Abstract
Background: The left hippocampus is believed to play an important role in memory function. Thus, left hippocampectomy should be carefully performed. However, the left hippocampus may be less important for memory in patients with right hemisphere language dominance. We performed left hippocampectomy in a patient with epilepsy and right hemisphere language dominance. Here we present the findings of our functional mapping and examine the relationship between memory and language dominance. Methods: The patient was a 32-year-old left-handed female implanted with subdural electrocorticography (ECoG) grids over both hemispheres to locate the epilepsy focus. Language dominance was determined by the Wada test. Functional localizations were identified with ECoG and electrocortical stimulation (ECS) mapping. The Wechsler Memory Scale-Revised (WMS-R) was used to evaluate the patient’s memory before and after surgery. Results: The epilepsy focus was located in the left mesial temporal lobe and hippocampus. The Wada test revealed right hemisphere language dominance. High gamma activity (HGA) mapping was consistent with ECS mapping in that both showed language-related activity in the right precentral gyrus. The WMS-R revealed improved memory after left hippocampectomy, without neurological deficits. The patient fully recovered without further epileptic seizures. Conclusion: Left hippocampectomy in a patient with epilepsy and right hemisphere language dominance did not cause memory dysfunction. Thus, memory function is possibly related to the language-dominant hemisphere. Furthermore, preoperative mapping and determining a patient’s language dominance can help to preserve language and memory in epilepsy surgery.
Public Interest Statement
Epilepsy, a group of neurological disorders which causes seizure or loss of consciousness, is one of the most common diseases in the world; however, its pathophysiology and surgical treatment have not been well known to the general public. The principal treatment for epilepsy is drug treatment. However, surgical treatment should be considered if drugs are ineffective. For surgical treatment, the most important points include identification of the epileptic lesion that should be removed and the brain functions that should be preserved. In this research, we presented a strategy of epilepsy surgery with latest pre-surgical brain functional mappings and resulted in better surgical outcomes. We believe that this article could help improve the surgical outcomes in patients with epilepsy and the advancement of brain functional mapping fields.
Competing Interests
The authors declare no competing interest.
1. Introduction
Mesial temporal lobectomy including the hippocampus is a common procedure in epilepsy surgery (Wieser, Citation1988). However, left hippocampectomy should be carefully performed because it often leads to memory dysfunction (Alpherts, Vermeulen, van Rijen, da Silva, & van Veelen, Citation2006; Berenbaum, Baxter, Seidenberg, & Hermann, Citation1997; Helmstaedter, Roeske, Kaaden, Elger, & Schramm, Citation2011). Although multiple subpial transection (MST) of the hippocampus has been noted for preserving memory function, the surgical procure is not the gold standard therapy in epilepsy surgery (Faught, Citation2002; Shimizu, Kawai, Sunaga, Sugano, & Yamada, Citation2006). Left hemisphere language dominance is common and reported in at least 90% of patients, whereas absolute right hemisphere language dominance is rare and reported in less than 10% of patients (Hardyck & Petrinovich, Citation1977; Knecht, Deppe, et al., Citation2000; Knecht, Drager, et al., Citation2000; Pujol, Deus, Losilla, & Capdevila, Citation1999). It is possible that patients with right hemisphere language dominance show similar lateralization for memory function. In this report, we present a patient with right hemisphere language dominance who suffered from left mesial temporal epilepsy. We performed functional brain mapping and used the Wada test to determine the language-dominant hemisphere, electrocorticography (ECoG) and electrocortical stimulation (ECS) mapping to localize language areas, and the Wechsler Memory Scale-Revised (WMS-R) to assess the patient’s memory. Using the findings from our functional mapping and the surgical outcome, we validated the relationship between memory function and language dominance.
