Abstract
This report describes an unusual presentation of a voice disorder arising from inability to coordinate the three components of motor speech: respiration, phonation, and articulation. These systems were individually intact, as demonstrated by laryngoscopy, motor speech examination, and treatment methods achieving success under controlled conditions. Following initial programming of his deep brain stimulation device, a 62-year-old male, diagnosed with Parkinson’s disease 14 years previously, abruptly experienced a vocal disorder characterized by pressed, very low frequency creaky voice produced on held breath. Evaluation and therapy sessions revealed intact respiration, phonation, and articulation as component systems of motor speech, while indicating a severe deficit in coordinating these systems for articulated speech. Performance varied with mode of vocal production. Vowel prolongation and singing were normal in contrast to severe impairment when respiration and phonation were integrated with articulated speech. A listening study utilizing speech samples from five spoken modes—conversation, repetition, formulaic expressions, continuously phonated material and singing, yielded higher intelligibility on sung and continuously phonated phrases, confirming clinical impressions. Acoustic measures of fundamental frequency, vowel quality (harmonic-to-noise ratios) and duration supported the intelligibility results. Repetition and conversation were similarly impaired, suggesting that the disability was not attributable to the basal ganglia. This case reveals the role of higher order management of respiration, articulation, and voice for speech and describes a successful treatment utilizing breath control.
Public Interest Statement
Speech requires a precise integration of respiration, phonation (vibratory action of the larynx), and articulation (movement of tongue and lips). This study reports a failure to coordinate these systems, although the individual modules were intact, implying a deficit in higher order programming of motor speech. A person who had undergone an adjustment of his therapeutic device, deep brain stimulation, for Parkinson’s disease, suffered a sudden inability to manage breath flow, phonation, and articulation. The result was a strained, nearly unintelligible “creaky voice” produced on a held breath. Vowel prolongation and singing were normal in contrast to severe impairment when respiration and phonation were integrated with articulated speech. Therapeutic intervention revealed improved speaking when breath flow was carefully controlled and articulation demands were minimal, as in “hello.” This case reveals the role of higher order management of respiration, articulation, and voice for speech and describes a successful treatment utilizing breath control.
Competing interests
The authors declare no competing interest.
1. Introduction
Speech requires a fast and efficient integration of three disparate systems: articulatory, laryngeal, and respiratory. The lungs supply on-going subglottal pressure to the laryngeal structures, which titrate puffs of air through intermittently vibrating vocal folds into the supraglottal vocal tract for continuous and dynamic articulatory shaping.Footnote1 The coordination of hundreds of muscular processes operating in millisecond time frames is sine que non for normal motor speech. This high level of coordination between breathing, phonating, and articulating emerges in the normal course of events for every normally developing child and adult.
Little is understood about the neurological substrate for the overall control of this physiological cooperation. Some insights into how speech systems are interleaved for fluent production can be gained by examining individual instances of damage to one or the other system. In an early study, a proportion of persons diagnosed with Parkinson’s disease, who had laryngeal dysfunction, were found also to have articulatory anomalies of tongue and lips (Logemann, Fisher, Boshes, & Blonsky, Citation1978). Apraxias of individual components of the speech system may be explained by specific anatomical abnormalities, such as buccofacial apraxia (Alexander, Baker, Naeser, Kaplan, & Palumbo, Citation1992). Speech breathing has been shown to be abnormal in some cases of Parkinson’s disease (Solomon & Hixon, Citation1993), and deep brain stimulation of the subthalamic nucleus (STN-DBS) alters respiratory and laryngeal control (Hammer, Barlow, Lyons, & Pahwa, Citation2010). Respiratory apraxia has been reported for several neurological disorders, including progressive supranuclear palsy and multiple sclerosis (Atack & Suranyi, Citation1975; Haouzi, Chenuel, & Barroche, Citation2006; Leiguarda et al., Citation1997; Pinto, Pinto, Atalaia, Peralta, & de Carvalho, Citation2007; Zadikoff & Lang, Citation2005). Apraxia of the respiratory system in amyotrophic lateral sclerosis (ALS), a disease of the motor neurons, was characterized by irregular breathing on command but not in automatic mode (Pinto et al., Citation2007). Close coupling of lip and laryngeal action was demonstrated in persons with spasmodic dysphonia and muscle tension dysphonia revealed lip gesture abnormalities; in that study, vocal therapy resulted in improvements in articulatory function (Maassen, Citation2010). However, the broad scope of unified motor speech coordination remains still evades easy explication.
