Abstract
Benign prostatic hyperplasia (BPH) is a common cause of lower urinary tract symptoms (LUTS) in men. In our practice, we employ Transurethral Microwave Thermotherapy (TUMT) as an effective and minimally invasive means of treating BPH for patients presenting with small to moderate prostate size. TUMT utilizes the transfer of heat to necrotize prostatic tissue in the treatment zone, resulting in prostatic edema that can temporarily exacerbate symptoms during the post-procedure recovery period. Management of post-procedure voiding symptoms often requires the use of an indwelling urinary catheter (IUC), in some cases, for an extended period. We systematically replace post-TUMT IUC with temporary prostatic stents (TPS) to alleviate voiding symptoms, reduce infection risk and improve the quality of life during recovery. The purpose of this study is to assess the clinical efficacy of TPS using The Spanner® Temporary Prostatic Stent in men with BPH, post initial catheterization following TUMT. This review documents the effects of TPS on post-void-residual (PVR), infection rate, and complication rate in 25 consecutive men treated with our TUMT/TPS protocol. Our data demonstrates that TPS had effectively reduced edema-related urethral resistance in the post-TUMT recovery period, resulting in reduced PVR and no incidence of infection.
Public Interest Statement
Non-malignant enlargement of the prostate, known as benign prostatic hyperplasia or BPH, is a common disease among aging males. The increase in prostate size often results in urinary symptoms as the urethra becomes obstructed. Transurethral Microwave Thermotherapy (TUMT) is one of several in-office therapies used to treat BPH. Energy applied by TUMT causes the prostate to temporarily swell in the post-procedure period, causing urinary symptoms to exacerbate as the prostate heals. This may prolong symptoms and lengthen the need for urinary catheterization.
To limit the use of urinary catheters, our practice routinely replaces indwelling urinary catheters with temporary prostate stents (TPS) at 48 h. This review is the first of its kind to demonstrate real-world evidence that TPS offer a safe and efficacious means of alleviating urinary symptoms; providing the ability to void naturally without the complications common with urinary catheterization.
Competing Interests
The authors declare no competing interest.
1. Introduction
Transurethral Microwave Thermotherapy (TUMT) is one of several in-office modalities for treating men with benign prostatic hyperplasia (BPH). TUMT employs heat to necrotize prostate tissue that is obstructing the prostatic urethra and inhibiting urine flow. The procedure is performed under local anesthesia in the office setting.
Heat-inducing procedures such as TUMT produce prostatic edema which can result in several weeks of persisting LUTS during the initial healing period. Patients often retain high levels of post-void residual (PVR) and require post-procedure catheterization, in some cases for extended periods (Dineen, Shore, Lumerman, Saslawsky, & Corica, Citation2008). While effective at draining the bladder, the use of indwelling urinary catheters (IUC) may result in complications or decreased patient satisfaction, particularly among patients with longer indwell periods (Saint, Veenstra, Sullivan, Chenoweth, & Fendrick, Citation2000). To minimize catheter indwell duration, we aim to remove the catheter after two days for every TUMT procedure. We do this by replacing the IUC with a temporary prostatic stent (TPS) to decrease urethral resistance and reduce the prevalence of voiding symptoms.
A multi-center, US clinical trial, assessed voiding parameters in 186 patients, 100 of whom had a TPS placed post-TUMT (Shore, Dineen, Saslawsky, Lumerman, & Corica, Citation2007). The study concluded that the replacing an IUC with a TPS improved patient voiding symptoms without exacerbating irritative symptoms. TPS patients demonstrated statistically significant improvements in PVR and Qmax, as well as improved quality of life (QOL) measured by the International Prostate Symptom Score (IPSS) and other QOL indices. These benefits were seen both while the TPS was in place as well as eight weeks afterwards. Patients rated the TPS to be comfortable and preferred over the use of an IUC.