2. Patient and methods
2.1. Patient
The patient was a 32-year-old left-handed female who suffered from a complex partial seizure during working time (Table ). When the seizure occurred, she fixed her gaze at one point and turned her head toward her right, with right upper limb movement. Magnetic resonance imaging (MRI) showed no hippocampal sclerosis (Figure (a) and (b)), and single-photon ECT (SPECT) showed no abnormal blood flow. We examined the patient with transcranial electroencephalography (EEG) for 1 week after her admission, but no seizure was detected. Spike-like waveforms were identified on F4 during sleep, but they were insufficient for identifying the epilepsy focus. After examination, the patient was discharged and she continued taking medication. However, the epileptic seizures did not diminish and occurred more than 10 times per month. The patient had elected for epilepsy surgery; therefore, we implanted subdural grids to identify the epilepsy focus. The study was approved by the ethics committees of the Asahikawa Medicl University Hospital. Written consent was obtained from the patients in accordance with the guidelines of the hospitals.
Table 1. Demographic and clinical characteristics of the patient
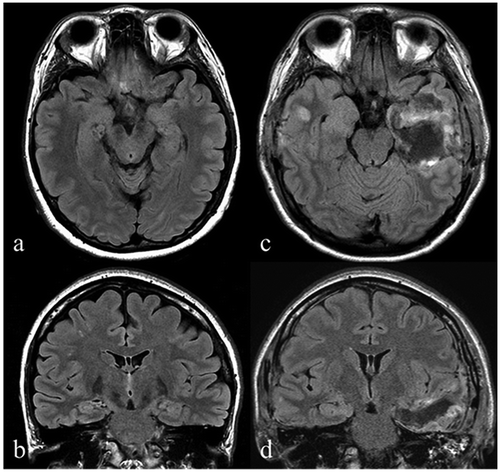
Figure 1. Anatomical imaging. The preoperative axial (a) and coronal (b) fluid-attenuated inversion recovery (FLAIR) MRI images demonstrate no hippocampal sclerosis. Post-operative axial (c) and coronal (d) FLAIR MRI images showing left-sided hippocampectomy including the amygdala and partial lateral temporal lobe.
2.2. Methods
2.2.1. Subdural grid placement and monitoring
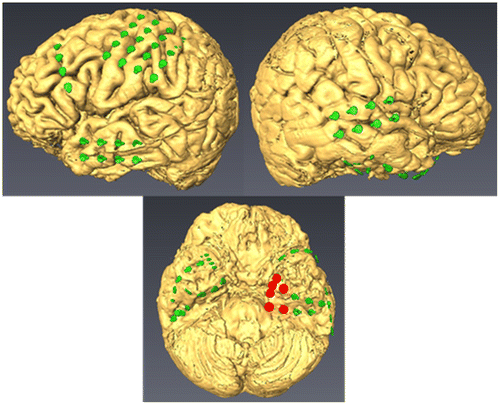
Because the seizure focus was not detected with transcranial EEG, we implanted subdural grids over both hemispheres in regions that included the temporal base. Each ECoG grid included 70 electrodes (2 were used as a reference and a ground) spaced at 1-cm inter-electrode intervals, with each electrode having a 4-mm diameter and a 2.3-mm exposed area (Unique medical, Japan). The layout drawing was created with AVIZO (Maxnet, Japan) by combining MRI brain surface images and CT electrode data (Figure ). After the subdural grids were implanted, the patient was monitored with Neurofax (Nihonkoden, Japan) to detect epileptic seizures. The data were sampled at 1,000 Hz with a 300-Hz high-cut filter and a 0.3-Hz low-cut filter; an AC filter also was used. The patient was monitored after the implant procedure until an epileptic seizure occurred.
Figure 2. Layout drawing. Left, ECoG electrodes were implanted over the motor area (20) and the lateral temporal lobe (8). The 8 triangle-shaped electrodes and 2 sets of 4 electrodes were implanted over the temporal base. Right, electrodes were implanted over the front temporal lobe (8). The 8 triangle-shaped electrodes and 2 sets of 4 electrodes were implanted over the temporal base. According to ECoG, the focus located on left mesial temporal (red circle).
2.2.2. Brain function measurements
2.2.2.1. Wada test
It was important to identify the language-dominant hemisphere before surgery because the patient was left handed. The Wada test is the gold standard technique for determining the language dominant hemisphere. We performed the Wada test in the angiography room, where we injected diluted propofol into the internal carotid arteries. The first injection was on the right side, and the second was on the left. Following the injection, the patient performed several language tasks. If the patient became aphasic after an injection, then the injected side was considered the language-dominant hemisphere. Thus, contralateral injection should not affect language function.