The purpose of this study was to describe an unusual case of a failure to successfully coordinate the three systems for speech, although each system was found to be healthy and capable of function. While the site of brain damage cannot be definitely described, the apractic loss of coordination of phonatory, respiratory, and articulatory systems occurred acutely within hours of initial programming of bilateral subthalamic nucleus stimulators and resumption of levodopa medication for Parkinson’s disease. Speech problems remained stable over the course of a year of modifications of his subthalamic nucleus-deep brain stimulation (STN-DBS) programming, including turning the stimulators off, and medication adjustments. Close examination of preserved and impaired speech performance modes led to a proposal of a site of lesion outside of the basal ganglia.
2. Participant description
This case study describes a 62-year-old, right-handed male, native speaker of British English, with 16 years of education, diagnosed with Parkinson’s disease (PD) 14 years previously, presenting originally with right sided tremor. The individual has lived in the United States of America for 30 years. On the Hoehn and Yahr (Citation1967) rating scale, he was at stage 2 (bilateral or midline involvement without impairment of balance). Medication dosage was 650 mg levodopa/carbidopa (Sinemet) per day. One of his primary symptoms was speech impairment. His spontaneous speech was characterized by mild monopitch, monoloudness, low vocal volume, hypernasal resonance, and articulatory imprecision, all typical of Parkinson’s disease. His speech impairment was mild and he was sometimes difficult to understand in conversation.
Speech and language were normal prior to the Parkinson’s disease diagnosis and continued post diagnosis to be functional in his profession as an educator in a large corporation, where he held teaching seminars during the course of the disease. First programming of the deep brain stimulation (DBS) implant was performed while the individual was on medication at 12 years into the course of the disease. Some minor speech changes and reduction of tremor were observed initially. The second programming occurred three days later. Within the same day, on the train home return, speech failed and swallowing problems arose. According to the patient’s report, the leg-foot tremor returned immediately.
On subsequent clinical examination, speech was observed to be produced on continuous, low amplitude creaky voice (vocal fry) resulting in a highly unusual vocal production, corroborated as extremely rare by Dr B. Gerratt (personal communication, 2014), Director of the Speech Pathology Clinic at the David Geffen School of Medicine at the University of Californiat at Los Angeles (UCLA) and voice expert, who listened to recordings of the voice disorder. The participant typically held his breath while talking, exhaling explosively at the end of the phrase. While speech coordination appeared overall to fail, the major contributor to the failed process was the respiratory component. Conversational speech was unintelligible. Oral-motor evaluation revealed normal lip and tongue movement. Videostroboscopy (direct examination) of the larynx revealed no abnormalities. Breathing was normal during nonspeech periods. A brain imaging procedure using magnetic resonance imaging performed one month later showed no abnormalities and the electrode placement at the subthalamic nucleus, the target site, was verified.
Intelligibility was formally assessed using the standardized battery, Assessment of Intelligiblity of Dysarthric Speech (38). Results indicated that on the single word intelligibility subtest, 26 out of 50 (52%) of words were correctly identified using a multiple choice judging format. Word recognition was better for two syllable words, with 64%, being identified correctly than for single syllable words where 41% of words were identified correctly. On the sentence intelligibility subtest, 39 out of 220 words were intelligible (18% of words were correctly transcribed). Out of 220 total words in the subtests, 181 were unintelligible, revealing a severe compromise to intelligibility of speech.
During evaluation and therapy sessions, relatively successful production could be achieved on continuously phonated or vocalized material, such as vowel prolongation (producing the vowels “ah” or “oo”) and singing. Given the abnormal breath flow during articulated speech, we explored methods of breath control, enhancing and practicing improvement of phonatory competence using singers’ techniques. “Dropping” the breath (“belly breath”) was practiced as was speaking on a long exhale. These activities resulted in stretches of intelligible speech, utilizing continuously phonated phrases, such as hello and how are you. These sessions were video and audio recorded for later analysis.
