figure (A) represents the recovery of the PAI-1 marker from 111 biopsies from two groups of patients diagnosed as BPH and PCa (Serafin et al., Citation2016). PAI-1 levels in patients diagnosed as BPH fall below a cut-off range of 4.5 ng mg−1 protein across an age span of 48–73 years, whereas patients diagnosed as PCa show an upward trend (2.6–9.1 ng mg−1 protein) across an age span of 56–75 years. Figure (B) represents the validation assessment of 217 prostate needle biopsy samples (114 diagnosed as PCa, and 103 diagnosed as BPH). PAI-1 values from the PCa samples, as before, showed an upward trend (3.8–9.6 ng mg−1 protein) above a cut-off range of 4.5 ng mg−1 protein, across an age span of 55–76 years. PAI-1 values from the BPH samples are consistent with those seen previously for mean ages up to 65 years, and are not significantly higher than 4.5 ng mg−1 protein (Serafin et al., Citation2016). However, at a mean age of 75 years, samples from BPH patients yield about 16 ng of PAI-1 per mg protein, on average.
Figure 1. Plot of PAI-1 marker concentration in BPH and PCa biopsy tissues against the mean age of patients: (A) initial data (Serafin et al., Citation2016) and (B) validation data.
4.2. Proposed malignancy index in prostate biopsy and TURP samples as a predictive marker
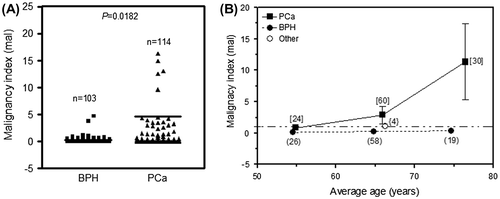
In order to address the disparity in PAI-1 levels seen in Figure for BPH patients, we propose a malignancy index which clearly significantly separates the two prostate pathologies of BPH and PCa with a p-value of 0.0182 (Figure (A)). In Figure (B), the malignancy indices obtained from prostate biopsies from 114 PCa patients increase with age and range from 0.86 ± 0.45 mal (for a mean age of 55 years) to 11.33 ± 6.02 mal (for a mean age of 76 years). The average malignancy indices for the 103 BPH patients, on the other hand, appear to be independent of age and range between 0.12 ± 0.03 and 0.36 ± 0.20 mal across a similar age span as for the PCa cohort.
Figure 2. (A) Comparison of malignancy indices in biopsy tissue from BPH (n = 103) and PCa (n = 114) patients. p < 0.05 indicates a statistically significant difference. (B) Plot of malignancy index against the mean age of patients grouped in 10-year intervals. “Other” refers to data from individuals presenting with non-BPH and non-PCa pathologies.
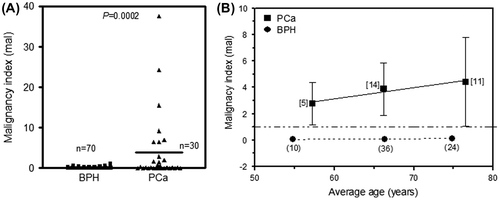
To further test the robustness of the proposed malignancy index, indices were derived from data obtained previously from a cohort of 100 TURP samples from the Tygerberg Academic Hospital, and the Gatesville Medical Center in Athlone, Cape Town, of which 30 were diagnosed as PCa and 70 as BPH (Akudugu et al., Citation2015), using a scoring system described elsewhere (Böhm et al., Citation2013). The mean ages of the BPH and PCa groups were 67.6 years (range 46–83 years) and 68.5 years (range 53–88 years), respectively.
Figure (A) shows a significantly higher malignancy index for PCa patients relative to their BPH counterparts (p = 0.0002). In Figure (B), the malignancy indices derived from TURP tissue increase with age and range from 2.78 ± 1.60 mal (for a mean age of 57 years) to 4.42 ± 3.36 mal (for a mean age of 77 years). The corresponding malignancy indices for the BPH patients, on the other hand, are constant (~0.1 mal) across a similar age span.
Figure 3. (A) Comparison of malignancy indices in TURP tissue from BPH (n = 70) and PCa (n = 30) patients. p < 0.05 indicates a statistically significant difference. (B) Plot of malignancy index against the mean age of patients grouped in 10-year intervals.
