 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The aim of the present study was to evaluate the potential anticancer action of extracts prepared from german chamomile (Matricaria recutita L.)and pot marigold (Calendula officinalis L.) to human melanoma and epidermoid carcinoma cells. Sulforhodamine B assay was used to measure the cytotoxic activity of methanolic extracts from flowers of chamomile and marigold. The cytotoxic activity of extract of chamomile flowers on melanoma cells (IC50 value 40.7 μg/ml) was approximately twofold higher than on epidermoid carcinoma cells (IC50 value 71.4 μg/ml). In the present study, the anticancer action of extracts prepared from german chamomile flowers on human melanoma and non-melanoma skin cancer cells (SK-MEL-2 melanoma and KB epidermoid carcinoma cells) is described for the first time.
Public Interest Statement
German chamomile is very popular medicinal plants in differents countries, expecially in Europe. The chamomile flowers are used for centuries for their antiinflammatory, anodyne, antimicrobial, antispasmic, calming and anticancer properties. Medicinal properties have made chamomile increasingly popular in the form of tea which is consumed at a rate of even more than one million cups per day. Pot marigold is also wellknown medicinal, as well as ornamental plant. The marigold flowers have been reported to exhibit various biological and medicinal properties, including antiinflammatory, antioxidant, antibacterial, antifungal, antiviral, diaphoretic, antispasmodic, antipyretic, antidiabetic, immunostimulant, antigenotoxic and even anticancer effects. We evaluated the potential anticancer action of extracts prepared from chamomile and marigold to different cancer cells: human melanoma and epidermoid carcinoma cells. The cytotoxic activity of extract of chamomile on melanoma cells was significant, approximately two-fold higher than on epidermoid carcinoma cells. The cytotoxic activity of marigold extracts was low.
Competing Interests
The authors declare no competing interest.
1. Introduction
The incidence of both non-melanoma and melanoma skin cancers is an increasing problem for health care services worldwide. The components of chamomile exhibit antioxidant properties in human skin fibroblasts, thus suggesting the anticancer effects of this herb (Mamalis, Nguyen, Brody, & Jagdeo, Citation2013).
German chamomile (Matricaria recutita L., Asteraceae) flowers have been used for centuries for their antiinflammatory, analgesic, antimicrobial, antispasmic and sedative properties. The infusion of chamomile has been traditionally used to calm nerves and reduce anxiety, and to treat hysteria, nightmares, insomnia and other sleep problems. The infusion has been also used for relieving gastrointestinal disorders. All these medicinal properties have made chamomile increasingly popular today, and for example, chamomile tea is globally consumed even more than one million cups per day (Srivastava & Gupta, Citation2009). Furthermore, chamomile is applicable for preventing and treating e.g. breast, ovarian and prostate cancer (Kozak, Sobczak, & Żukiewicz-Sobczak, Citation2016). Although the extracts of chamomile flowers have been shown to inhibit cell growth and to induce apoptosis in some human cancer cell lines (i.e. prostate, cervix, colon, breast cancer cells, and fibrosarcoma cells), these effects were exerted at rather high concentrations (IC50 in the range of more than 100 μg/ml) (Srivastava & Gupta, Citation2009; Matić et al., Citation2013). To date, no studies have been published reporting the activity of chamomile extracts on human skin cancer cells.
Pot marigold (Calendula officinalis L., Asteraceae) has been shown to have a clear cytotoxic activity on malignant cells including skin cancer cells (Jiménez-Medina et al., Citation2006; Matić et al., Citation2013; Muley, Khadabadi, & Banarase, Citation2009; Preethi, Siveen, Kuttan, & Kuttan, Citation2010; Wegiera, Smolarz, Jedruch, Korczak, & Kopron, Citation2012). The extracts and compounds derived from pot marigold have numerous biological and medicinal properties. These include e.g. anti-inflammatory, antioxidant, antibacterial, antifungal, antiviral, diaphoretic, antiedematous, antispasmodic, antipyretic, hypoglycemic, immunostimulant, antigenotoxic and even antitumor properties (Jiménez-Medina et al., Citation2006; Matić et al., Citation2013; Muley et al., Citation2009; Preethi, Kuttan, & Kuttan, Citation2009, Citation2010; Sak, Jürisoo, & Raal, Citation2014; Ukiya et al., Citation2006; Wegiera et al., Citation2012). The extracts derived from pot marigold have also hepato-, gastro-, reno-, and nephron-protective capability. Furthermore, the preparations obtained from marigold flowers are widely used for the treatment of dermatological disorders, such as skin inflammations, burns, wounds, bruises, rashes and eczema. Such extracts can exhibit also in vitro cytotoxic action on melanoma cells of human and mouse origin (Matić et al., Citation2013; Preethi et al., Citation2010). In addition, the formation of metastases hindered with these extracts, thus increasing the life span of tumor bearing C57BL/6 mice (Preethi et al., Citation2010). Alnuqaydan, Lenehan, Hughes, and Sanderson (Citation2015) reported that the marigold flower extract can significantly protect against oxidative stress in a human skin cell culture model. Marigold flower extract provided also better therapeutic response in the prevention and treatment of radiodermatitis compared to that obtained with essential fatty acids (Schneider, Danski, & Vayego, Citation2015).