Given that performance varied with speech modes, as has been reported (Boersma and Weenink, Citation2009; Huber & Darling, Citation2011; Kempler & Lancker, Citation2002; Tjaden & Wilding, Citation2011; Van Lancker Sidtis, Cameron, Bonura, & Sidtis, Citation2011; Van Lancker Sidtis, Cameron, & Sidtis, Citation2012; Van Lancker Sidtis, Choi, Alken, & Sidtis, Citation2015; Van Lancker Sidtis, Rogers, Godier, Tagliati, & Sidtis, Citation2010), structured sets representing selected differing modes were created and offered during therapy. These previous studies have revealed that motor speech efficacy differs for conversation and repetition (Huber & Darling, Citation2011; Kempler & Lancker, Citation2002; Tjaden & Wilding, Citation2011; Van Lancker Sidtis et al., Citation2010, Citation2011, Citation2012, Citation2015). Other studies suggested that formulaic and novel expressions are differently processed in the brain (Boersma and Weenink, Citation2009). Current work with the participant indicated more successful production on continuously voiced material. We selected excerpts representing these modes to test our clinical impressions, using listeners.
Types of phrases probed in therapy were spontaneous speech, sentence repetition, singing, formulaic expressions, and continuously voiced phrases. As mentioned above, phonatory creak with extreme breath control was most extreme when speech required coordination of breathing, phonation and articulation involving a range of phonetic inventory including voiceless sounds (f, t, p, k, th). In order to verify the impression that some speech modes were more successfully produced, and therefore more intelligible than others, a listening study was prepared for healthy listeners to transcribe utterances from spontaneous speech, sentence repetition, singing, formulaic expressions, and continuously phonated phrases. These utterances were excerpted from several months of videotaped treatment sessions with the participant.
3. Methods and materials
3.1. Listening study
Fifty-six utterances consisting of lengths of from 2 to 6 words representing the five speech modes taken from recorded sessions were randomized for the listening study. Background noise on the recording was reduced through acoustic manipulation in the Praat environment (Rammell, Pisoni, & Van Lancker Sidtis, Citationin press). Eighteen native English-speaking listeners, mean age of 28 years, born and educated in the United States, without history of confounding conditions, transcribed each utterance and provided a difficulty rating from 1 (easy) to 5 (difficult). The purpose was to quantify the intelligibility of the different modes of speech: spontaneous speech, sentence repetition, singing, formulaic expressions, and continuously phonated phrases.
3.2. Acoustic analysis
Six speech samples were submitted to analysis using Praat software (Rammell et al., Citationin press). In addition to the five modes utilized in the listening protocol, vowel prolongation was measured acoustically. Because the fundamental frequency was abnormally low and irregular, leading to artifacts in the pitch analysis program, ten individual pitch periods, identified at the central portion of each utterance, were measured on the wave form and averaged to derive a mean F0 value. Voice and resonance in the speech samples were measured using mean harmonic to noise ratios (HNR), providing measures of the amplitude of periodic portions of the signal relative to aperiodic (Yorkston & Beukelman, Citation2012).
4. Results
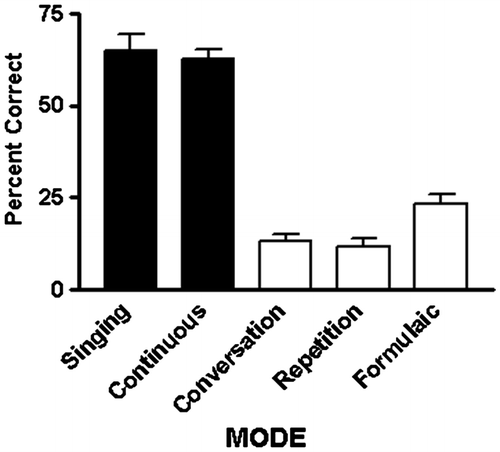
The effect of speech mode was significant for both transcription accuracy [F(4,68) = 118.76; p < 0.001] and perceived difficulty of the task [F(4,68) = 54.9; p < 0.001] (Figure ). Conversational speech was less accurately transcribed than formulaic speech [t(17) = 3.61; p = 0.002], singing [t(17) = 12.12; p < 0.001], and therapy-directed speech [t(17) = 20.08; p < 0.001]. Conversational speech and repetition did not differ. Formulaic speech transcriptions were more accurate than those for repeated speech [t(17) = 3.82; p = 0.001], but less accurate than transcriptions of sung [t(17) = 10.18; p < 0.001] or therapy-directed speech [t(17) = 17.15; p < 0.001]. Transcriptions of repeated speech were less accurate than those for sung [t(17) = 11.63; p < 0.001] or therapy-directed speech [t(17) = 15.94; p < 0.001]. Sung and therapy-directed speech did not differ.