2. Material and methods
We treated 25 consecutive BPH patients with Cooled ThermoTherapy™ TUMT (Urologix, Minneapolis, MN, USA) who presented to our practice from March to October 2014. The TUMT procedure is performed in the urology office under local anesthesia with a typical treatment duration lasting less than one hour. Prostate size was measured using transrectal ultrasound, and treatment was provided via a small flexible catheter inserted into the urethra. The flexible catheter contains a heat-emitting antenna that necrotizes excess prostatic tissue to de-obstruct the prostatic urethra. Immediately following TUMT, an IUC was placed and the patient was sent home from the office.
We replace the IUC with The Spanner® Temporary Prostatic Stent (SRS Medical, North Billerica, MA, USA), post-TUMT procedure. The intra-urethral TPS holds open the prostatic urethra, maintaining urine flow and promoting voluntary urination in patients following TUMT for a period of up to 30 days. The mean number of days between TUMT and TPS placement was 1.7 (range 1–5; SD ±1.3) and the TPS remained in place for a mean of 16.4 days (range 2–28; SD ±7.3).
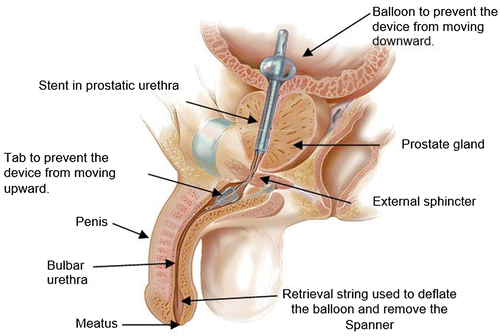
Placed in the urologist office, the TPS procedure is similar to introducing a standard IUC and uses only topical anesthesia. It is inserted using a detachable introducer, held in the bladder by an inflatable balloon and stationed within the bulbar urethra by a soft distal anchor. Tethers traverse the external sphincter, allowing normal sphincter function. The TPS is removed by traction on the retrieval tether which deflates the balloon and then withdraws the TPS from the urethra (Figure ).
BPH symptoms data pre-TUMT were also collected for all 25 patients (Table ).
Table 1. Pre-TUMT BPH symptoms
PVR was measured via bladder ultrasound before TUMT, immediately following TPS placement, and immediately following TPS removal. We performed cystoscopy on all patients, post-TPS removal, to confirm urethral patency.
3. Results
TUMT was performed on 25 consecutive patients with BPH symptoms. The patients had a mean age of 72.6 years (range 48–91), and a mean prostate size of 46.9 mL3 (range 20.5–155.1). Statistical analysis of PVR measurements was performed using a non-parametric paired t-test.
Prior to the TUMT, mean PVR urine volume was 178.4 mL (SD ±136.1). PVR measurements immediately following TPS placement were significantly decreased (p < 0.001) as compared to pre-TUMT, with a mean PVR of 47.4 mL (SD ±48.0). PVR assessed immediately following TPS removal was 107.7 mL (SD ±169.7). PVR following TPS removal were also significantly decreased (p = 0.001) as compared to PVR pre-TUMT (Table ).
Table 2. PVR (mL) pre-TUMT and post-TUMT at TPS placement and at TPS removal
None of the patients had symptomatic urinary tract infections (SUTI) prior to TPS removal and no SUTI were reported after TPS removal. Patients returned to the office for a follow-up consultation one month after TPS removal, and no complications or infections were found.
4. Discussion
Our study presents real-world clinical efficacy data that are consistent with observations in a similarly designed recent TPS study (Abdul-Muhsin, Jakob, McLemore, McAdams, & Humphreys, Citation2016). In our experience, TPS placement resulted in a statistically significant reduction in PVR, and despite a mean indwell of 16.4 days for TPS (range 2–28), none of our patients experienced SUTI. Our findings on infection rate are consistent with results of a recent study (Abdul-Muhsin et al., Citation2016) that found no symptomatic infection detected in patients wearing TPS for less than 20 days.