Recently, Matić et al. (Citation2013) reported that the tea prepared from marigold flowers exhibited significantly stronger cytotoxic action against several target malignant cell lines in comparison to the tea obtained from chamomile flowers.
The aim of the present study was to investigate and gain understanding of the potential action of chamomile flower extract on human skin cancer cells (melanoma SK-MEL-2 and epidermoid carcinoma KB cells). Marigold flower extract was used as a reference plant material for the evaluation of cytotoxic activity.
2. Results
The effect of methanol extracts prepared from the dried flowers of chamomile and marigold was studied against human melanoma SK-MEL-2 and oral epidermoid carcinoma KB cells. Sulforhodamine B cytotoxic assay was conducted in the concentration range of 0.8–100 μg/ml to determine the doses at which 50% of the cell growth was inhibited (Figure ).
Figure 1. Cytotoxic effect of German chamomile and pot marigold extracts on human SK-MEL-2 melanoma and KB oral epidermoid carcinoma cells.
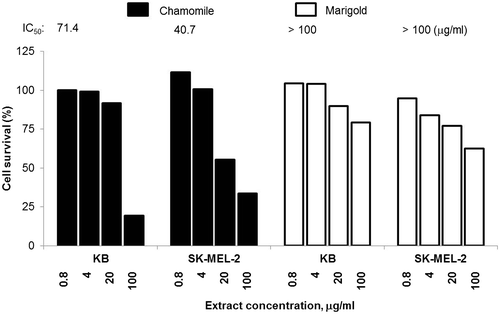
The strongest anticancer effect was found with the methanol extract of chamomile flowers against SK-MEL-2 cells (IC50 40.68 ± 2.92 μg/ml). The effect of this extract on the KB cells was somewhat weaker (IC50 71.42 ± 2.34 μg/ml). With the marigold flower extracts, the half maximal cell growth inhibitory concentrations exceeded the pre-determined threshold (100 μg/ml) in both cell lines studied. At this concentration (100 μg/ml) the methanol extract of marigold flowers provided only 37.4 and 20.8% growth inhibition on SK-MEL-2 and KB cells, respectively (Figure ).
In this study, the significant antimelanoma effect of chamomile extract was shown for the first time. Although the in vitro screening is a robust tool providing preliminary data about the potential cytotoxic effects of herbal extracts, there are several limitations in transferring these results to in vivo conditions. It is well-known that in vivo conditions the bioavailability, distribution, metabolism and excretion of the active ingredient(s) affect the final therapeutic efficacy, and a wide range of side effects can also appear.
3. Experimental
3.1. Cell culture
SK-MEL-2 (human melanoma) cells were cultured in DMEM and KB (human oral epidermoid carcinoma) cells in RPMI-1640 cell culture medium, both supplemented with 10% fetal bovine serum. The cells were cultivated at 37°C in a humidified atmosphere containing 5% carbon dioxide.
3.2. Plant material and preparation of extracts
Dried chamomile and marigold flowers were cultivated in Estonia and harvested in the vegetation period by herb farm Kubja Ürditalu, Estonia, N59.054344, E25.963234. The plant material was identified by a phytochemical profile (Orav, Raal, & Arak, Citation2010; Raal, Orav, Nesterovitsch, & Maidla, Citation2016). The voucher specimens (No Asteraceae/Cham27—Chamomilla recutita; No Asteraceae/Cal43—Calendula officinalis) have been deposited in the Institute of Pharmacy, University of Tartu, Estonia.