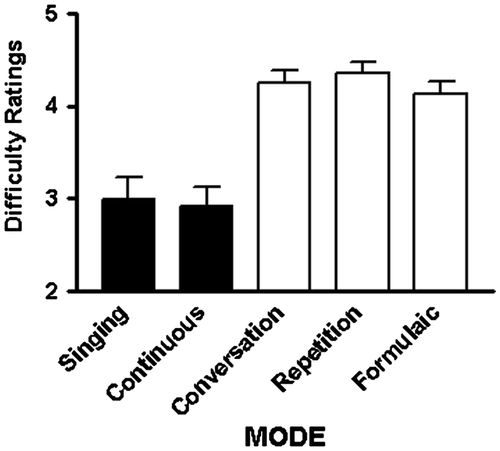
Difficulty ratings tracked the accuracy measures (Figure ). Transcribing conversational speech was not judged to be more difficult than transcribing formulaic or repeated speech, but was more difficult than transcribing sung [t(17) = 8.02; p < 0.001] or therapy-directed speech [t(17) = 8.11; p < 0.001]. Transcribing formulaic speech was judged to be less difficult than transcribing repeated speech [t(17) = 3.22; p = 0.005], but more difficult than sung [t(17) = 7.63; p < 0.001] or therapy-directed speech [t(17) = 8.92; p < 0.001]. Repeated speech was judged to be more difficult to transcribe than sung [t(17) = 7.4; p < 0.001] or therapy-directed speech [t(17) = 8.51; p < 0.001]. Sung and therapy-directed speech did not differ.
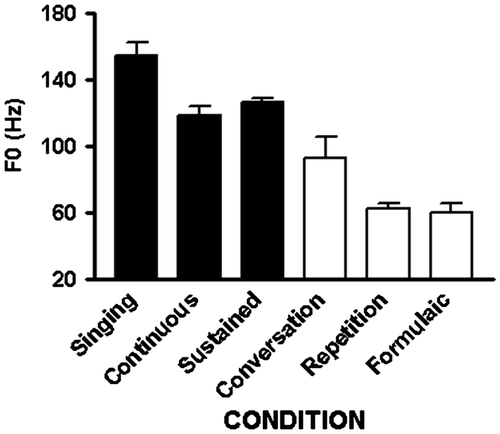
For the fundamental frequency measure, there was a main effect of task [F(5,25) = 27.5; p < 0.001]. As can be seen on Figure , singing, continuously voiced speech, and vowel prolongation yielded F0 measures at125 Hz and above, while the other three speaking modes, which require articulation of voiceless consonants, were produced at 95 Hz or below. Post hoc procedures using paired sample t-tests revealed that singing F0 was significantly higher than all other conditions: sustained vowels [t(5) = 3.79; p = 0.013], continuously voiced utterances [t(5) = 2.93; p = 0.032], spontaneous speech [t(5) = 4.13; p = 0.009], repetition [t(5) = 12.23; p < 0.001], and formulaic expressions [t(5) = 8.36; p < 0.001]. Sustained vowels had a higher F0 than repetition [t(5) = 15.57; p < 0.001], and formulaic expressions [t(5) = 11.62; p < 0.001]. Continuously voiced utterances had higher F0 than repetition [t(5) = 6.89; p < 0.001] and formulaic expressions [t(5) = 6.43; p < 0.001]. Finally, spontaneous speech had higher F0 than repetition [t(5) = 2.84; p = 0.036].