2.2.2.2. High gamma activity (HGA) mapping
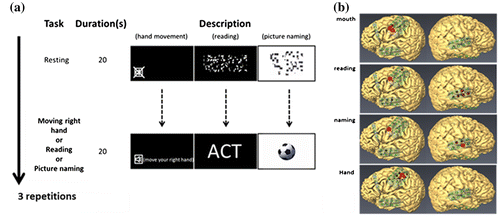
During the ECoG recordings, the patient received visual stimuli that were presented with a monitor positioned in front of her. The patient was first asked to relax during a 20-s rest period (baseline) and then to perform the task during a subsequent 20-s period. The task consisted of hand movements, mouth movements, reading words, and naming pictures (Figure (a)). The ECoG data were digitally recorded at a 1,200-Hz sampling rate using a 256-channel g.HIamp amplifier (Guger Technologies, OG). The ECoG data were analyzed with CortiQ software in g.Hiamp (Prueckl et al., Citation2013). CortiQ performed a common average reference (CAR) to suppress common mode signals and also calculated the baseline ECoG activity for each channel in the gamma frequency range of 60–120 Hz. A t-test was used to determine whether HGA values in the active phases were significantly different from those in the baseline. p-values of <0.05 were considered statistically significant. Finally, the results were visualized in real time as a red circle presented on a monitor.
Figure 3. Results of ECoG real-time mapping. (a) Schematic representation of the tasks. The task stimuli were presented on an LCD monitor positioned in front of the patient. The patient performed tasks in response to the cues. (b) The red circle indicated significant HGA during task performance ranging from 60 to 120 Hz. Language activity was located in the right precentral gyrus, whereas the motor areas were revealed over the left motor cortex. The mouth area was also activated in the language tasks.
2.2.2.3. ECS mapping
ECS mapping was performed after ECoG mapping to confirm whether HGA mapping was consistent with the gold standard mapping. Electrical stimuli were delivered using Neuromaster (Nihonkoden, Japan) and with two electrodes positioned at a short inter-electrode distance. The electrical stimuli were bipolar square-wave pulses, each with a 200-μs duration, delivered at 50 Hz over a 1-ms duration at 4–8 mA. When hand or mouth movements were evoked during stimulation, we considered the stimulation site was located in the hand or mouth motor representation, respectively. For language mapping, we presented 3 pictures or words to the patient. The first picture or word was used as a reference. After the first presentation, we performed ECS to determine whether the patient could correctly identify the second and third pictures or words during ECS. The stimulation sites at which speech arrest or difficulties were evoked were marked as language areas. In the present study, we attempted to identify memory functional areas using ECS. Before stimulation, the patient was asked to memorize 3 out of 20 randomly presented pictures. We then delivered ECS using the same parameters used in the language tasks and asked the patient to recall the memorized pictures. When the patient could not recall at least 1 picture, we regarded that area as a memory functional area.
2.2.2.4. Memory test
Memory function was tested before and after surgery using the WMS-R.