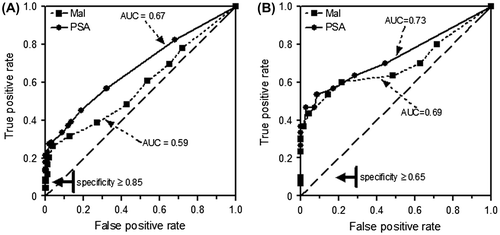
The predictive powers of the malignancy index and PSA levels are compared on the basis of ROC in Figure . The data indicate that the malignancy index has a potential to discriminate between patients with and without prostate disease. For biopsies, the c-indices were 0.59 and 0.67 when the malignancy index and PSA level were used as predictors, respectively (Figure (A)). For TURP tissue, the corresponding c-indices were found to be 0.69 and 0.73 (Figure (B)).
Figure 4. Comparison of ROC curves for malignancy index (Mal) and PSA level. (A) Mal obtained from needle biopsies of 217 patients. (B) Mal obtained from TURP tissues of 100 patients. PSA was routinely measured.
5. Discussion
In a series of studies, the uPA/PAI-1 ratio in TURP tissue (Akudugu et al., Citation2015; Böhm et al., Citation2013) and PAI-1 level in prostate biopsy tissue (Serafin et al., Citation2016) were suggested as being capable of distinguishing between benign and malignant prostate pathology. The purpose of this study was to validate the latter using a larger cohort of patients from whom biopsies were obtained. The data presented in Figure (B) corroborate the initial findings for PCa patients as shown in Figure (A) (Serafin et al., Citation2016). However, the average PAI-1 expression in biopsy tissue from BPH patients within the 70–80 year age bracket is about 2-fold higher than the initially suggested cut-off of 4.5 ng mg−1 protein for a diagnosis of PCa (Serafin et al., Citation2016). This incidence of false-positivity, and therefore lack of specificity, weakens the capacity of PAI-1 in prostate biopsy to serve as a sole reliable biomarker for PCa. The current finding is not peculiar. The use of PSA as a screening tool for PCa is controversial because of concerns about a lack of specificity, and subsequent overtreatment. Despite its limitations, delayed diagnosis of PCa may lead to poor outcomes, greater health care costs, and a lower quality of life (Van Neste et al., Citation2016). As with false-positive (raised PSA in the absence of prostate disease) and false-negative PSA (low PSA in the presence of disease) readings, the possibility exists for PAI-1 levels to be raised in the absence of histologically proven PCa.
The Gleason score, often divided into three groups (6, 7, and 8–10), is very important in predicting the behavior of PCa, and that a biomarker separating, in particular, Gleason score (3 + 4 = 7) from (4 + 3 = 7), the latter having a poorer prognosis, is very important. That being said, there are publications that question the subjectivity and inter-observer variation of Gleason grading (Fournier & Narayan, Citation1993; Helin et al., Citation2005; Tabesh et al., Citation2007). Gonzalgo and colleagues investigated the relationship between biopsy Gleason score, the Gleason score from an entire prostate removed during surgery, and the recurrence of PSA amongst men who were diagnosed with Gleason 7 cancer in a needle biopsy, and found that the vast majority (75%) of those turned out to have the less aggressive type of Gleason 7 cancer (Gonzalgo et al., Citation2006). Nevertheless, it is important to counsel patients with Gleason 7 PCa on the history of the disease and treatment decisions.
The phenomenon of false-positivity or false-negativity in single marker expression, which often makes diagnosis of disease difficult, may be circumvented by considering multi-parameter approaches as it is likely that the interaction between biomarkers drives their capacity to reliably predict disease. Therefore, in the current study, we propose the use of a malignancy index, calculated by dividing the product of the concentrations of three popular markers (PSA, PAI-1 and uPA) by patient age. As may be seen, the malignancy index clearly separates BPH from malignant prostate pathology in tissue from core needle biopsies, especially for individuals older than 60 years (Figure (B)). It is interesting to note that malignancy indices derived from biopsy tissue from patients with pathologies other than BPH and PCa are low and comparable to those for their BPH counterparts (Figure (B)). A mean malignancy index of 1.09 ± 0.44 mal was obtained for four patients (mean age: 66 years) presenting with atrophic prostate, bladder hyperplasia, chronic prostatitis, and inflammation, suggesting that the index has the potential of reliably identifying PCa in elderly individuals.
To test the robustness of the proposed index, data from TURP tissue, previously presented in the uPA/PAI-1 ratio studies (Akudugu et al., Citation2015; Böhm et al., Citation2013), were used to derive indices, as shown in Figure . In addition to the clear distinction between the BPH and PCa patient groups, the index shows potential in separating benign from malignant tumours, regardless of age. This differs from the finding that it only appears to be capable of distinguishing between BPH and PCa in the elderly (Figure ), suggesting that this approach may be more appropriate when evaluating TURP tissue than biopsy tissue.