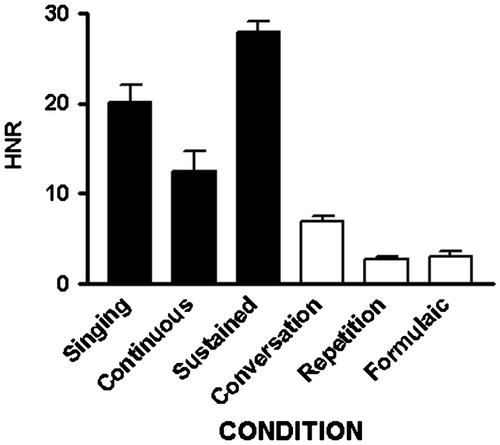
The harmonic-to-noise ratio measures reflected the F0 values also having a main effect of task [F(5,25) = 89.3; p < 0.001] (Figure ). Sustained vowels had higher HNR than singing [t(5) = 3.54; p < 0.017], continuously voiced utterances [t(5) = 9.52; p < 0.001], spontaneous speech [t(5) = 14.92; p < 0.001], repetition [t(5) = 23.47; p < 0.001], and formulaic expressions [t(5) = 26.17; p < 0.001]. Singing had higher HNR than continuously voiced utterances [t(5) = 2.69; p < 0.043], spontaneous speech [t(5) = 5.34; p = 0.003], repetition [t(5) = 9.24; p < 0.001], and formulaic expressions [t(5) = 9.91; p < 0.001]. Continuous voiced utterances had higher HNR than spontaneous speech [t(5) = 5.12; p = 0.004], repetition [t(5) = 11.1; p < 0.001], and formulaic expression [t(5) = 9.65; p < 0.001]. Spontaneous speech had higher HNR than repetition [t(5) = 6.42; p = 0.001] and formulaic expressions [t(5) = 3.53; p = 0.017].
The modes in which utterances were based on continuous phonation with those produced with varied phonetic demands (intermittent condition) were collapsed (singing, continuous phonation, and sustained vowel production vs. conversation, repetition, and formulaic utterance production). The average durations of the samples in the two modes did not differ. As apparent from the figures, however, the average F0 in the continuously phonated condition (133.3 ± 20.6) was significantly greater [t(34) = −8.16; p < 0.001] than in the intermittent condition (72.0 ± 24.3). Similarly, the average HNR in the continuously phonated condition (20.2 ± 7.8) was significantly greater [t(34) = −8.37; p < 0.001] than in the intermittent condition (4.2 ± 2.3). These acoustic results closely track the performance measures, accuracy and confidence, from the intelligibility study.
5. Discussion
This description presents an apractic (motor speech planning disorder) deficit in voluntarily coordinating respiratory, laryngeal, and articulatory systems, with a focused disability on breath control. As such, this description moves beyond reports of deficient systems or impaired cooperation of two systems. The case reported here represents a form of motor speech system apraxia following STN-DBS programming, affecting overall coordination of systems underlying speech. The participant was acutely compromised in his ability to integrate respiratory, phonatory, and articulatory systems volitionally, leading to a strategy of breath-holding during articulated speech while adducting the vocal folds, producing a low amplitude vocal fry. Clinical evaluation indicated that some speech modes were more successful than others. The most successful speech production occurred on treatment items that included words and phrases designed to be produced on a consistent stream of air across the glottis with minimal articulatory demand. Articulation was normal when continuously phonated utterances were elicited. These, together with sung items, were also most accurately transcribed when presented in a controlled listening protocol. This result leads to the conclusion that the disability involved central coordination of the three systems of speech. Slightly better performance for formulaic expressions is likely attributable to their familiarity; in a previous study, formulaic expressions heard in noise were significantly better transcribed than matched grammatical expressions (Iulianella, Adams, & Gow, Citation2008). For all test items in the listening study, difficulty ratings mirrored transcription performance. Acoustic measures supported the intelligibility results by indicating that adequate F0 and HNR measures occurred primarily on the continuously phonated elements but were impaired on speech requiring a range of phonetic inventory and concomitantly more precise motor speech control.
The observation that repetition and conversation were similarly poorly transcribed, compared to the other modes, differs from other motor speech disorders due to basal ganglia disease, in which repeated utterances are more successfully produced (and understood) than conversational ones (Baev, Citation1995; Rosen, Kent, Delaney, & Duffy, Citation2006; Rusz, Cmejla, & Tykalova, Citation2013; Tjaden & Wilding, Citation2011; Van Lancker Sidtis et al., Citation2005; Van Lancker Sidtis et al., Citation2015). In persons with Parkinson’s disease, repeated utterances are significantly more intelligible than matched spontaneous, conversational utterances, and motor speech measures, such as vowel quality and fluency, support this observation (Van Lancker Sidtis et al., Citation2011). This finding is consistent in basal ganglia dysfunction, and has been attributed to the benefit in repetition of the availability of a model in the motor production process. Deficits in Parkinson’s disease are more severe in when motor tasks must be internally modeled as compared to being provided with an external model (Baev, Citation1997; Georgiou et al., Citation1994; Lewis et al., Citation2007; Schenk, Baur, Steude, & Bötzel, Citation2003). This has been documented for gait and arm reach (Atchison, Thompson, Frackowiak, & Marsden, Citation1993; Burleigh, Horak, Nutt, & Obeso, Citation1997; Fimm, Bartl, Zimmermann, & Wallesch, Citation1994; Georgiou et al., Citation1993; Rice, Antic, & Thompson, Citation2002). In speech, repetition presents an external model, reducing the burden of motor planning in the basal ganglia. The pattern for this participant, for whom a voice disturbance in conversation and repetition appeared equally impaired, suggests a level of impairment for this specific dyskinesia elsewhere than the basal ganglia.