3. Results
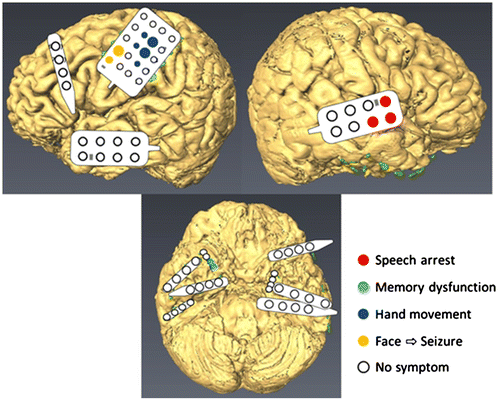
One epileptic seizure occurred during the monitoring period. According to the ECoG recordings, the seizure focus was located in the left mesial temporal lobe surrounding the left hippocampus (Figure ). During the Wada test, we observed no aphasia after left side injection, but we observed total aphasia after right side injection. HGA and ECS mapping also revealed language-related activity in the right precentral gyrus (Figures (b) and ). From these results, we determined the patient to have right hemisphere language dominance. Although left frontal activity appeared to be related to language, HGA mapping of mouth movement showed similar activation in the left frontal lobe, which suggested motor-related activity. HGA mapping also revealed a hand motor representation above the mouth motor representation (Figure (b)). The results of HGA mapping were compared with those of ECS mapping by calculating the sensitivity and specificity. For motor mapping, the sensitivity was 100% and specificity was 84%, whereas for language mapping, the sensitivity was 78% and the specificity was 73%. In addition, we identified memory functional areas using ECS. When the stimuli were delivered through electrodes positioned over the right temporal base, the patient forgot the memorized pictures (Figure ). This phenomenon lasted for 2 min, but then naturally subsided. Stimulation applied at other areas failed to evoke memory dysfunction. We assumed that language and memory functions could be preserved if the left hippocampus was resected because functional mapping indicated that language and memory functional areas were both located in the right hemisphere.
Figure 4. Speech was influenced by ECS only for the right precentral gyrus (Red). Finger movement was evoked from the motor areas (Purple). ECS evoked seizures when the facial area was stimulated (Yellow). Memory dysfunction was induced when the right temporal base was stimulated (Blue).
We subsequently performed surgery in which we resected the entire left hippocampus, amygdala, and a part of the lateral temporal lobe (Figure (c) and (d)). During surgery, we monitored ECoG activity recorded with subdural grids and confirmed that no epileptogenic activity was present after resection. The patient’s post-surgical condition was good without neurological dysfunction. As the condition improved, the WMS-R was again used to examine the patient’s memory. All scores (verbal memory, visual memory, and ordinary memory) were higher than those recorded before surgery (Table ). No seizure occurred after surgery and the patient was discharged from the hospital.
Table 2. Pre- and post-operative WMS-R scores
4. Discussion
In this study, a left-sided hippocampectomy was performed in a patient with epilepsy and right hemisphere language dominance. It was observed that the procedure did not cause any memory dysfunction. Thus, the present findings indicate that memory function is mainly related to the language-dominant hemisphere. In addition, we performed multimodality functional mapping, including the Wada test, HGA mapping, ECS mapping, and WMS-R. These measurements helped detect brain function and preserve memory function. Detailed presurgical functional mapping should be necessary in patients with epilepsy.
In the present case, the patient was left handed and showed right hemisphere language dominance. Statistical studies suggest that 70–90% of the world population is right handed, whereas approximately 10% is left handed (Ardila, Rosselli, de Estudios, & de Neuroepidemiologicos, Citation2001; Dane & Gümüstekin, Citation2002). Other types of handedness included mixed handedness and ambidexterity (Holder & Kateeba, Citation2004). In left-handed people, the incidence of right hemisphere language dominance has been reported as 15% (Rasmussen & Milner, Citation1977) and 27% (Knecht, Drager, et al., Citation2000). In those reports, only few epilepsy cases demonstrated right hemisphere language dominance with left mesial temporal epilepsy.
The left hippocampus is believed to plays an important role in verbal memory (Novelly et al., Citation1984). The other study reported memory mapping using ECS for six left language dominant subject and showed left hippocampal ECS impaired verbal memory (Coleshill et al., Citation2004). However, in our case, right hippocampal ECS impaired verbal memory and no memory dysfunction occurred after left hippocampectomy. For now, the laterality of memory function was not disclosed, but this result suggests that laterality of memory function is not determined by anatomical laterality but language dominance. Of course, non-dominant side was less important for verbal memory function than dominant hemisphere, but this side thought to play a role in visual memory (Cutting, Citation1978; Stepankova, Fenton, Pastalkova, Kalina, & Bohbot, Citation2004). In this case, however, visual memory was not affected even though the left hippocampus (non-dominant side) was totally resected. Other reports have indicated that no visual memory dysfunction occurred following non-dominant side hyppocampectomy (Hamberger, Seidel, McKhann, & Goodman, Citation2010). These findings support our present results. The post-surgical WMS-R showed improvement in all memory scores (verbal, visual, and general). The same outcome was reported in a previous study, which was due to the extinction of abnormal waveforms recorded from the contralateral hippocampus (Patrikelis et al., Citation2013).