PSA is and has been the biomarker of choice for PCa screening. A new group of biomarkers has emerged, consisting of urine- and serum- and tissue-based assays (Prensner, Rubin, Wei, & Chinnaiyan, Citation2012). The introduction of new biomarkers will likely supplement PSA until one is found to be superior, and replaces it. What are the requirements of an ideal biomarker? The ideal biomarker needs to be specific for a particular tumour type, sensitive, should accurately depict disease progression or regression, be inexpensive, and quantifiable in biological samples. For PCa biomarkers used in early detection or monitoring of the disease, the candidate should be prostate-specific and able to distinguish between BPH and cancerous prostate tissue. In light of this, ROC analysis shows that at high levels of specificity, the proposed malignancy index and the commonly used PSA marker are equally sensitive as predictors of prostatic disease (Figure ). High specificity is of the essence for diseases with severe prognoses, such as cancer, for which an ideal “rule-in” test would be required (Florkowski, Citation2008). Therefore, within the ranges of specificities identified in Figure , malignancy index and PSA are equally discriminating, and the former merits further and more extensive evaluation.
The index proposed, herein, should not be confused with malignancy related indices suggested elsewhere (Jayson, Kohn, Kitchener, & Ledermann, Citation2014; Matchariyakul, Kochakarn, Chaimuangraj, Leenanupunth, & Lertsithichai, Citation2004). For instance, the malignancy risk index used for estimating the risk of malignant ovarian cancer is based on a combination of radiological findings, menstrual status, and serum CA-125 concentration (Jayson et al., Citation2014). A second, though unvalidated, numerical malignancy index suggested for identifying candidates for prostate biopsy from a high risk group of individuals is based on age, DRE, PSA, prostate volume, and transrectal ultrasonography (Matchariyakul et al., Citation2004).
6. Conclusions
Although the assertion that PAI-1 expression in prostate biopsies is generally high in elderly PCa patients has been validated in a larger cohort, a significant level of false-positivity was observed. To address this, a simple but promising malignancy index was proposed to discriminate benign from malignant in prostate tissue (both biopsy and TURP), without the use of DRE, prostate volume, and ultrasound. It would be of significant interest to test the malignancy index described here, using venous blood samples from patients providing TURP or biopsy tissue samples, across different age groups and tumour stages.
Funding
Cancer Association of South Africa (CANSA) to AS, and the National Research Foundation of South Africa (NRF: grants numbers 85703 and 92741) to JA.
Acknowledgements
We thank Professor A van der Merwe, and the medical registrars of the Urology Department, University of Stellenbosch, and Dr NA Aziz of the Gatesville Medical Centre, Athlone, SA for prostate biopsy samples.
Additional information
Notes on contributors
Antonio Serafin
Antonio Serafin has retired from the Division of Radiobiology at the University of Stellenbosch, after 32 years. His interest in prostate cancer dates back to his PhD work which centered on the cell biological responses of novel prostate tumour cell lines to radiation and anticancer drugs.
The primary objective of the current prostate biomarker project is to move from more invasive to less invasive medical procedures (from transurethral resection of the prostate tissue to prostate biopsy tissue), and to eventually test the research observations using venous blood samples.
Antonio Serafin is currently a member of a small team investigating biomarkers in prostate cancer, antibody cocktails for targeted therapy, the cytotoxicity and radiomodifying effects of aqueous plant extracts, the effect of radio frequency electromagnetic waves on radiosensitivity, and high throughput biodosimetry tools.
References
- Akudugu, J., Serafin, A., & Böhm, L. (2015). Further evaluation of uPA and PAI-1 as biomarkers for prostatic diseases. Journal of Cancer Research and Clinical Oncology, 141, 627–631. doi:10.1007/s00432-014-1848-3
- Böhm, L., Serafin, A., Akudugu, J., Fernandez, P., van der Merwe, A., & Aziz, N. A. (2013). uPA/PAI-1 ratios distinguish benign prostatic hyperplasia and prostate cancer. Journal of Cancer Research and Clinical Oncology, 139, 1221–1228. doi:10.1007/s00432-013-1428-y
- Duffy, M. J., McGowan, P. M., Harbeck, N., Thomssen, C., & Schmitt, M. (2014). uPA and PAI-1 as biomarkers in breast cancer: Validated for clinical use in level-of-evidence-1 studies. Breast Cancer Research, 16, 428. doi:10.1186/s13058-014-0428-4
- Florkowski, C. M. (2008). Sensitivity, specificity, receiver-operating characteristic (ROC) curves and likelihood ratios: Communicating the performance of diagnostic tests. The Clinical Biochemist Reviews, 29, S83–S87.