The plant samples were dried and crushed to a fine powder, and then extracted with methanol for three times (48 h per time) at room temperature (20°C). The filtrates were then concentrated in a rotary evaporator under a reduced pressure to obtain crude extracts which were used in the cytotoxic assays. For preparing 4 mg/ml stock solutions, the extracts were dissolved in dimethyl sulfoxide (DMSO). The stock solutions were later mixed with cell culture medium to achieve the desired concentrations. The final test concentrations were 0.8, 4, 20 and 100 μg/ml.
3.3. In vitro cytotoxic assay
The effects of chamomile and marigold extracts on the viability of malignant cells was determined by sulforhodamine B cytotoxic assay (Monks et al., Citation1991). Briefly, the cells were grown in 96-well microtiter plates with each well containing 190 μl medium. After 24 h, 10 μl of test samples dissolved in DMSO were added to each well. One plate with no samples served as a day 0 control. The cells were continuously cultured for additional 48 h, fixed with trichloroacetic acid and stained with sulforhodamine B, followed by the determination of optical densities at 515 nm using a Microplate Reader (Bio-Rad Laboratories, USA). The percentage of growth inhibition was calculated using the following equation:
where OD is an optical density or absorbance value. The potent anticancer agent ellipticine was used as a positive control (showing the IC50 value for SK-MEL-2 cells of 0.39 ± 0.04 μg/ml and for KB cells of 0.35 ± 0.05 μg/ml).
3.4. Statistical analysis
Cytotoxic data were calculated and expressed as concentrations at which 50% of cell growth was inhibited (IC50 values ± SD). All experiments were carried out in triplicate, and a TableCurve 2Dv4 software (System software Inc., San Jose, California, USA) was used for calculating IC50 values. P values less than 0.01 were considered as statistically significant.
4. Discussion
To our best knowledge, this is the first study describing the anti-proliferative effect of chamomile extract on human skin cancer cells. Melanoma SK-MEL-2 and epidermoid carcinoma KB cells were used as model systems. Significant cytotoxic action on melanoma cells appeared already at the dose of 20 μg/ml, thus revealing the half maximal inhibitory concentration for methanol extract of chamomile flowers at about 40 μg/ml. It has been presented earlier that the exposure of human prostate cancer (PC-3, LNCaP, DU145), breast carcinoma (T-47D), colon carcinoma (RKO), cervical adenocarcinoma (HeLa) and fibrosarcoma (HT1080) cells to methanolic chamomile extracts induce the decrease in cell viability and apoptosis with IC50 values in the range of 100–300 μg/ml. The respective effects for aqueous extracts appeared at even higher doses with the IC50 values ranging from 2,000 to 4,000 μg/ml (Srivastava & Gupta, Citation2009). Furthermore, the tea obtained from chamomile flowers revealed the cytotoxic action on various malignant cells at the doses higher than some mg/ml (Matić et al., Citation2013). Thus, the cytotoxic action of chamomile extracts described in this work is the strongest anticancer effect at all measured for this herb so far.
Chamomile is known to contain several classes of biologically active compounds including essential oils and several polyphenols. Phenolic fraction of chamomile extract contains several common flavonoids, such as apigenin, luteolin, quercetin and patuletin, and these flavonoids exist mostly as glucosides in natural conditions (Bulgari et al., Citation2012; Srivastava & Gupta, Citation2009). Apigenin-7-O-glucoside is the major constituent of chamomile, and its anticancer effect occurs through the deconjugation to active aglycone, apigenin (Srivastava & Gupta, Citation2009). Apigenin reduces the expression of DNA methyltransferases (DNMT1 and DNMT3b) epigenetic proteins and some histone deacetylases (1–8), and consequently, could prevent e.g. skin cancer (Paredes-Gonzalez, Fuentes, Su, & Kong, Citation2014). In addition, apigenin and the extract of chamomile ligulate flowers were shown to have a cytotoxic activity against human rhabdomyosarcoma and human cervix carcinoma Hep2c (Cvetanović et al., Citation2015). One cup (200 ml) of chamomile tea contains up to 9.3 mg of apigenin glucosides (Raal et al., Citation2012).