Regarding functional mapping, it has been suggested that the Wada test and ECS should be replaced because of their invasiveness (Papanicolaou et al., Citation2014). The Wada test has a risk of vascular complication (1.3–11%) and the anesthetics used in the procedure may cause somnolence, agitation, and confusion (Bauer, Reitsma, Houweling, Ferrier, & Ramsey, Citation2014). On the other hand, ECS has a risk of seizure and is a time-consuming procedure (Ojemann, Ojemann, Lettich, & Berger, Citation2008; Pouratian, Cannestra, Bookheimer, Martin, & Toga, Citation2004). In this study, we performed functional brain mapping with real-time HGA mapping and confirmed consistency among HGA and ECS mapping and the results of the Wada test. The usefulness of HGA mapping, including real-time mapping, has been widely reported (Hill et al., Citation2012; Ogawa et al., Citation2014; Qian et al., Citation2013; Roland, Brunner, Johnston, Schalk, & Leuthardt, Citation2010). The biggest advantage of HGA mapping is that it has low invasiveness. Although subdural grid implantation is invasive, subdural grids are necessary for epilepsy diagnosis (Bekelis et al., Citation2012). In terms of functional mapping, HGA mapping is a much safer technique than the Wada test and ECS mapping because it requires only spontaneous brain activity. Therefore, HGA mapping is a useful technique and has the potential to be an alternative to ECS mapping and the Wada test.
Although ECS alone was used for memory mapping, HGA mapping also could be used for memory mapping. A recent report described HGA mapping with recall activity (Kunii, Kawai, Kamada, Ota, & Saito, Citation2014). According to this report, memory-related HGA also showed a dominance that was similar to that for language, but the two did not always correspond. The report concluded that memory-related HGA dominance plays a more important role in memory function than language dominance. In future studies, we will develop tasks that examine memory function and that can realize real-time memory functional mapping using HGA. Our next challenge is to map language dominance and memory functional areas using HGA.
5. Conclusion
This study indicates that memory function has a strong connection to language dominance. Determining language dominance before performing hippocampectomy is useful for determining whether the resection will affect the patient’s memory. Although memory functional mapping procedure has not been established, ECS mapping allowed us to detect the site. In the future, we plan to establish HGA real-time memory mapping with visualization.
Ethical standard
This study was approved by the Ethics Committee of Asahikawa Medical University.
Funding
This work was supported by Grant-in-Aid for Young Scientists (B) [grant number 16K20887] from 2016 to 2017.
Acknowledgments
The authors thank the patient for participating in this study and Enago for the English language review.
Additional information
Notes on contributors
Hiroshi Ogawa
We have studied about brain functional mapping using electrocorticography (ECoG), particularly the high frequency band of ECoG, which is thought to reflect task-related brain functional activities, such as motor or language functions. Until now, the gold standard of brain functional mapping has been electrical stimulation mapping. However, it is a time-consuming procedure and has a risk of evoking seizures, particularly in the patients with epilepsy. To overcome these limitations, we paid attention to ECoG and created a real-time ECoG functional mapping system, which enabled rapid, precise, and minimally invasive mapping. In this research, the results of ECoG mapping were consistent with that of the gold standard mappings. We assume that ECoG mapping is a powerful brain mapping procedure and has the potential to be the gold standard. We hope that many readers are interested in our research and that the ECoG mapping will be a popular technique in the future.