Common IUC concerns such as low patient tolerance and high rates of complication (Gould, Umscheid, Agarwal, Kuntz, & Pegues, Citation2010) do not apply to TPS. As such, we leave the TPS in place, because of its low infection risk and high patient tolerance (Shore et al., Citation2007). PVR with TPS in place was lower than PVR after TPS removal. Literature shows that post-TUMT retention symptoms improve over the first six weeks as edema subsides (Larson et al., Citation1998). The increase in PVR post-TPS removal may be explained by the loss of scaffolding as tissue once again partially obstructs the bladder outlet, creating urethral resistance. One may expect to see further improvements beyond six weeks as tissue healing continues and urethral resistance is further reduced.
To this end, we have performed post-TUMT cystoscopy on patients who have been treated with TPS, as well as in patients treated prior to this study who have had only an IUC placed after the procedure for less than a week. We observe that patients treated with TPS have improved urethral patency over those treated with IUC alone, and postulate that leaving the TPS in place during the post-treatment healing period allows for controlled remodeling of the prostate tissue, as the 20 Fr stent acts as a scaffold upon which prostatic tissue may heal. The ability of TPS to improve urethral patency, and thereby enhance procedure durability should be considered for future research.
5. Conclusion
The use of TPS in post-TUMT patients effectively addresses post-procedure edema by ensuring patency as the urethra heals. Our data is consistent with Shore et al. (Citation2007), which demonstrated that the use of a TPS is an effective alternative to IUC following TUMT for BPH.
Placing a TPS after TUMT results in many patient benefits that include reduced infection risk, improved PVR during and after TPS use, improved QOL and the creation of a more patent urethral channel. In our practice, we have adopted the technique of inserting a TPS following TUMT procedures as we believe this treatment protocol provides the highest quality of care for our patients.
Funding
The authors received no direct funding for this research.
Additional information
Notes on contributors
Dimitri Kessaris
Dimitri Kessaris is the Medical Director at Progressive Urology in New York. He is board certified by the American Board of Urology. Kessaris received his Bachelors of Arts in Chemistry at New York University and Medical degree from Stony Brook University Medical School. He did his general surgery and urology residency training at Long Island Jewish Medical Center.
He is also a member of professional organizations including the American Urological Association, the Hellenic Medical Society, and the Medical Society of the County of Queens.
References
- Abdul-Muhsin, H. M., Jakob, N. J., McLemore, R. M., McAdams, S. B., & Humphreys, M. R. (2016). Infectious complications associated with the use of temporary prostatic urethral stents in patients with benign prostatic hyperplasia. The Canadian Journal of Urology, 23, 8465.
- Dineen, M. K., Shore, N. D., Lumerman, J. H., Saslawsky, M. J., & Corica, A. P. (2008). Use of a temporary prostatic stent after transurethral microwave thermotherapy reduced voiding symptoms and bother without exacerbating irritative symptoms. Urology, 71, 873–877.10.1016/j.urology.2007.12.015
- Gould, C. V., Umscheid, C. A., Agarwal, R. K., Kuntz, G., & Pegues, D. A. (2010). Guideline for prevention of catheter-associated urinary tract infections 2009. Infection Control & Hospital Epidemiology, 31, 319–326.10.1086/651091
- Larson, T. R., Blute, M. L., Bruskewitz, R. C., Mayer, R. D., Ugarte, R. R., & Utz, W. J. (1998). A high-efficiency microwave thermoablation system for the treatment of benign prostatic hyperplasia: Results of a randomized, sham-controlled, prospective, double-blind, multicenter clinical trial. Urology, 51, 731–742.10.1016/S0090-4295(97)00710-3
- Saint, S., Veenstra, D. L., Sullivan, S. D., Chenoweth, C., & Fendrick, A. M. (2000). The potential clinical and economic benefits of silver alloy urinary catheters in preventing urinary tract infection. Archives of Internal Medicine, 160, 2670–2675.10.1001/archinte.160.17.2670
- Shore, N. D., Dineen, M. K., Saslawsky, M. J., Lumerman, J. H., & Corica, A. P. (2007). A temporary intraurethral prostatic stent relieves prostatic obstruction following transurethral microwave thermotherapy. The Journal of Urology, 177, 1040–1046.10.1016/j.juro.2006.10.059