According to the literature, the cytotoxic activity of the tea prepared from marigold flowers is much higher on various cancer cells than that obtained with the chamomile tea (Matić et al., Citation2013). Our studies with the methanol extracts of these plants, however, showed completely contrary results revealing significantly higher cytotoxic activity with chamomile extracts. The spectrum of bioactive compounds in the extracts obtained with different solvents may greatly vary, thus affecting the respective cytotoxic profiles. Moreover, the additive or synergistic combinations between individual constituents of plant extracts are obviously important for the ultimate biological activity. Therefore, the observed differences in the intensities of tumoricidal activity between the chamomile and marigold specimens are most likely caused by the specific constituents of the corresponding extracts. The cytotoxicity of marigold extracts is attributed at least partially to some phenolic compounds, such as narcissin and isorhamnetin 3-O-glucoside as well as several triterpene glycosides including calenduloside F6′-O-n butyl ester and calenduloside G6′-O-methyl ester (Matić et al., Citation2013; Ukiya et al., Citation2006). To date, the chemical content of chamomile (Orav et al., Citation2010; Raal et al., Citation2012) and marigold (Raal & Kirsipuu, Citation2011; Raal et al., Citation2016) flowers is well documented. The total content of flavonoids (0.21–0.68%) in marigold flowers depends both on the cultivated varieties as well as growth conditions (Raal & Kirsipuu, Citation2011). The content of terpenoids in essential oils of marigold does not depend on different cultivars (Raal et al., Citation2016).
Interestingly, the cytotoxic effects of the marigold extracts on melanoma cells determined previously with human melanoma Fem-x cells (IC50 360 μg/ml) (Matić et al., Citation2013) and in our current study with human SK-MEL-2 cells (IC50 > 100 μg/ml), were significantly weaker compared to those determined with mouse melanoma B16F-10 cells (IC50 ~50 μg/ml) (Preethi et al., Citation2010).
5. Conclusions
The science-based and systematic search of new compounds from medicinal plants and other natural sources is a rapidly growing field in the pharmaceutical industry. Today, the new native-origin compounds hold great promises as potential leads of next-generation chemopreventive agents and chemotherapeutics. Since chamomile extracts have a proven antioxidant, anti-inflammatory and antimelanoma activity, it is expected that the use of chamomile supplements in the conventional and popular sunscreen products will be increased in the near future. The advantages of chamomile extracts include the abundance of the herbal material in the nature and its cheapness. To our best knowledge, this is the first study describing the anti-proliferative effect of chamomile extract on human skin cancer cells.
Acknowledgements
We would like to thank the herb farm Kubja Ürditalu for kindly providing the plants material for this study.
Additional information
Funding
Notes on contributors
Ain Raal
Ain Raal has graduated as a pharmacist at the University of Tartu (Estonia), and has completed his PhD in pharmaceutical chemistry and pharmacognosy (“The pharmacognostical study of Matricaria matricarioides of Estonian origin”) at the Institute of Chemistry and Pharmacy of Leningrad (Soviet Union). He is the Head of the Institute of Pharmacy, University of Tartu, Estonia, and the Professor of Pharmacognosy of the same Institute. His main fields of research are pharmacognosy and phytochemistry, history of pharmacy and ethnomedicine, as well as natural medicines in social pharmacy. He has published more than 80 scientific papers in international scientific journals indexed by the Web of Science, Scopus and other databases, more than 130 conference abstracts, as well as 23 textbooks, monographs, handbooks and popular-scientific books such as Pharmacognosy and Encyclopedia of Medicinal Plants of the World (in Estonian).
References
- Alnuqaydan, A. M., Lenehan, C. E., Hughes, R. R., & Sanderson, B. J. (2015). Extracts from Calendula officinalis offer in vitro protection against H2O2 induced oxidative stress cell killing of human skin cells. Phytotherapy Research, 29, 120–124.10.1002/ptr.5236
- Bulgari, M., Sangiovanni, E., Colombo, E., Maschi, O., Caruso, D., Bosisio, E., & Dell’Agli, M. (2012). Inhibition of neutrophils elastase and metalloprotease-9 human adenocarcinoma gastric cells by chamomile (Matricaria recutita L.) infusion. Phytotherapy Research, 26, 1817–1822.10.1002/ptr.v26.12
- Cvetanović, A., Švarc-Gajić, J., Zeković, Z., Savić, S., Vulić, J., Mašković, P., & Ćetković, G. (2015). Comparative analysis of antioxidant, antimicrobiological and cytotoxic activities of native and fermented chamomile ligulate flower extracts. Planta, 242, 721–732.10.1007/s00425-015-2308-2
- Jiménez-Medina, E., Garcia-Lora, A., Paco, L., Algarra, I., Collado, A., & Garrido, F. (2006). A new extract of the plant Calendula officinalis produces a dual in vitro effect: Cytotoxic anti-tumor activity and lymphocytes activation. BMC Cancer, 6, 119.10.1186/1471-2407-6-119
- Kozak, M., Sobczak, P., & Żukiewicz-Sobczak, W. (2016). Health properties of selected herbal plants. Health Problems of Civilization, 2, 64–70.10.5114/hpc.2016.59635
- Mamalis, A., Nguyen, D. H., Brody, N., & Jagdeo, J. (2013). The active natural anti-oxidant properties of chamomile, milk thistle, and halophilic bacterial components in human skin in vitro. Journal of Drugs in Dermatology, 12, 780–784.