References
- Alpherts, W. C., Vermeulen, J., van Rijen, P. C., da Silva, F. H., & van Veelen, C. W. (2006). Verbal memory decline after temporal epilepsy surgery? A 6-year multiple assessments follow-up study. Neurology, 67, 626–631. doi:10.1212/01.wnl.0000230139.45304.eb
- Ardila, A., Rosselli, D., de Estudios, Grupo, & de Neuroepidemiologicos, C. (2001). Handedness in Colombia: Some associated conditions. Laterality: Asymmetries of Body, Brain and Cognition, 6, 77–87. doi:10.1080/713754398
- Bauer, P. R., Reitsma, J. B., Houweling, B. M., Ferrier, C. H., & Ramsey, N. F. (2014). Can fMRI safely replace the Wada test for preoperative assessment of language lateralisation? A meta-analysis and systematic review. Journal of Neurology, Neurosurgery & Psychiatry, 85, 581–588. doi:10.1136/jnnp-2013-305659
- Bekelis, K., Radwan, T. A., Desai, A., Moses, Z. B., Thadani, V. M., Jobst, B. C., … Roberts, D. W. (2012). Subdural interhemispheric grid electrodes for intracranial epilepsy monitoring: Feasibility, safety, and utility. Journal of Neurosurgery, 117, 1182–1188. doi:10.3171/2012.8.JNS12258
- Berenbaum, S. A., Baxter, L., Seidenberg, M., & Hermann, B. (1997). Role of the hippocampus in sex differences in verbal memory: Memory outcome following left anterior temporal lobectomy. Neuropsychology, 11, 585–591.10.1037/0894-4105.11.4.585
- Coleshill, S. G., Binnie, C. D., Morris, R. G., Alarcon, G., van Emde Boas, W., Velis, D. N., … van Rijen, P. C. (2004). Material-specific recognition memory deficits elicited by unilateral hippocampal electrical stimulation. Journal of Neuroscience, 24, 1612–1616. doi:10.1523/JNEUROSCI.4352-03.2004
- Cutting, J. (1978). Patterns of performance in amnesic subjects. Journal of Neurology, Neurosurgery & Psychiatry, 41, 278–282.10.1136/jnnp.41.3.278
- Dane, S., & Gümüstekin, K. (2002). Handedness in deaf and normal children. International Journal of Neuroscience, 112, 995–998.10.1080/00207450290025996
- Faught, E. (2002). Collective data supports efficacy of multiple subpial transection. Epilepsy Currents, 2, 108. doi:10.1046/j.1535-7597.2002.t01-1-00039.x
- Hamberger, M. J., Seidel, W. T., McKhann, G. M., & Goodman, R. R. (2010). Hippocampal removal affects visual but not auditory naming. Neurology, 74, 1488–1493. doi:10.1212/WNL.0b013e3181dd40f0
- Hardyck, C., & Petrinovich, L. F. (1977). Left-handedness. Psychological Bulletin, 84, 385–404.10.1037/0033-2909.84.3.385
- Helmstaedter, C., Roeske, S., Kaaden, S., Elger, C. E., & Schramm, J. (2011). Hippocampal resection length and memory outcome in selective epilepsy surgery. Journal of Neurology, Neurosurgery & Psychiatry, 82, 1375–1381. doi:10.1136/jnnp.2010.240176
- Hill, N. J., Gupta, D., Brunner, P., Gunduz, A., Adamo, M. A., Ritaccio, A., & Schalk, G. (2012). Recording human electrocorticographic (ECoG) signals for neuroscientific research and real-time functional cortical mapping. J Vis Exp, 64. doi:10.3791/3993
- Holder, M. K., & Kateeba, D. (2004). Hand preference survey of 5136 school children in Western Uganda. Laterality: Asymmetries of Body, Brain and Cognition, 9, 201–207. doi:10.1080/13576500342000059
- Knecht, S., Deppe, M., Dräger, B., Bobe, L., Lohmann, H., Ringelstein, E., & Henningsen, H. (2000). Language lateralization in healthy right-handers. Brain, 123, 74–81.10.1093/brain/123.1.74
- Knecht, S., Drager, B., Deppe, M., Bobe, L., Lohmann, H., Floel, A., … Henningsen, H. (2000). Handedness and hemispheric language dominance in healthy humans. Brain, 123, 2512–2518.10.1093/brain/123.12.2512