This case is unlike previously reported PD presentations, where levodopa induced respiratory dyskinesia was successfully treated by DBS (Oyama et al., Citation2011; Xie, Guan, Staisch, Towle, & Warnke, Citation2015; Yorkston & Beukelman, Citation2012). Stimulation during initial programming plus the effects of reinstating medication may have resulted in a delayed lesion effect along the tracts of one of the electrodes. Consistent use of creaky voice likely represented a compensatory process by lowering demands inherent in coordinating the breath stream for phonation with the overlay of articulation. The disability described here went beyond previous observations of abnormalities in one or two components, rather, affecting voluntary coordination of all three speech systems. Further detection, awareness and study of this unique disability may shed light on the site and function of higher order speech coordinating mechanisms. In addition, this paper describes a treatment plan, incremental application of breath control from simple to more complex phonated utterances, that led to successful production of intelligible speech.
Funding
This study was supported by a grant from the National Institute of Deafness and Communicative Disorders [grant number R01 DC 007658].
Acknowledgements
We appreciate the contributions of Angela Ferranti, Michele Burgevin, Amy Alken, and the Brain and Behavior Laboratory research associates at various stages of this study.
Additional information
Notes on contributors
Diana Van Lancker Sidtis
John J. Sidtis, Director, and Diana Van Lancker Sidtis, Associate Director oversee projects in The Brain and Behavior Laboratory, NKI, Orangeburg, NY. The laboratory studies studying the relationships between human behavior and the brain through measuring physiological parameters and examining behaviors after brain injury. Our focus is on speech and language. Our current funding specifies studies of the effects of deep brain stimulation (DBS), a therapy for Parkinson’s disease, on speech. The ability to evaluate speech samples produced with the DBS stimulators turned on or off allows for closer scrutiny of the role of subcortical structures in motor speech performance. In addition to group studies, we perform examinations of individuals with anomalous conditions. Single cases can contribute to better understanding of how speech and language are processed in the brain. The case study presented here represents a rare pathological condition in a person diagnosed with Parkinson’s disease arising from adjustment of the DBS stimulators, and one that offers insight into overall neurological control of motor speech.
Notes
1. For a review of this process, see Kreiman and Sidtis (Citation2011, Chapter 2).
References
- Alexander, M. P., Baker, E., Naeser, M. A., Kaplan, E., & Palumbo, C. (1992, February). Neuropsychological and neuroanatomical dimensions of ideomotor apraxia. Brain, 115(1), 87–107.10.1093/brain/115.1.87
- Atack, E. A., & Suranyi, L. (1975). Respiratory inhibitory apraxia. Canadian Journal of Neurological Sciences, 2(10), 37–43.10.1017/S031716710001996X
- Atchison, P. R., Thompson, P. D., Frackowiak, R. S., & Marsden, C. D. (1993, July). The syndrome of gait ignition failure: A report of six cases. Movement Disorders, 8(3), 285–292.10.1002/(ISSN)1531-8257
- Baev, K. V. (1995, February). Disturbances of learning processes in the basal ganglia in the pathogenesis of Parkinson’s disease: A novel theory. Neurological Research, 17(1), 38–48.10.1080/01616412.1995.11740285
- Baev, K. V. (1997, February). Highest level automatisms in the nervous system: A theory of functional principles underlying the highest forms of brain function. Progress in Neurobiology, 51(2), 129–166.10.1016/S0301-0082(96)00053-6
- Boersma, P., & Weenink, D. (2009). Praat: Doing phonetics by computer (Version 5.1.11) [Computer program]. Retrieved July 19, 2009, from http://www.praat.org/