- Matić, I. Z., Juranić, Z., Šavikin, K., Zdunić, G., Nađvinski, N., & Gođevac, D. (2013). Chamomile and marigold tea: Chemical characterization and evaluation of anticancer activity. Phytotherapy Research, 27, 852–858.10.1002/ptr.v27.6
- Monks, A., Scudiero, D., Skehan, P., Shoemaker, R., Paull, K., Vistica, D., … Boyd, M. (1991). Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. JNCI Journal of the National Cancer Institute, 83, 757–766.10.1093/jnci/83.11.757
- Muley, B. P., Khadabadi, S. S., & Banarase, N. B. (2009). Phytochemical constituents and pharmacological activities of Calendula officinalis Linn (Asteraceae): A review. Trop J Pharm Res., 8, 455–465.
- Orav, A., Raal, A., & Arak, E. (2010). Content and composition of the essential oil of Chamomilla recutitia (L.) Rauschert from some European countries. Natural Product Research, 24, 48–55.10.1080/14786410802560690
- Paredes-Gonzalez, X., Fuentes, F., Su, Z.-Y., & Kong, A.-N. (2014). Apigenin reactivates Nrf2 anti-oxidative stress signaling in mouse skin epidermal JB6 P + cells through epigenetics modifications. The AAPS Journal, 16, 727–735.10.1208/s12248-014-9613-8
- Preethi, K. C., Kuttan, G., & Kuttan, R. (2009). Anti-inflammatory activity of flower extract of Calendula officinalis Linn. and its possible mechanism of action. Indian Journal of Experimental Biology, 47, 113–120.
- Preethi, K. C., Siveen, K. S., Kuttan, R., & Kuttan, G. (2010). Inhibition of metastasis of B16F-10 melanoma cells in C57BL/6 mice by an extract of Calendula officinalis L flowers. Asian Pacific Journal of Cancer Prevention, 11, 1773–1779.
- Raal, A., & Kirsipuu, K. (2011). Total flavonoid content in varieties of Calendula officinalis L. originating from different countries and cultivated in Estonia. Natural Product Research, 25, 658–662.10.1080/14786419.2010.528417
- Raal, A., Orav, A., Püssa, T., Valner, C., Malmiste, B., & Arak, E. (2012). Content of essential oil, terpenoids and polyphenols in commercial chamomile (Chamomilla recutita L. Rauschert) teas from different countries. Food Chemistry, 131, 632–638.10.1016/j.foodchem.2011.09.042
- Raal, A., Orav, A., Nesterovitsch, J., & Maidla, K. (2016). Analysis of carotenoids, flavonoids and essential oil of Calendula officinalis cultivars growing in Estonia. Natural Product Communications, 11, 1157–1160.
- Sak, K., Jürisoo, K., & Raal, A. (2014). Estonian folk traditional experiences on natural anticancer remedies: From past to the future. Pharmaceutical Biology, 52, 855–866.10.3109/13880209.2013.871641
- Schneider, F., Danski, M. T. R., & Vayego, S. A. (2015). Usage of Calendula officinalis in the prevention and treatment of radiodermatitis: A randomized double-blind controlled clinical trial. Revista Da Escola De Enfermagem Da USP, 49, 220–226.
- Srivastava, J. K., & Gupta, S. (2009). Extraction, characterization, stability and biological activity of flavonoids isolated from chamomile flowers. Molecular and Cellular Pharmacology, 1, 138–147.10.4255/mcpharmacol
- Ukiya, M., Akihisa, T., Yasukawa, K., Tokuda, H., Suzuki, T., & Kimura, Y. (2006). Anti-inflammatory, anti-tumor-promoting, and cytotoxic activities of constituents of marigold (Calendula officinalis) flowers. Journal of Natural Products, 69, 1692–1696.10.1021/np068016b
- Wegiera, M., Smolarz, H. D., Jedruch, M., Korczak, M., & Kopron, K. (2012). Cytotoxic effect of some medicinal plants from Asteraceae family on J-45.01 leukemic cell line – pilot study. Acta Poloniae Pharmaceutica, 69, 263–268.