- Kunii, N., Kawai, K., Kamada, K., Ota, T., & Saito, N. (2014). The significance of parahippocampal high gamma activity for memory preservation in surgical treatment of atypical temporal lobe epilepsy. Epilepsia, 55, 1594–1601. doi:10.1111/epi.12764
- Novelly, R. A., Augustine, E. A., Mattson, R. H., Glaser, G. H., Williamson, P. D., Spencer, D. D., & Spencer, S. S. (1984). Selective memory improvement and impairment in temporal lobectomy for epilepsy. Annals of Neurology, 15, 64–67. doi:10.1002/ana.410150112
- Ogawa, H., Kamada, K., Kapeller, C., Hiroshima, S., Prueckl, R., & Guger, C. (2014). Rapid and minimum invasive functional brain mapping by realtime visualization of high gamma activity during awake craniotomy. World Neurosurg. doi:10.1016/j.wneu.2014.08.009
- Ojemann, G., Ojemann, J., Lettich, E., & Berger, M. (2008). Cortical language localization in left, dominant hemisphere. Journal of Neurosurgery, 108, 411–421. doi:10.3171/JNS/2008/108/2/0411
- Papanicolaou, A. C., Rezaie, R., Narayana, S., Choudhri, A. F., Wheless, J. W., Castillo, E. M., … Boop, F. A. (2014). Is it time to replace the Wada test and put awake craniotomy to sleep? Epilepsia, 55, 629–632. doi:10.1111/epi.12569
- Patrikelis, P., Lucci, G., Siatouni, A., Zalonis, I., Sakas, D. E., & Gatzonis, S. (2013). Simulating memory outcome before right selective amygdalohippocampectomy. Neuropsychological Rehabilitation, 23, 401–415. doi:10.1080/09602011.2013.772065
- Pouratian, N., Cannestra, A. F., Bookheimer, S. Y., Martin, N. A., & Toga, A. W. (2004). Variability of intraoperative electrocortical stimulation mapping parameters across and within individuals. Journal of Neurosurgery, 101, 458–466. doi:10.3171/jns.2004.101.3.0458
- Prueckl, R., Kapeller, C., Potes, C., Korostenskaja, M., Schalk, G., Lee, K. H., & Guger, C. (2013). CortiQ – Clinical software for electrocorticographic real-time functional mapping of the eloquent cortex. In Engineering in Medicine and Biology Society (EMBC), 2013 35th Annual International Conference of the IEEE (pp. 6365–6368). doi:10.1109/EMBC.2013.6611010
- Pujol, J., Deus, J., Losilla, J. M., & Capdevila, A. (1999). Cerebral lateralization of language in normal left-handed people studied by functional MRI. Neurology, 52, 1038–1038.10.1212/WNL.52.5.1038
- Qian, T., Zhou, W., Ling, Z., Gao, S., Liu, H., & Hong, B. (2013). Fast presurgical functional mapping using task-related intracranial high gamma activity. Journal of Neurosurgery, 119, 26–36. doi:10.3171/2013.2.jns12843
- Rasmussen, T., & Milner, B. (1977). The role of early left-brain injury in determining lateralization of cerebral speech functions. Annals of the New York Academy of Sciences, 299, 355–369.10.1111/nyas.1977.299.issue-1
- Roland, J., Brunner, P., Johnston, J., Schalk, G., & Leuthardt, E. C. (2010). Passive real-time identification of speech and motor cortex during an awake craniotomy. Epilepsy & Behavior, 18, 123–128. doi:10.1016/j.yebeh.2010.02.017
- Shimizu, H., Kawai, K., Sunaga, S., Sugano, H., & Yamada, T. (2006). Hippocampal transection for treatment of left temporal lobe epilepsy with preservation of verbal memory. Journal of Clinical Neuroscience, 13, 322–328. doi:10.1016/j.jocn.2005.04.020
- Stepankova, K., Fenton, A. A., Pastalkova, E., Kalina, M., & Bohbot, V. D. (2004). Object-location memory impairment in patients with thermal lesions to the right or left hippocampus. Neuropsychologia, 42, 1017–1028. doi:10.1016/j.neuropsychologia.2004.01.002
- Wieser, H. G. (1988). Selective amygdalo-hippocampectomy for temporal lobe epilepsy. Epilepsia, 29, S100–S113.10.1111/epi.1988.29.issue-s2