- Burleigh, J. A., Horak, F. B., Nutt, J. G., & Obeso, J. A. (1997, March). Step initiation in Parkinson’s disease: Influence of levodopa and external sensory triggers. Movement Disorders, 12(2), 206–215.10.1002/(ISSN)1531-8257
- Fimm, B., Bartl, G., Zimmermann, P., & Wallesch, C. W. (1994). Different mechanisms underlie shifting set on external and internal cues in Parkinson’s disease. Brain and Cognition, 25(2), 287–304.10.1006/brcg.1994.1037
- Georgiou, N., Bradshaw, J. L., Iansek, R., Phillips, J. G., Mattingley, J. B., & Bradshaw, J. A. (1994, March). Reduction in external cues and movement sequencing in Parkinson’s disease. Journal of Neurology, Neurosurgery & Psychiatry, 57(3), 368–370.10.1136/jnnp.57.3.368
- Georgiou, N., Iansek, R., Bradshaw, J. L., Phillips, J. G., Mattingley, J. B., & Bradshaw, J. A. (1993, December). An evaluation of the role of internal cues in the pathogenesis of parkinsonian hypokinesia. Brain, 116(6), 1575–1587.10.1093/brain/116.6.1575
- Hammer, J. J., Barlow, S. M., Lyons, K. E., & Pahwa, R. (2010, October). Subthalamic nucleus deep brain stimulation changes speech respiratory and laryngeal control in Parkinson’s disease. Journal of Neurology, 257(10), 1692–1702.10.1007/s00415-010-5605-5
- Haouzi, P., Chenuel, B., & Barroche, G. (2006, June). Interactions between volitional and automatic breathing during respiratory apraxia. Respiratory Physiology & Neurobiology, 152(2), 169–175.10.1016/j.resp.2005.08.004
- Hoehn, M. M., & Yahr, M. D. (1967). Parkinsonism: Onset, progression, and mortality. Neurology, 17, 427–442.
- Huber, J. E., & Darling, M. (2011, February). Effect of Parkinson’s disease on the production of structured and unstructured speaking tasks: Respiratory physiologic and linguistic considerations. Journal of Speech Language and Hearing Research, 54(1), 33–46.10.1044/1092-4388(2010/09-0184)
- Iulianella, I., Adams, S. G., & Gow, A. K. (2008). Effects of sub-thalamic deep brain stimulation on speech production in Parkinson’s disease: A critical review of the literature. Canadian Journal of Speech-Language Pathology & Audiology, 32(2), 85–91.
- Kempler, D., & Lancker, D. (2002, March). The effect of speech task on intelligibility in dysarthria: Case study of Parkinson’s disease. Brain and Language, 80(3), 449–464. PMID: 11896652.10.1006/brln.2001.2602
- Kreiman, J., & Sidtis, D. (2011). Foundations of voice studies. Hoboken, NJ: Blackwell-Wiley.
- Leiguarda, R. C., Pramstaller, P. P., Merello, M., Starkstein, S., Lees, A. J., & Marsden, C. D. (1997, January). Apraxia in Parkinson's disease, progressive supranuclear palsy, multiple system atrophy and neuroleptic-induced parkinsonism. Brain, 120(1), 75–90.10.1093/brain/120.1.75
- Lewis, M. M., Slagle, C. G., Smith, A. B., Truong, Y., Bai, P., McKeown, M. J., … Huang, X. (2007, June). Task specific influences of Parkinson’s disease on the striato-thalamo-cortical and cerebello-thalamo-cortical motor circuitries. Neuroscience, 147(1), 224–235.10.1016/j.neuroscience.2007.04.006
- Logemann, J. A., Fisher, H. B., Boshes, B., & Blonsky, E. R. (1978, February). Frequency and co-occurrence of vocal tract dysfunctions in the speech of a large sample of Parkinson’s patients. Journal of Speech and Hearing Disorders, 43(1), 47–57.10.1044/jshd.4301.47
- Maassen, B. (2010). Laryngeal articulatory coupling in three speech disorders. In P. Van Lieshout, B. Maassen, & H. Terband (Eds.), Speech motor control: New developments in basic and applied research (pp. 283–296). Oxford: Oxford University Press.10.1093/acprof:oso/9780199235797.001.0001
- Oyama, G., Foote, K. D., Iyer, S. S., Zeilman, P., Hwynn, N., Jacobson, C. E., … Okun, M. S. (2011). Unilateral GPiDBS as treatment for levodopa-induced respiratory dyskinesia in Parkinson Disease, 17(5), 282–285.