- Fournier Jr., G. R., & Narayan, P. (1993). Re-evaluation of the need for pelvic lymphadenectomy in low grade prostate cancer. British Journal of Cancer, 72, 484–488.
- Gondek, T., Szajewski, M., Szefel, J., Aleksandrowicz-Wrona, E., Skrzypczak-Jankun, E., Jankun, J., & Lysiak-Szydlowska, W. (2014). Evaluation of 12-Lipoxygenase (12-LOX) and Plasminogen Activator Inhibitor 1 (PAI-1) as prognostic markers in prostate cancer. Biomedical Research International, 2014, 102478. doi:10.1155/2014/102478
- Gonzalgo, M. L., Bastian, P. J., Mangold, L. A., Trock, B. J., Epstein, J. I., Walsh, P. C., & Partin, A. W. (2006). Relationship between primary Gleason pattern on needle biopsy and clinicopathologic outcomes among men with Gleason score 7 adenocarcinoma of the prostate. Urology, 67, 115–119.10.1016/j.urology.2005.07.037
- Harbeck, N., Schmitt, M., Meisner, C., Friedel, C., Untch, M., Schmidt, M., … Thomssen, C. (2013). Ten-year analysis of the prospective multicentre Chemo-N0 trial validates American Society of Clinical Oncology (ASCO)-recommended biomarkers uPA and PAI-1 for therapy decision making in node-negative breast cancer patients. European Journal of Cancer, 49, 1825–1835. doi:10.1016/j.ejca.2013.01.007
- Helin, H., Lundin, M., Lundin, J., Martikainen, P., Tammela, T., Helin, H., … Isola, J. (2005). Web-based virtual microscopy in teaching and standardizing Gleason grading. Human Pathology, 36, 381–386.10.1016/j.humpath.2005.01.020
- Jänicke, F., Pache, L., Schmitt, M., Ulm, K., Thomssen, C., Prechtl, A., & Graeff, H. (1994). Both the cytosols and detergent extracts of breast cancer tissues are suited to evaluate the prognostic impact of the urokinase-type plasminogen activator and its inhibitor, plasminogen activator inhibitor type 1. Cancer Research, 54, 2527–2530.
- Jayson, G. C., Kohn, E. C., Kitchener, H. C., & Ledermann, J. A. (2014). Ovarian cancer. The Lancet, 384, 1376–1388. doi:10.1016/S0140-6736(13)62146-7
- Matchariyakul, C., Kochakarn, W., Chaimuangraj, S., Leenanupunth, C., & Lertsithichai, P. (2004). A risk index for prostate cancer. International Journal of Urology, 11, 310–315.10.1111/iju.2004.11.issue-5
- Prensner, J. R., Rubin, M. A., Wei, J. T., & Chinnaiyan, A. M. (2012). Beyond PSA: The next generation of prostate cancer biomarkers. Science Translational Medicine, 4, 127rv3. doi:10.1126/scitranslmed.3003180
- Schmitt, M., Harbeck, N., Brünner, N., Jänicke, F., Meisner, C., Mühlenweg, B. … Thomssen, C. (2011). Cancer therapy trials employing level-of-evidence-1 disease forecast cancer biomarkers uPA and its inhibitor PAI-1. Expert Review of Molecular Diagnostics, 11, 617–634. doi:10.1586/erm.11.47
- Serafin, A., Böhm, L., Fernandez, P., Achel, D., & Akudugu, J. (2016). The potential of PAI-1 expression in needle biopsies as a predictive marker for prostate cancer. Cogent Medicine, 3, 1183275.
- Shariat, S. F., & Roehrborn, C. G. (2008). Using biopsy to detect prostate cancer. Reviews in Urology, 10, 262–280.
- Tabesh, A., Teverovskiy, M., Pang, H.-Y., Kumar, V. P., Verbel, D., Kotsianti, A., & Saidi, O. (2007). Multifeature prostate cancer diagnosis and gleason grading of histological images. IEEE Transactions on Medical Imaging, 26, 1366–1378.10.1109/TMI.2007.898536
- Van Neste, L., Hendriks, R. J., Dijkstra, S., Trooskens, G., Cornel, E. B., Jannink, S. A., … Schalken, J. A. (2016). Detection of high-grade prostate cancer using a urinary molecular biomarker–based risk score. European Urology, 70, 740–748. doi:10.1016/j.eururo.2016.04.012