- Pinto, S., Pinto, A., Atalaia, A., Peralta, R., & de Carvalho, M. (2007, June). Respiratory apraxia in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis, 8(3), 180–184.10.1080/17482960701249340
- Rammell, C. S., Pisoni, D., & Van Lancker Sidtis, D. (in press). Perception of formulaic and novel expressions under acoustic degradation: Evidence for a unitary memory trace.
- Rice, J., Antic, R., & Thompson, P. D. (2002). Disordered respiration as a levodopa-induced dyskinesia in Parkinson’s disease. Movement Disorders, 17(3), 524–527.10.1002/(ISSN)1531-8257
- Rosen, K. M., Kent, R. D., Delaney, A. L., & Duffy, J. R. (2006). Parametric quantitative acoustic analysis of conversation produced by speakers with dysarthria and healthy speakers. Journal of Speech Language and Hearing Research, 49(2), 395–411.10.1044/1092-4388(2006/031)
- Rusz, J., Cmejla, R., & Tykalova, T. (2013). Imprecise vowel articulation as a potential early marker of Parkinson’s disease: Effect of speaking task. The Journal of the Acoustical Society of America, 134(3), 2171–2181.10.1121/1.4816541
- Schenk, T., Baur, B., Steude, U., & Bötzel, K. (2003). Effects of deep brain stimulation on prehensile movements in PD patients are less pronounced when external timing cues are provided. Neuropsychologia, 41(7), 783–794.10.1016/S0028-3932(02)00286-5
- Solomon, N. P., & Hixon, T. J. (1993, April). Speech breathing in Parkinson’s disease. Journal of Speech Language and Hearing Research, 36(2), 294–310.10.1044/jshr.3602.294
- Tjaden, K., & Wilding, G. (2011, February). Effects of speaking task on intelligibility in Parkinson’s disease. Clinical Linguistics & Phonetics, 25(2), 155–168.10.3109/02699206.2010.520185
- Van Lancker Sidtis, D., Cameron, K., Bonura, L., & Sidtis, J. J. (2011). Speech intelligibility by listening in Parkinson speech with and without deep brain stimulation. Journal of Neurolinguistics, 25(2), 121–132. PMID: 21971352.
- Van Lancker Sidtis, D., Cameron, K., & Sidtis, J. J. (2012, August). Dramatic effects of speech task on motor and linguistic planning in severely dysfluent parkinsonian speech. Clinical Linguistics & Phonetics, 26(8), 695–711 . PMID: 22774929.10.3109/02699206.2012.696307
- Van Lancker Sidtis, D., Choi, J.-H., Alken, A., & Sidtis, J. J. (2015, October). Formulaic language in Parkinson’s and Alzheimer’s disease: Complementary effects of subcortical and cortical dysfunction. Journal of Speech Language and Hearing Research, 58(5), 1493–1507 . PMID: 26183940.10.1044/2015_JSLHR-L-14-0341
- Van Lancker Sidtis, D., Hanson, W., Jackson, C., Lanto, A., Kempler, D., & Metter, E. J. (2005). Fundamental frequency (f0) measures comparing speech tasks in aphasia and parkinson’s disease. Journal of Medical Speech-Language Pathology, 12(4), 207–212.
- Van Lancker Sidtis, D., Rogers, T., Godier, V., Tagliati, M., & Sidtis, J. J. (2010, October). Voice and fluency changes as a function of speech task and deep brain stimulation. Journal of Speech Language and Hearing Research, 53(5), 1167–1177. PMID: 20643796.10.1044/1092-4388(2010/09-0154)
- Xie, T., Guan, R., Staisch, J., Towle, V. L., & Warnke, P. (2015, August). Respiratory dyskinesia in a patient with Parkinson disease successfully treated with STN DBS. Neurology, 85, 479–480.10.1212/WNL.0000000000001809
- Yorkston, K., & Beukelman, D. (2012). Assessment of intelligibility of dysarthric speech. Boston, MA: ProEd.
- Zadikoff, C., & Lang, A. E. (2005). Apraxia in movement disorders. Brain, 128(7), 1480–1497.10.1093/brain/awh560